Abstract
Human cytomegalovirus (HCMV) is ubiquitous in all populations, and is the most commonly recognized cause of congenital viral infection in developed countries. On the basis of the economic costs saved and the improvement in quality of life that could potentially be conferred by a successful vaccine for prevention of congenital HCMV infection, the Institute of Medicine has identified HCMV vaccine development as a major public health priority. An effective vaccine could potentially also be beneficial in preventing or ameliorating HCMV disease in immunocompromised individuals. Although there are no licensed HCMV vaccines currently available, enormous progress has been made in the last decade, as evidenced by the recently reported results of a Phase II trial of a glycoprotein B vaccine for the prevention of HCMV infection in seronegative women of childbearing age. HCMV vaccines currently in clinical trials include: glycoprotein B subunit vaccines; alphavirus replicon particle vaccines; DNA vaccines; and live-attenuated vaccines. A variety of vaccine strategies are also being examined in preclinical systems and animal models of infection. These include: recombinant vesicular stomatitis virus vaccines; recombinant modified vaccinia virus Ankara; replication-deficient adenovirus-vectored vaccines; and recombinant live-attenuated virus vaccines generated by mutagenesis of cloned rodent CMV genomes maintained as bacterial artificial chromosomes in Escherichia coli. In this article, we provide an overview of the current state of clinical trials and preclinical development of vaccines against HCMV, with an emphasis on studies that have been conducted in the past 5 years. We also summarize a number of recent advances in the study of the biology of HCMV, particularly with respect to epithelial and endothelial cell entry of the virus, which have implications for future vaccine design.
Keywords: congenital cytomegalovirus infection, cytomegalovirus glycoprotein B, cytomegalovirus vaccine, endothelial cell entry, epithelial cell entry, human cytomegalovirus
Overview of the clinical problem of congenital cytomegalovirus infection: the rationale for a cytomegalovirus vaccine
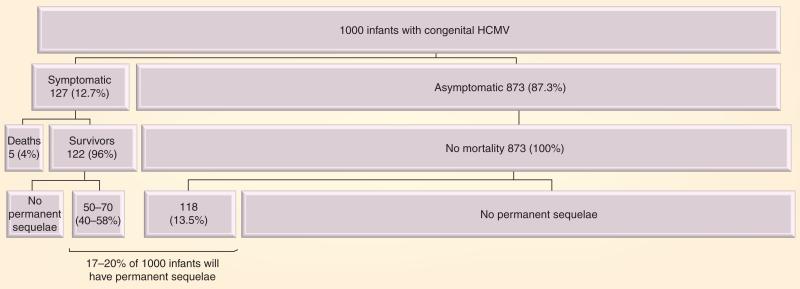
Human cytomegalovirus (HCMV) is a ubiquitous β-herpesvirus that leads to congenital infection in 0.5–2% of all pregnancies, often with devastating consequences for the developing fetus [1]. Transmission of HCMV to the fetus can occur during any trimester, but the risk of adverse fetal effects appears to be highest when primary infection occurs in the first half of pregnancy [2]. Among congenitally infected infants, approximately 10% have signs and symptoms of disease at birth. Although the remaining 90% of infants are asymptomatic at birth, 10–15% will subsequently develop permanent sequelae, including sensorineural hearing loss and mental retardation (Figure 1) [3–5]. The public health impact of congenital HCMV infection is substantial and under-recognized: in fact, more children suffer from long-term neurodevelopmental handicaps as a result of congenital HCMV infection than either Down syndrome or fetal alcohol syndrome [6].
Figure 1. Estimates of long-term sequelae in infants with congenital human cytomegalovirus.
In a given cohort of 1000 infants with congenital HCMV, 170–190 will have permanent sequelae, of whom one out of three is from the symptomatic group and two out of three are from the asymptomatic group.
HCMV: Human cytomegalovirus.
Data from [3].
Congenital HCMV infection can occur as the result of a primary maternal infection, re-activation of a latent infection, or re-infection with a new strain of virus [7–9]. Although imperfect, preconception maternal immunity to HCMV provides some degree of protection against vertical transmission of the virus; this is the key observation driving interest in development of a preconception vaccine for HCMV. Reports in the literature regarding transmission of HCMV to the fetus in the setting of primary maternal infection vary widely, with a range of 24–75%, but the risk is high, with an overall risk of transmission of approximately 30% reported in a recent meta-ana lysis [10]. By contrast, the risk of HCMV transmission to the fetus after a recurrent maternal infection during pregnancy appears to be in the range of 0.15–2% [11]. The incidence of congenital HCMV infection is dependent on the overall seroprevalence in the population, with highly seropositive populations exhibiting the highest rates of infection [12]. The total number of congenitally infected infants born in the setting of recurrent maternal infection is 4–5-fold greater than those born following primary maternal infection [13]. These observations suggest that current vaccine strategies for young women, which have focused on vaccination of the seronegative woman, may require re-evaluation. Indeed, a HCMV vaccine may ideally need to prevent re-infection, by ‘boosting’ immunity in seropositive women. A recent multicenter study of newborn screening for congenital HCMV infection identified an overall prevalence of 0.45% in the seven sites surveyed; although the serological status of women in this study was not described, this provides a useful benchmark for overall population-based estimates of the impact of congenital infection on pregnancies in the USA [14]. The impact of preconception immunity was demonstrated in a study by Fowler et al., which reported a 69% reduction in the risk of congenital HCMV infection in future pregnancies in women who were seropositive for HCMV when compared with seronegative women [15]. Not only do women with preconception immunity to HCMV have a significant reduction in transmission of virus to the fetus, but some studies also suggest that there is a decreased severity of disease if their infants are congenitally infected, compared to infected infants born to women who had primary infection in pregnancy. It has been reported that 25% of congenitally infected infants whose mother had a primary HCMV infection during pregnancy had at least one sequela, compared with 8% in infants born to women with recurrent infection [11].
These observations regarding the protective effect of preconception maternal immunity have bolstered the case for development of a maternal vaccine, toward the goal of reducing both the incidence of congenital HCMV and the severity of disease if vertical transmission occurs. A HCMV vaccine could also, in theory, benefit other patient populations at high risk for HCMV disease, including allograft recipients, hematopoietic stem cell transplant patients and patients with malignancies [16]. However, the magnitude of the neurodevelopmental injury associated with congenital HCMV infection makes women of childbearing age the most important target population for vaccine development from both an economic and and a public health perspective. A recent Institute of Medicine report, ‘Vaccines for the 21st Century’, characterized a HCMV vaccine as the ‘highest-level (level 1) priority for vaccine development in the new millennium’ [17,18]. A recent review published in 2005 summarized the status of HCMV vaccines [19], but since publication of this article, there has been a significant surge in interest in moving vaccines forward in clinical trials, driven largely by the success of a purified recombinant glyco protein B (gB) vaccine in a Phase II study (described in the following section) [20]. In this article, we summarize recent activity in HCMV vaccines since 2005 and consider preclinical strategies that may move forward in future clinical trials.
What has gone into people – an update on the status of recent and ongoing clinical trials
A number of HCMV vaccines have been evaluated in clinical trials and substantial advances have been made in the field of HCMV vaccine development. Here, we summarize the data from clinical trials since 2005 (Table 1).
Table 1.
Human cytomegalovirus vaccines that have undergone evaluation in clinical trials since 2005.
| Vaccines | Status | Characteristics and results | Ref. |
|---|---|---|---|
| gB/MF59 adjuvant | Phase II study completed | Acceptable safety for further studies Evaluated in HCMV-seronegative women within 1 year after they had given birth Vaccine efficacy of 50% on the basis of infection rates per 100 person years |
[20] |
| gB/pp65/IE1 alphavirus replicon trivalent vaccine | Phase I study completed | Favorable safety profile Evaluated in healthy, nonpregnant adults Elicits humoral and cellular immune responses Based on replication-deficient alphavirus technology |
[39] |
| gB/pp65 bivalent DNA vaccine | Ongoing Phase II study | Well tolerated with no serious adverse events in a Phase I study HCMV-seropositive or -seronegative healthy adults in Phase I and HCT recipients in Phase II studies Higher frequencies of HCMV-specific pp65 and gB T cells compared with placebo |
[42,47] |
| Towne ± rhIL-12 ± priming by DNA vaccine encoding pp65, IE1 and gB | Phase I studies completed | Favorable safety profile; no evidence for viral latency or viral shedding in recipients Evaluated in HCMV-seronegative healthy adults Augmentation of immunogenicity by inclusion of rhIL-12 or DNA vaccine in Phase I studies |
[52,53] |
gB: Glycoprotein B; HCMV: Human cytomegalovirus; HCT: Hematopoietic stem cell transplant; IE1: Immediate–early protein with a molecular mass of 72 kDa; pp65: Phosphoprotein 65 (ppUL83); rhIL-12: Recombinant human IL-12.
Trial of a subunit vaccine consisting of recombinant HCMV envelope gB with MF59 adjuvant
All HCMV-infected individuals have antibody to gB, an essential viral glycoprotein that is necessary for viral infectivity [21]. A significant proportion of neutralizing antibodies to HCMV in human serum are specific for epitopes on gB [21–25]. A recent study of the use of HCMV gB vaccine plus MF59 adjuvant was reported. This study was a Phase II, randomized, double-blind, placebo-controlled clinical trial in seronegative women of childbearing age, in which vaccine was administered following a 0-, 1- and 6-month schedule [20]. The major experimental end point reported in this study was the time to primary HCMV infection, documented by seroconversion using an assay in which viral target antigens were depleted of gB protein [26,27]. Primary HCMV infection was confirmed by virus culture or immunoblotting in 18 out of 225 (8%) of the vaccine group compared with 31 out of 216 (14%) of the placebo group, an overall efficacy of 50% (95% CI: 7–73%).
One question raised by the results of this study is whether or not a vaccine efficacy of 50% would be adequate for eventual widespread implementation of the gB vaccine into clinical practice. Insight into this question can be gained by considering the overall force of infection of HCMV in the population. The basic reproductive number (R0) of HCMV is estimated to be approximately 1.7–2.5, meaning that only 1.7–2.5 of individuals will become infected from exposure to any single person infected [28,29]. As calculated in one study [30], HCMV could be eradicated from a community via ‘herd immunity’ if 50% (if R0 = 1.7) or 60% (if R0 = 2.5) of the population is protected from primary infection. Thus, the 50% level of gB vaccine efficacy demonstrated in the recently reported Phase II study, performed in a population with intense exposure to HCMV, may already be sufficient to prevent HCMV transmission within a community [20,31,32]. Although the basic reproductive number of HCMV is similar to that of smallpox (R0 = 2.3–2.4), Thus, the 50% level of gB vaccine efficacy demonstrated in the recently reported phase II study, performed in a population with intense exposure to HCMV, may already be sufficient to prevent HCMV transmission within a community [20, 31, 32].
Only limited information could be gleaned from this study about the impact of gB vaccination on congenital HCMV infection. Congenital HCMV infection occurred in one out of 81 (1.2%) and three out of 97 (3.1%) infants born to gB vaccine and placebo recipients, respectively. One infant in the placebo group had severe symptomatic congenital HCMV infection. However, the sample size for the study was too small to support any conclusions about efficacy on the basis of the infection rate in newborns [20,33]. Therefore, although the study demonstrated that the gB vaccine could significantly reduce the risk of acquiring primary maternal HCMV infection, the study did not address the question of whether vaccine-induced HCMV immunity was equivalent to natural immunity in modulating either infection rate or sequelae for the fetus [33]. Future studies, such as a Phase III clinical trial with the rate of congenital infection as the primary end point, would be required to determine the possibility of protection of women of childbearing age (and more importantly, their newborns) through universal immunization [31].
More local and systemic reactions occurred in the gB vaccine group than in the placebo group, but the majority of these reactions were mild and short lived. There were no significant differences between the vaccine and placebo groups in overall rates and severity of adverse events, indicating that the safety profile of gB vaccine is outstanding and that further studies are warranted [20,33]. Further studies are needed to define the duration of protection and the correlation between antibody level and subsequent protection, and to optimize the immunization schedule. Since re-infection with new strains of HCMV with which the host has no prior experience can lead to transmission to the fetus with subsequent sequelae [8,34], the issue of cross-protection against diverse clinical isolates following administration of gB vaccine from a single genotype must also be defined in future studies.
Clinical trial evaluation of a two-component alphavirus replicon particle vaccine containing HCMV gB and phosphoprotein 65 (pp65)/immediate early fusion proteins
The gB and the tegument phosphoprotein 65 (pp65, also known as ppUL83) are the HCMV antigens most frequently recognized by CD4+ T cells, and pp65 is also one of the antigens most frequently recognized by CD8+ T cells in immune individuals [35]. Therefore, for vaccination strategies aimed at eliciting T-cell responses, most attention has focused on the pp65 protein [36]. The HCMV immediate–early protein with a molecular mass of 72 kDa (IE1) is also an important target of the CD8+ T-cell response to HCMV infection, and IE1-specific responses have been identified in up to 40% of HCMV-seropositive subjects [37]. Thus, the IE1 gene product is also being evaluated as a candidate vaccine immunogen in a number of clinical trials.
Alphavirus replicon vector systems are being used as platforms for the development of many prophylactic and therapeutic vaccines for infectious diseases and cancer [38], and this platform has also been applied to HCMV vaccines. The initial report of the use of an alphavirus vaccine against HCMV was a randomized, double-blind, Phase I study of an alphavirus replicon in healthy, HCMV-seronegative adult volunteers [39]. AVX601 is a two-component alphavirus replicon particle vaccine expressing HCMV gB and an in-frame fusion protein of pp65–IE1 [40]. In a recently described clinical trial with two groups of 20 HCMV-seronegative individuals, subjects in group 1 received the lower dose of vaccine (1 × 107 infectious units) and subjects in group 2 received the higher dose of vaccine (1 × 108 infectious units). For each dosage level, 16 participants received the study vaccine and four participants received placebo by intramuscular or subcutaneous injection at 0, 8 and 24 weeks after enrollment [39]. A total of 4 weeks after the third dose, 93% of subjects in group 1 and 100% of subjects in group 2 developed neutralizing antibodies detected by microneutralization assay. T-cell responses to each of the HCMV antigens measured by ex vivo, direct IFN-γ enzyme-linked immunospot (ELISpot) assay were detected in 90–97% of vaccine recipients after the second dose, with similar rates of response in groups 1 and 2. The magnitude of the T-cell response was greatest for pp65 followed by gB and IE1. Polychromatic flow cytometry demonstrated that HCMV antigen-specific CD4+ and CD8+ effector T cells producing multiple cytokines in response to HCMV peptide stimulation were induced in all subjects immunized with AVX601.
The vaccine was well tolerated, with only mild-to-moderate local reactogenicity, although erythema and induration began or persisted for more than 7 days in half of the subjects after subcutaneous injection. Mild-to-moderate systemic reactogenicity was reported in some subjects, with no clinically important changes in laboratory parameters. These results support acceptable safety for further evaluation of AVX601. Further studies are required to demonstrate the vaccine's ability to prevent HCMV infection in women of childbearing age and to prevent congenital HCMV disease.
Bivalent HCMV DNA vaccine
As already discussed, the potential value of HCMV vaccines is not limited to prevention of congenital infection [36]. Since immunocompromised patients, such as transplant recipients, are also a target population for HCMV vaccination, the use of a HCMV DNA vaccine in immunocompromised subjects would eliminate the safety concerns of live-attenuated HCMV or live recombinant viral-vectored vaccines [41]. DNA vaccines elicit robust CD4+ and CD8+ T-cell and antibody responses and are, in principle, well suited to specificity and precision in vaccine design [101]. Although the favorable safety profile of DNA vaccines favors the transplantation/oncology patient setting as a logical target population for evaluation and possible licensure, efficacy in this patient population could lead to future studies for prevention of HCMV infection and disease in mothers and infants.
As with the alphavirus platform, DNA vaccines for HCMV have focused on immunogens gB and pp65. VCL-CB01, a bivalent HCMV DNA vaccine that contains two plasmids encoding HCMV pp65 and gB (along with poloxamer CRL1005 and benzalkonium chloride to increase immunogenicity) was evaluated in a Phase I, open-label, dose-escalating trial in healthy HCMV-seropositive and HCMV-seronegative adults [42]. Through week 16 of the study, immunogenicity was documented in 45.5% of HCMV-seronegative subjects and in 25% of HCMV-seropositive subjects who received the full vaccine series, defined by gB-binding ELISA and/or ex vivo IFN-γ ELISpot assay with pp65 or gB antigens. An additional assay, the cultured IFN-γ ELISpot assay, was employed to evaluate whether the vaccine primed the memory T-cell response; this assay demonstrated a positive response to pp65 and/or gB in 68.2% (15 out of 22) of HCMV-seronegative subjects, including subjects who did not have detectable responses by ex vivo IFN-γ ELISpot assay or ELISA. These results suggest that the VCL-CB01 vaccine has the ability to prime antigen-specific T cells, with the capacity to proliferate and secrete IFN-γ on restimulation with antigen [43, 44], and that the cultured ELISpot assay appears to be more sensitive than the ex vivo ELISpot assay for the detection of vaccine-induced antigen-specific T-cell responses [42]. A limitation of the VCL-CB01 vaccine was the lack of gB antibody response in HCMV-seropositive subjects, although the vaccine boosted the existing pp65 T-cell response. Further modifications of this vaccine may be required to optimize immunogenicity, particularly to the gB moiety and amongst HCMV-seropositive individuals.
VCL-CB01 was generally well tolerated. The most common adverse events (AEs) were mild and moderate site injection pain, and no serious AEs were observed. Although a grade 1 AE (transient or mild discomfort) developed in 81.8% of study subjects, and a grade 2 AE (mild-to-moderate limitation in activity) developed in 40.9% of study subjects, the severity of AEs did not increase significantly with increasing dose or accelerated vaccine schedule, and all AEs resolved without sequelae [42]. These safety data are comparable to those observed in other Phase I clinical trials of HCMV vaccines, and support the continued investigation of VCL-CB01 in future studies.
A Phase II, randomized, double-blind, placebo-controlled clinical trial to assess the safety and immunogenicity of VCL-CB01 is ongoing in donors and recipients undergoing allogeneic hemato poietic stem cell transplantation (HCT) [45–47]. In preliminary immunogenicity analyses, higher frequencies of HCMV-specific pp65 and gB T cells were observed at days 56 and 84 after transplantation in 33 HCMV-seropositive recipient-only individuals, immunized at 3–5 days prior to, and 3–6, 12 and 28 weeks after transplantation compared with placebo [45,46]. However, there were no differences in anti-gB antibody levels after transplantation. The VCL-CB01 vaccine had an impact on HCMV disease in this population. Interim results from a Phase II study with 80 HCT recipients demonstrated 24–70% reductions in the occurrence of HCMV infection, recurrence of HCMV infection, duration of DNAemia, area under the curve for cumulative viral load post-HCT and peak magnitude of DNAemia in VCL-CB01-vaccinated patients compared with recipients receiving placebo. In addition, the time to initial viral reactivation was reduced, and a site-by-site ana lysis suggested that vaccine recipients required a shorter duration of antiviral therapy and were less likely to initiate antiviral therapy compared with placebo [46,47]. The final results from the Phase II trial will be of value for developing strategies to prevent HCMV disease in HCMV-seropositive transplant recipients, and may lead to other trials of VCL-CB01 or related vaccines for the prevention of congenital HCMV infection [47].
Live-attenuated HCMV Towne vaccine with or without adjuvant recombinant IL-12 and/or priming by DNA vaccine
Although several HCMV vaccines have been developed and tested in humans, the vaccine that has been most extensively evaluated for the longest period of time is the live-attenuated Towne vaccine [48–50]. Immunization with Towne vaccine prevented HCMV disease in seronegative renal transplant recipients, although it did not prevent infection in these patients or in parents of HCMV-infected children [48,50]. Recent evidence suggests that the relative defect in Towne vaccine may be related to inadequate antigen-specific IFN-γ responses by CD4+ and CD8+ T cells following vaccination [51]. A few approaches to improve the immunogenicity of the Towne vaccine are currently being explored [52–54]. One approach, reviewed in the previous update on CMV vaccines published in 2005 [19], was to generate a series of genetic recombinant vaccines containing regions from the genome of the unattenuated Toledo strain of HCMV, substituted for the corresponding regions of the Towne genome [54]. In another approach, HCMV DNA vaccine is used to prime for memory immune responses to Towne vaccine [53]. A third approach is to co-administer Towne with recombinant human (rh)IL-12 [52]. Each of these approaches is briefly reviewed in the following sections.
The immunogenicity and safety of the Towne vaccine with adjuvant rhIL-12 were evaluated in a Phase I, dose-escalation, randomized clinical trial in HCMV-seronegative healthy volunteers [52]. The adjuvant effect of rhIL-12 was associated with dose-related increases in peak anti-HCMV gB IgG titers and improved viral lysate-specific CD4+ T-cell proliferation responses. There was a trend (p = 0.09) toward a higher proportion of subjects developing a positive CD8+ T-cell IFN-γ response to the pp65 peptide pool at any time point after Towne vaccine administration in the groups assigned to rhIL-12 doses of 0.5 μg or higher. The vaccine with adjuvant rhIL-12 at doses up to 2 μg was well tolerated, and rIL-12 co-administration with Towne did not lead to Towne persistence in vaccinees, as confirmed by whole-blood HCMV DNA PCR and urine HCMV culture. Thus, this adjuvanted Towne vaccine is sufficiently safe to warrant future studies in HCMV-seronegative individuals.
As noted, another approach to improve the immunogenicity of Towne has recently been reported, in which a HCMV DNA vaccine was used to prime for memory responses [53]. The priming effect of VCL-CT02, a DNA vaccine containing HCMV genes pp65, IE1 and gB (cloned from the AD169 strain), along with Towne vaccination, was evaluated in a series of Phase I clinical trials in healthy HCMV-seronegative volunteers [53]. HCMV-specific memory T cells were detected by cultured INF-γ ELISpot assay in 60% of subjects (three out of five) primed intramuscularly. The median time to first pp65 T-cell response and gB antibody response after Towne vaccination was 14 days for DNA-primed subjects and 28 days for controls administered Towne only, suggesting a more rapid induction of antigen-specific responses (all p < 0.05 by log-rank test). Although the DNA vaccine alone had minimal immunogenicity, vaccination with VCL-CT02 was found to be able to safely prime for an anamnestic response to administration of the Towne vaccine. In the intramuscular or intradermal trial, VCL-CT02 was well tolerated with no severe AEs. The most common AEs observed were mild and transient local reactions, such as pain, erythema and swelling at the injection site. All urine HCMV cultures obtained after Towne vaccination were negative in all subjects. Further studies attempting to increase and optimize immunogenicity and to test its capability of moderating the course of HCMV infection would be beneficial.
The other approach to improve the immunogenicity of the Towne vaccine that continues to be evaluated in clinical trials is the family of intertypic ‘chimeric’ vaccines generated as mixtures of Towne and Toledo genome [54]. These vaccines retain some, but not all, of the mutations that apparently contribute to Towne vaccine attenuation and were hypothesized to be less attenuated and, hence, presumably more immunogenic than the Towne vaccine. Four independent chimeric vaccines were produced and tested in a double-blind, placebo-controlled study [54]. Although vaccinations with the Towne/Toledo chimeras failed to boost humoral and cellular immune responses to HCMV in healthy sero positive volunteers, these four vaccine candidates were well tolerated and did not cause systemic infection [54]. Future studies are warranted to ascertain the safety and immunogenicity of these vaccine candidates in HCMV-seronegative individuals who would likely represent the eventual target of a HCMV vaccine program [19].
Preclinical vaccine development – what is in the pipeline?
Several other vaccination strategies have been proposed for prevention of HCMV infection and disease and some have been validated, to varying degrees, in animal models using rodent CMVs. The following section summarizes work from these model systems, with an emphasis on novel strategies developed and evaluated in vivo, and published since 2005 (Table 2).
Table 2.
Alternative cytomegalovirus vaccine strategies that have been explored in preclinical/animal model studies since 2005.
| Vaccine strategies | Characteristics and results | Ref. |
|---|---|---|
| Recombinant vesicular stomatitis virus | Favorable safety profile – minimal adverse effects in a mouse model Neutralizing antibody response and CD8+ IFN-γ response to gB Protection against challenge with MCMV in a mouse model Potential for induction of mucosal immune responses through mucosal vaccination |
[56] |
| Recombinant modified vaccinia virus Ankara | Good safety profile Excellent humoral and cellular immune responses in HLA A2 Tg mice and rhesus macaques Protective efficacy in rhesus macaque models Large foreign gene capacity enables expression of multiple immunogens |
[58,64,67,68] |
| Replication-deficient adenovirus | Favorable safety profile owing to inability to replicate in human cells Neutralizing antibody responses and virus-specific T-cell responses in HLA A2 Tg mice Efficacy in a HLA A2 mouse model Potential for induction of mucosal immune responses |
[72,73] |
| Recombinant live CMV by BAC mutagenesis | Severely attenuated in vivo, even in immunodeficient mice MCMV-specific antibodies and a strong T-cell response Protective in a mouse model with replication-competent mutant Offers theoretical potential for specifically engineered vaccines with deletions in putative pathogenesis or immune evasion genes |
[79] |
BAC: Bacterial artificial chromosome; CMV: Cytomegalovirus; gB: Glycoprotein B; MCMV: Murine cytomegalovirus; Tg: Transgenic.
Recombinant vesicular stomatitis virus expressing murine cytomegalovirus gB
Mucosal surfaces play a key role in acquisition of HCMV infection. HCMV can be transmitted via saliva, sexual contact, placental transfer, blood transfusion, solid-organ transplantation or HCT [55]. The nasal, oral and genital mucosas are natural routes for horizontal HCMV infection. As a recombinant vaccine vector, vesicular stomatitis virus (VSV) can induce strong humoral and cellular immunity, particularly at mucosal surfaces. This attribute makes recombinant VSV (rVSV) an attractive candidate for development of a vectored HCMV vaccine [56].
Toward the goal of validating this strategy, live rVSV vector expressing a murine CMV (MCMV) homolog of the gB protein has been developed and tested in the mouse model [56]. To assess the efficacy of a mucosal rVSV-gB vaccine in protection against infection with MCMV, a single intranasal dose of rVSV-gB was administered and a challenge dose of live MCMV was injected intraperitoneally into mice 4 weeks after immunization. All of the immunized mice developed serum antibodies to gB. Immunization with rVSV-gB induced neutralizing antibody responses, and resulted in reduced viral titers in lung homogenates following MCMV challenge infection, compared with the unvaccinated controls. Splenocytes from VSV-gB immunized mice produced a CD8+ IFN-γ response to gB, suggesting that rVSV-gB stimulates a cellular immune response. Immunized mice exhibited minimal AEs and none of the mice required veterinary intervention. The safety and immunogenicity data from this animal trial provides support for potential further studies of this strategy in human clinical trials.
Recombinant modified vaccinia virus Ankara
The attenuated poxvirus, modified vaccinia virus Ankara (MVA), was developed as a safer alternative to licensed vaccinia virus derivatives as a potential smallpox vaccine, and has been well established as a safe and potent antigen delivery system [57]. An attractive feature of MVA for delivery of heterologous antigens as potential vaccines is its potential for accomodating a large complement of foreign genes. This appears to be related to the fact that the MVA genome has undergone six major deletions during serial passage [57], which, in turn, allows the insertion of multiple HCMV genes without impacting replication or packaging of subsequent antigens [58].
A recombinant MVA vaccine has been constructed that expresses a soluble, secreted form of HCMV gB, based on the AD169 strain sequence [59]. In preclinical studies, high levels of gB-specific neutralizing antibodies, which appear to be equivalent to levels induced by adjuvanted subunit gB protein immunization or by natural HCMV infection [60–62] in humans, were elicited in mice vaccinated with gB-MVA [59]. The gB-MVA immunization produced very similar levels of gB-specific antibody response and had similar booster efficacy regardless of pre-existing MVA or vaccinia virus immunity, suggesting utility for prospective vaccinees who have received the smallpox vaccine [36,59,63]. A trivalent MVA expressing gB, pp65 and IE1 has been developed and proposed for clinical studies [64]. This trivalent MVA vaccine potently induced humoral and cellular immunity to gB after immunization of mice. Further support for human studies comes from an experiment using two healthy volunteers in whom their peripheral blood mononuclear cells (PBMCs) were stimulated with the trivalent MVA vaccine; in this experiment, specific cytotoxic T-cell responses to pp65 and IE1 were elicited [64]. Recombinant MVAs have similarly been generated expressing both full-length pp65 and exon 4 of IE1, and are capable of inducing robust primary cell-mediated immunity and stimulating vigorous expansion of memory T-cell responses to both antigens in PBMCs of HCMV-seropositive donors [58]. These properties make the MVA-based vaccine approach a potentially ideal strategy for generation of antigen-specific T cells for adoptive transfer therapy [64–66], as well as for priming and boosting immunity in transplant recipients [58]. Another recombinant MVA expressing pp65 and a fusion protein of HCMV IE1 exon 4 and IE2 exon 5 was constructed to maximize the representation of IE-specific immunity [67]. This approach is based on the observation that the IE2 stimulated a vigorous CD8+ and a smaller CD4+ T-cell memory response in a large percentage of asymptomatic HCMV-seropositive adults [35]. In an experiment using two different types of humanized transgenic mice that express human HLA A2 or B7 without expressing murine class I MHC-1 alleles, the recombinant MVA was processed efficiently by multiple HLA alleles [67]. Evaluation of human PBMCs from healthy HCMV-seropositive donors or patients within 6 months of receiving HCT showed robust stimulation of existing HCMV-specific CD4+ and CD8+ T cells [67].
A recombinant MVA vaccine was evaluated in a rhesus macaque model of CMV infection. In this study, an MVA expressing the rhesus CMV gB, pp65-2 and IE1 homologs was generated and administered to rhesus macaques with or without prior priming with DNA plasmid vaccines for the same antigens [68]. In the study, following the first recombinant MVA boosting, the rhesus macaques primed with only a single dose of DNA plasmids elicited earlier and stronger antibody and cellular immune responses than those without priming. Similar levels of antibodies to anti-gB and pp65-2, neutralizing antibody response, and pp65-2- and IE1-specific CD4+ and CD8+ T cells were detected in both vaccinated groups after the second MVA boost, with the exception of anti-IE1 antibodies, which were not detectable in the MVA group. Plasma peak viral loads were reduced in both vaccine groups compared with untreated controls [68]. These promising data from this highly relevant nonhuman primate model underscore the potential of MVA-vectored vaccines for prevention of HCMV infection and disease, and justify continued development of these vaccines for eventual study in clinical trials.
Replication-deficient adenovirus-vectored polyepitope vaccine
Another vectored vaccine approach that has recently been pursued in preclinical studies is that of utilization of a replication-deficient adenoviral vector for expression of CMV subunit vaccine candidates [69–73]. Similar to the rVSV vaccines [56], systemic and mucosal immunity to MCMV could be induced by intranasal immunization using a replication-deficient adenoviral vector-expressing MCMV gB or glycoprotein H in a murine model [70,71]. More recently, modified adenoviral vector Ad5F35, Ad5F35-AD-1, has been generated, expressing the immunodominant antigenic domain-1 epitope of HCMV gB based on the sequence from the AD169 strain [72]. After in vitro infection with Ad5F35-AD-1, PBMCs from healthy blood donors expressed AD-1 antigen to high levels with little detectable cytopathic effect. Since the AD-1 epitope is well conserved between different strains of HCMV [74], expression of the AD-1 epitope from AD5F35 is expected to elicit neutralizing antibody responses to diverse clinical isolates [72].
As noted, most efforts in clinical trials of candidate subunit HCMV vaccine development and testing have focused on gB, pp65 and IE1 [36]. However, many other HCMV-encoded proteins play a key role in the host immune response [35,75–77]. A novel replication-deficient adenoviral-vectored vaccine, Ad-gBCMVpoly, was designed to induce a broad repertoire of HCMV-specific immune responses [73]. Ad-gBCMVpoly encodes 46 HCMV T-cell epitopes from multiple antigens, such as IE1, IE2, pp50, pp65, pp150, gB, gH and DNase. This HLA class I- and class II-restricted T-cell polyepitope was covalently linked to the extracellular domain of HCMV gB antigen, which allowed the expression of the HCMV polypeptide and gB proteins as a single fusion protein [73]. Immunization with this chimeric vaccine elicited neutralizing antibody responses and virus-specific CD4+ and CD8+ T-cell responses in a murine model [69,73]. Mice immunized with this chimeric vaccine strongly resisted infection with recombinant vaccinia virus expressing HCMV antigens gB and IE1 [69,73]. Following in vitro stimulation with the vaccine, there was a rapid expansion of multiple antigen-specific human CD4+ and CD8+ T cells from healthy HCMV-seropositive individuals. As the Ad-gBCMVpoly vaccine can induce both humoral and cellular immunity, it has the potential to be useful in different clinical settings, ranging from congenital infection (where IgG plays a key role in protecting the fetus) to primary infection or reactivation of HCMV in immunocompromised patients (where T cells may be the key effectors of protection) [69,73,78]. This polyepitope vaccine could be delivered with any of a number of vectors, including DNA vaccines or recombinant protein vaccines, further underscoring its potential value in a variety of approaches designed to protect the at-risk host against HCMV infection and/or disease [69].
Recombinant live CMV vaccine by bacterial artificial chromosome mutagenesis
An ideal live-attenuated HCMV vaccine should grow to high titers in cell culture for easy production, should be severely attenuated in vivo, even in immunocompromised hosts, and should elicit a strong immune response sufficient to protect against HCMV-associated disease [79,80]. A novel approach to the generation of such a vaccine is the targeted deletion of CMV genes modulating the host immune response [79]. This approach has been facilitated by the advances in mutagenesis of cloned CMV genomes maintained as bacterial artificial chromosomes (BACs) in Escherichia coli as well as the rapidly expanding knowledge about the role of viral genes in immunopathogenesis and immune evasion [79,81–83].
To test this concept, a recombinant MCMV lacking a total of 32 genes as 31.2 kb at the left and right genome termini of the wild-type genome, Δm01–17+m144–158-MCMV, was generated by MCMV BAC mutagenesis [79]. This MCMV mutant has lost the genes m01–17 and m144–158 and, thereby, most of the known immune modulators regulating functions in MHC-1 presentation (m04, m06 and m152) and natural killer (NK) cell response (m144, m145, m152, m155 and m157) [79,80]. The recombinant deletion mutant replicated to wild-type levels in vitro but was severely attenuated in vivo in immunocompetent BALB/c mice and even in the SCID/bg mouse, which lacks NK, B and T cells and which is the mouse strain most sensitive to MCMV [84]. It was well-tolerated in mice deficient for the type I IFN receptor [79]. The recombinant deletion mutant induced MCMV-specific antibodies and a specific cellular immune response detectable by tetramer staining of peripheral cytotoxic CD8+ T lymphocytes and protected mice from challenge with wild-type MCMV [79]. Immunization with the UV-inactivated recombinant deletion mutant induced no protective immunity to subsequent challenge with wild-type MCMV, indicating that viral replication in vivo was necessary for successful protection [79,80]. Targeted deletion of CMV genes by BAC mutagenesis offers a novel and valuable tool for rationale design of live-attenuated HCMV vaccines. Preclinical evaluation in animal models can aid in generating recombinant vaccines by experimental determination of the attenuation and immunogenicity of candidate vaccines following removal of viral genes that plays roles in pathogenesis and/or immune evasion [79,80].
Advances in understanding the basic biology of HCMV applied to novel vaccine design
A number of recent advances in the study of the biology of HCMV have identified novel potential vaccine targets, particularly those involved in epithelial/endothelial cell entry, as areas for future vaccine design. Another intriguing area of HCMV biology that has recently been elucidated is the impact of HCMV genes on host antiviral defense mechanisms mediated through dsRNA-activated protein kinase (PKR). The possible utility of future vaccine strategies based on these areas of new knowledge is considered in this section of the article.
Epithelial/endothelial cell entry & the HCMV UL128–131 protein complex
Recent studies into the attachment and entry of HCMV into epithelial and endothelial cells have demonstrated that the pathways by which the virus enters fibroblasts compared with endothelial/epithelial cells are different. Moreover, the host neutralizing antibody response is more complex than previously thought, with some antibody targeting and neutralizing virus at the interface with epithelial/endothelial cells. These observations suggest that vaccine design may need to anticipate and prevent these distinct pathways of infection. Cell type-specific mechanisms of entry were initially identified by Hahn and colleagues [85]. This work, using an endotheliotropic strain of HCMV cloned as a BAC (FIX-BAC), demonstrated that three HCMV genes, UL128, UL130, and UL131, were critical for endothelial cell tropism. These genetic determinants of endothelial tropism were subsequently also found to be responsible for HCMV entry into dendritic and epithelial cells [86,87]. Endocytic entry of HCMV requires a complex of HCMV proteins: gH, gL, UL128, UL130 and UL131. Evidence to suggest that a HCMV vaccine targeting the endocytic pathway of entry would provide advantages over existing HCMV vaccine strategies has been provided by the work of the McVoy [88] and Gerna [89] laboratories. These studies demonstrated that the majority of the neutralizing activity of convalescent human sera from HCMV-seropositive individuals targets the endocytic pathway of entry. Sera from recipients of the Towne vaccine or the gB/MF59 vaccine showed that Towne recipient sera and gB/MF59 recipient sera had epithelial neutralizing titers that were, on average, 28-fold and 15-fold lower than titers from seropositive subjects with HCMV infection, respectively [88]. Accordingly, subunit expression of the gH/gL/UL128/UL130/UL131 proteins as vaccine candidates merits consideration for future preclinical and clinical development.
Viral immune modulation genes: the key role of PKR
Human cytomegalovirus-encoded genes that inhibit the PKR host defense pathway play a critical role in HCMV immune evasion. PKR is a major effector of IFN-induced host defenses following viral infection. A key element triggering this host defense cascade is the production of dsRNA, synthesized following infection of cells by many viruses, including the CMVs [90]. The presence of dsRNA functions as an ‘alarm signal’ to the host, resulting in activation of cellular pathways designed to inhibit viral replication, including PKR, which functions by shutting off protein synthesis. In the presence of dsRNA, PKR undergoes a conformational change, dimerizes, autophosphorylates and then phosphorylates the subunit of eukaryotic initiation factor 2 leading to inhibition of translation initiation and of viral replication. Evolution of dsRNA-activated host antiviral pathways has been matched by the evolution of viral mechanisms that thwart these pathways [91,92]. One strategy that appears to have evolved in multiple viral lineages is the sequestration of dsRNA by dsRNA binding proteins. For example, the vaccinia virus E3L protein binds to dsRNA and contributes to repression of PKR, and a vaccinia virus lacking E3L (VVΔE3L) has an extremely limited host range for replication in cell culture and is avirulent in mice [93]. In the case of the two HCMV genes, TRS1 and IRS1, each binds to dsRNA resulting in suppression of PKR activation, and deletion of these genes results in highly attenuated HCMV that has its replication severely impaired by activation of the PKR pathway [94,95].
The notion that elimination of a viral PKR antagonist will make a useful HCMV vaccine has precedence from the vaccinia system [96]. A recombinant vaccinia virus with a deletion of the viral PKR antagonist, VVDE3L, had negligible virulence, even in immunodeficient animals, and yet induced a strong immune response that was protective against subsequent challenge with wild-type virus. Extension of these observations toward the long-term aim of designing a fully protective but highly attenuated HCMV vaccine would be a logical approach to live-attenuated vaccine design.
Expert commentary
A vaccine comprised of a recombinant HCMV envelope gB with MF59 adjuvant was recently found to have efficacy for prevention of HCMV infection in a Phase II clinical trial in young mothers. The report of 50% efficacy is a promising development in the long search for an effective vaccine to protect against congenital HCMV infection.
The optimal HCMV vaccine strategy remains to be identified. Ideally, an HCMV vaccine would elicit humoral and cell-mediated immunity without the potential risk of establishing a latent HCMV infection or risks of a live viral vector. An optimal vaccine could be administered to either immunocompetent females of childbearing age or to the immunocompromised patients who underwent organ transplantation.
A number of recent advances in the study of the biology of HCMV have identified novel potential vaccine targets, particularly those involved in epithelial/endothelial cell entry, as areas for future vaccine design. The subunit expression of the gH/gL/UL128/UL130/UL131 proteins, which are essential for entry into epithelial and endothelial cells, could be exploited for vaccine design, and this approach merits consideration for future preclinical evaluation.
The ideal target population for an HCMV vaccine remains to be determined. Although the Institute of Medicine modeled its ana lysis of the potential benefits of an HCMV vaccine program upon hypothetical administration of a vaccine to adolescents, the health benefits of universal immunization in early life could extend beyond the prevention of congenital HCMV disease and could theoretically impact long-term health consequences of HCMV infection, which may include certain types of malignancy (such as glioblastomas), atherosclerosis and immunosenescence.
Five-year view
Continued evaluation of the HCMV gB vaccine will lead to a definitive Phase III clinical trial that will provide a robust test of the vaccine's ability to prevent HCMV infection in women of childbearing age. The results of a Phase II clinical trial of bivalent HCMV DNA vaccine will be available and will provide information about the safety and immunogenicity of this vaccine in transplant recipients. Clinical trials of vectored-vaccine approaches and recombinant live vaccines will be conducted in adolescent and young adult populations. Preclinical evaluation of subunit vaccines based on novel potential vaccine targets, particularly those involved in epithelial/endothelial cell entry, will be performed in animal models. These studies may help justify and prioritize future human clinical trials.
Key issues.
A vaccine for congenital human cytomegalovirus (HCMV) infection is a major public health priority. Analysis of cost–effectiveness indicates that such a vaccine could have a major economic impact on the very high costs of caring for children with symptomatic congenital HCMV infection.
A breakthrough for HCMV vaccines came in 2009 with the publication of a Phase II study of a purified, recombinant vaccine based on adjuvanted glycoprotein B. This vaccine demonstrated an efficacy of approximately 50% against acquisition of HCMV infection in a clinical trial in young women.
Novel approaches to HCMV vaccines include vectored vaccines, DNA vaccines, BAC-based vaccines and genetically engineered, live attenuated vaccines. These vaccines are in various stages of preclinical and clinical development.
Until a HCMV vaccine is licensed, continued efforts must be made in education. Increasing public awareness of HCMV can help reduce the acquisition of infection among susceptible women and may help decrease the burden of congenital HCMV infection and disease.
Acknowledgments
This work was supported by NIH grants HD038416 and 044864. Mark R Schleiss holds the American Legion Endowed Chair in Pediatric Infectious Diseases at the University of Minnesota.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References/Website
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Demmler GJ. Congenital cytomegalovirus infection and disease. Semin. Pediatr. Infect. Dis. 1999;10(3):195–200. [PubMed] [Google Scholar]
- 2.Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256(14):1904–1908. [PubMed] [Google Scholar]
- 3.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007;17(5):355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 4.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 2009;22(1):99–126. doi: 10.1128/CMR.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bale JF, Miner L, Petheram SJ. Congenital cytomegalovirus infection. Curr. Treat. Options Neurol. 2002;4(3):225–230. doi: 10.1007/s11940-002-0039-8. [DOI] [PubMed] [Google Scholar]
- 6•.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70. [The CDC has demonstrated considerable interest in expanding public knowledge about the problem of congenital cytomegalovirus (CMV) in recent years. Simple hygienic measures can help decrease infections in young women.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand. J. Infect. Dis. 1999;31(5):443–457. doi: 10.1080/00365549950163969. [This landmark paper was the first to suggest that re-infection of women immune to CMV can lead to congenital CMV transmission, attendant symptoms and sequelae in infants. It poses the question, will a CMV vaccine have to be ‘better than natural immunity’?.] [DOI] [PubMed] [Google Scholar]
- 8.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 2001;344(18):1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 9.Novak Z, Ross SA, Patro RK, et al. Cytomegalovirus strain diversity in seropositive women. J. Clin. Microbiol. 2008;46(3):882–886. doi: 10.1128/JCM.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 11••.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 1992;326(10):663–667. doi: 10.1056/NEJM199203053261003. [The impetus for CMV vaccination owes a great deal to this paper, which indicated that the neurodevelopmental outcome of congenital CMV infection is largely dependent upon the immune status of the mother.] [DOI] [PubMed] [Google Scholar]
- 12.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 2008;325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 13.Stagno S, Pass RF, Dworsky ME, et al. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 1982;306(16):945–949. doi: 10.1056/NEJM198204223061601. [DOI] [PubMed] [Google Scholar]
- 14.Boppana SB, Ross SA, Novak Z, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. 2003;289(8):1008–1011. doi: 10.1001/jama.289.8.1008. [Provides a yardstick of protection conferred by maternal immunity against which vaccine studies may be compared.] [DOI] [PubMed] [Google Scholar]
- 16.Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009;11(1):1–9. doi: 10.1593/neo.81178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st Century: a Tool for Decisionmaking. Committee to Study Priorities for Vaccine Development Division of Health Promotion and Disease Prevention, Institute of Medicine; Washington, DC, USA: 1999. [Google Scholar]
- 18.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 2004;39(2):233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 19.Schleiss MR, Heineman TC. Progress toward an elusive goal: current status of cytomegalovirus vaccines. Expert Rev. Vaccines. 2005;4(3):381–406. doi: 10.1586/14760584.4.3.381. [DOI] [PubMed] [Google Scholar]
- 20••.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [For the first time, a clinical trial demonstrates efficacy for a CMV vaccine. This vaccine – purified recombinant glycoprotein B vaccine with MF59 adjuvant – demonstrated an approximate efficacy of 50% against primary infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197(1):143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 22.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology. 1994;201(2):263–276. doi: 10.1006/viro.1994.1291. [DOI] [PubMed] [Google Scholar]
- 23.Marshall GS, Rabalais GP, Stout GG, Waldeyer SL. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J. Infect. Dis. 1992;165(2):381–384. doi: 10.1093/infdis/165.2.381. [DOI] [PubMed] [Google Scholar]
- 24.Marshall GS, Stout GG, Knights ME, et al. Ontogeny of glycoprotein gB-specific antibody and neutralizing activity during natural cytomegalovirus infection. J. Med. Virol. 1994;43(1):77–83. doi: 10.1002/jmv.1890430115. [DOI] [PubMed] [Google Scholar]
- 25.Navarro D, Lennette E, Tugizov S, Pereira L. Humoral immune response to functional regions of human cytomegalovirus glycoprotein B. J. Med. Virol. 1997;52(4):451–459. [PubMed] [Google Scholar]
- 26.Zhang C, Buchanan H, Andrews W, Evans A, Pass RF. Detection of cytomegalovirus infection during a vaccine clinical trial in healthy young women: seroconversion and viral shedding. J. Clin. Virol. 2006;35(3):338–342. doi: 10.1016/j.jcv.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Pass RF. Detection of cytomegalovirus infection during clinical trials of glycoprotein B vaccine. Vaccine. 2004;23(4):507–510. doi: 10.1016/j.vaccine.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect. Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths PD, McLean A, Emery VC. Encouraging prospects for immunisation against primary cytomegalovirus infection. Vaccine. 2001;19(11–12):1356–1362. doi: 10.1016/s0264-410x(00)00377-7. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths P. The beginning of the end of a long wait for a vaccine against cytomegalovirus. Rev. Med. Virol. 2009;19(3):117–119. doi: 10.1002/rmv.616. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths PD. CMV vaccine trial endpoints. J. Clin. Virol. 2009;46(Suppl. 4):S64–S67. doi: 10.1016/j.jcv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Pass RF. Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant. J. Clin. Virol. 2009;46(Suppl. 4):S73–S76. doi: 10.1016/j.jcv.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekker CL, Arvin AM. One step closer to a CMV vaccine. N. Engl. J. Med. 2009;360(12):1250–1252. doi: 10.1056/NEJMe0900230. [DOI] [PubMed] [Google Scholar]
- 34.Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics. 1999;104(1 Pt 1):55–60. doi: 10.1542/peds.104.1.55. [DOI] [PubMed] [Google Scholar]
- 35•.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [Vaccine studies based on elicitation of cellular responses have focused on the pp65 (UL83) protein and the IE1 protein; this study, however, shows that the repertoire of cellular responses to CMV is far greater than has been previously appreciated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleiss MR. Cytomegalovirus vaccine development. Curr. Top. Microbiol. Immunol. 2008;325:361–382. doi: 10.1007/978-3-540-77349-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slezak SL, Bettinotti M, Selleri S, Adams S, Marincola FM, Stroncek DF. CMV pp65 and IE-1 T cell epitopes recognized by healthy subjects. J. Transl. Med. 2007;5:17. doi: 10.1186/1479-5876-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkins GJ, Fleeton MN, Sheahan BJ. Therapeutic and prophylactic applications of alphavirus vectors. Expert Rev. Mol. Med. 2008;10:e33. doi: 10.1017/S1462399408000859. [DOI] [PubMed] [Google Scholar]
- 39•.Bernstein DI, Reap EA, Katen K, et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009;28(2):484–493. doi: 10.1016/j.vaccine.2009.09.135. [Study indicating safety and immunogenicity of a multivalent vectored vaccine approach; encouraging results suggest that an efficacy study is warranted.] [DOI] [PubMed] [Google Scholar]
- 40.Reap EA, Morris J, Dryga SA, et al. Development and preclinical evaluation of an alphavirus replicon particle vaccine for cytomegalovirus. Vaccine. 2007;25(42):7441–7449. doi: 10.1016/j.vaccine.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selinsky C, Luke C, Wloch M, et al. A DNA-based vaccine for the prevention of human cytomegalovirus-associated diseases. Hum. Vaccin. 2006;1(1):16–23. doi: 10.4161/hv.1.1.1335. [DOI] [PubMed] [Google Scholar]
- 42.Liu MA, Ulmer JB. Human clinical trials of plasmid DNA vaccines. Adv. Genet. 2005;55:25–40. doi: 10.1016/S0065-2660(05)55002-8. [DOI] [PubMed] [Google Scholar]
- 43•.Wloch MK, Smith LR, Boutsaboualoy S, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J. Infect. Dis. 2008;197(12):1634–1642. doi: 10.1086/588385. [Study indicating the safety and immunogenicity of a multivalent DNA vaccine approach. Although these results focused on the bone marrow transplant population as a target for CMV vaccination, could this vaccine be extrapolated to women of childbearing age?.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Go V, Pollard RB. A cytomegalovirus vaccine for transplantation: are we closer? J. Infect. Dis. 2008;197(12):1631–1633. doi: 10.1086/588386. [DOI] [PubMed] [Google Scholar]
- 45.Goonetilleke N, Moore S, Dally L, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime–boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 2006;80(10):4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith LR, Wloch M, Guterwill D, Rolland A, Chu A, Moss R. Preliminary Phase 2 immunogenicity results of a CMV DNA vaccine in hematopoietic cell transplant (HCT) recipients. Ann. Conf. Vaccine Res. 2009;25:S23. [Google Scholar]
- 47.Schleiss MR. VCL-CB01, an injectable bivalent plasmid DNA vaccine for potential protection against CMV disease and infection. Curr. Opin. Mol. Ther. 2009;11(5):572–578. [PMC free article] [PubMed] [Google Scholar]
- 48.Plotkin SA, Higgins R, Kurtz JB, et al. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation. 1994;58(11):1176–1178. [PubMed] [Google Scholar]
- 49.Plotkin SA, Farquhar J, Horberger E. Clinical trials of immunization with the Towne 125 strain of human cytomegalovirus. J. Infect. Dis. 1976;134(5):470–475. doi: 10.1093/infdis/134.5.470. [DOI] [PubMed] [Google Scholar]
- 50.Adler SP, Starr SE, Plotkin SA, et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J. Infect. Dis. 1995;171(1):26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson MA, Sinclair E, Bredt B, et al. Antigen-specific T cell responses induced by Towne cytomegalovirus (CMV) vaccine in CMV-seronegative vaccine recipients. J. Clin. Virol. 2006;35(3):332–337. doi: 10.1016/j.jcv.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson MA, Sinclair E, Bredt B, et al. Safety and immunogenicity of Towne cytomegalovirus vaccine with or without adjuvant recombinant interleukin-12. Vaccine. 2006;24(25):5311–5319. doi: 10.1016/j.vaccine.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 53.Jacobson MA, Adler SP, Sinclair E, et al. A CMV DNA vaccine primes for memory immune responses to live-attenuated CMV (Towne strain). Vaccine. 2009;27(10):1540–1548. doi: 10.1016/j.vaccine.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 54•.Heineman TC, Schleiss M, Bernstein DI, et al. A Phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. J. Infect. Dis. 2006;193(10):1350–1360. doi: 10.1086/503365. [Despite safety concerns, these novel recombinant live-attenuated vaccines were well tolerated, were not shed by vaccine recipients and should be evaluated further in clinical trials in seronegative individuals.] [DOI] [PubMed] [Google Scholar]
- 55.Sia IG, Patel R. New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin. Microbiol. Rev. 2000;13(1):83–121. doi: 10.1128/cmr.13.1.83-121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson SR, Wilson JH, Buonocore L, Palin A, Rose JK, Reuter JD. Intranasal immunization with recombinant vesicular stomatitis virus expressing murine cytomegalovirus glycoprotein B induces humoral and cellular immunity. Comp. Med. 2008;58(2):129–139. [PMC free article] [PubMed] [Google Scholar]
- 57.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl Acad. Sci. USA. 1992;89(22):10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, La Rosa C, Li Z, et al. Vaccine properties of a novel marker gene-free recombinant modified vaccinia Ankara expressing immunodominant CMV antigens pp65 and IE1. Vaccine. 2007;25(6):1132–1141. doi: 10.1016/j.vaccine.2006.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, La Rosa C, Maas R, et al. Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J. Virol. 2004;78(8):3965–3976. doi: 10.1128/JVI.78.8.3965-3976.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frey SE, Harrison C, Pass RF, et al. Effects of antigen dose and immunization regimens on antibody responses to a cytomegalovirus glycoprotein B subunit vaccine. J. Infect. Dis. 1999;180(5):1700–1703. doi: 10.1086/315060. [DOI] [PubMed] [Google Scholar]
- 61.Pass RF, Duliege AM, Boppana S, et al. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J. Infect. Dis. 1999;180(4):970–975. doi: 10.1086/315022. [DOI] [PubMed] [Google Scholar]
- 62.Rasmussen L, Matkin C, Spaete R, Pachl C, Merigan TC. Antibody response to human cytomegalovirus glycoproteins gB and gH after natural infection in humans. J. Infect. Dis. 1991;164(5):835–842. doi: 10.1093/infdis/164.5.835. [DOI] [PubMed] [Google Scholar]
- 63.Frey SE, Newman FK, Yan L, Lottenbach KR, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. JAMA. 2003;289(24):3295–3299. doi: 10.1001/jama.289.24.3295. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, La Rosa C, Lacey SF, et al. Attenuated poxvirus expressing three immunodominant CMV antigens as a vaccine strategy for CMV infection. J. Clin. Virol. 2006;35(3):324–331. doi: 10.1016/j.jcv.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, La Rosa C, Mekhoubad S, et al. Attenuated poxviruses generate clinically relevant frequencies of CMV-specific T cells. Blood. 2004;104(3):847–856. doi: 10.1182/blood-2003-10-3469. [DOI] [PubMed] [Google Scholar]
- 66.La Rosa C, Wang Z, Lacey SF, et al. In vitro expansion of polyclonal T-cell subsets for adoptive immunotherapy by recombinant modified vaccinia Ankara. Exp. Hematol. 2006;34(4):497–507. doi: 10.1016/j.exphem.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, Zhou W, Srivastava T, et al. A fusion protein of HCMV IE1 exon4 and IE2 exon5 stimulates potent cellular immunity in an MVA vaccine vector. Virology. 2008;377(2):379–390. doi: 10.1016/j.virol.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yue Y, Wang Z, Abel K, et al. Evaluation of recombinant modified vaccinia Ankara virus-based rhesus cytomegalovirus vaccines in rhesus macaques. Med. Microbiol. Immunol. 2008;197(2):117–123. doi: 10.1007/s00430-008-0074-5. [DOI] [PubMed] [Google Scholar]
- 69.Zhong J, Khanna R. Ad-gBCMVpoly: a novel chimeric vaccine strategy for human cytomegalovirus-associated diseases. J. Clin. Virol. 2009;46(Suppl. 4):S68–S72. doi: 10.1016/j.jcv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Shanley JD, Wu CA. Mucosal immunization with a replication-deficient adenovirus vector expressing murine cytomegalovirus glycoprotein B induces mucosal and systemic immunity. Vaccine. 2003;21(19–20):2632–2642. doi: 10.1016/s0264-410x(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 71.Shanley JD, Wu CA. Intranasal immunization with a replication-deficient adenovirus vector expressing glycoprotein H of murine cytomegalovirus induces mucosal and systemic immunity. Vaccine. 2005;23(8):996–1003. doi: 10.1016/j.vaccine.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 72.Zhao P, Ma D, Yan S, et al. Towards a novel vaccine against human cytomegalovirus based on a chimeric Ad5F35 adenovirus vector expressing the immunodominant antigenic domain 1 epitope. Intervirology. 2009;52(1):35–42. doi: 10.1159/000212989. [DOI] [PubMed] [Google Scholar]
- 73.Zhong J, Rist M, Cooper L, Smith C, Khanna R. Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus. PLoS One. 2008;3(9):e3256. doi: 10.1371/journal.pone.0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Britt WJ, Jarvis MA, Drummond DD, Mach M. Antigenic domain 1 is required for oligomerization of human cytomegalovirus glycoprotein B. J. Virol. 2005;79(7):4066–4079. doi: 10.1128/JVI.79.7.4066-4079.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Elkington R, Walker S, Crough T, et al. Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers. J. Virol. 2003;77(9):5226–5240. doi: 10.1128/JVI.77.9.5226-5240.2003. [Similarly to [35], this study demonstrated a previously unappreciated diversity in cellular immune responses to CMV infection in seropositive individuals, thereby complicating the vaccine design field.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elkington R, Shoukry NH, Walker S, et al. Cross-reactive recognition of human and primate cytomegalovirus sequences by human CD4 cytotoxic T lymphocytes specific for glycoprotein B and H. Eur. J. Immunol. 2004;34(11):3216–3226. doi: 10.1002/eji.200425203. [DOI] [PubMed] [Google Scholar]
- 77.Manley TJ, Luy L, Jones T, Boeckh M, Mutimer H, Riddell SR. Immune evasion proteins of human cytomegalovirus do not prevent a diverse CD8+ cytotoxic T-cell response in natural infection. Blood. 2004;104(4):1075–1082. doi: 10.1182/blood-2003-06-1937. [DOI] [PubMed] [Google Scholar]
- 78.Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. New advances in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 2008;41(3):192–197. doi: 10.1016/j.jcv.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Cicin-Sain L, Bubic I, Schnee M, et al. Targeted deletion of regions rich in immune-evasive genes from the cytomegalovirus genome as a novel vaccine strategy. J. Virol. 2007;81(24):13825–13834. doi: 10.1128/JVI.01911-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohr CA, Cicin-Sain L, Wagner M, et al. Engineering of cytomegalovirus genomes for recombinant live herpesvirus vaccines. Int. J. Med. Microbiol. 2008;298(1–2):115–125. doi: 10.1016/j.ijmm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Messerle M, Crnkovic I, Hammerschmidt W, Ziegler H, Koszinowski UH. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl Acad. Sci. USA. 1997;94(26):14759–14763. doi: 10.1073/pnas.94.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagner M, Ruzsics Z, Koszinowski UH. Herpesvirus genetics has come of age. Trends Microbiol. 2002;10(7):318–324. doi: 10.1016/s0966-842x(02)02394-6. [DOI] [PubMed] [Google Scholar]
- 83.Dunn W, Chou C, Li H, et al. Functional profiling of a human cytomegalovirus genome. Proc. Natl Acad. Sci. USA. 2003;100(24):14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kobayashi H, Kobayashi M, McCauley RL, Herndon DN, Pollard RB, Suzuki F. Cadaveric skin allograft-associated cytomegalovirus transmission in a mouse model of thermal injury. Clin. Immunol. 1999;92(2):181–187. doi: 10.1006/clim.1999.4735. [DOI] [PubMed] [Google Scholar]
- 85.Hahn G, Revello MG, Patrone M, et al. Human cytomegalovirus UL131–128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 2004;78(18):10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerna G, Percivalle E, Lilleri D, et al. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131–128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 2005;86(Pt 2):275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 87•.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl Acad. Sci. USA. 2005;102(50):18153–18158. doi: 10.1073/pnas.0509201102. [Revealed the importance of the UL128–131 locus in eliciting antibodies that neutralize CMV at the epithelial interface, where most infections are acquired.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine. 2008;26(45):5760–5766. doi: 10.1016/j.vaccine.2008.07.092. [Revealed the importance of the UL128–131 locus in eliciting antibodies that neutralize CMV at the epithelial interface, where most infections are acquired.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Gerna G, Sarasini A, Patrone M, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J. Gen. Virol. 2008;89(Pt 4):853–865. doi: 10.1099/vir.0.83523-0. [Revealed the importance of the UL128–131 locus in eliciting antibodies that neutralize CMV at the epithelial interface, where most infections are acquired.] [DOI] [PubMed] [Google Scholar]
- 90.Haller O, Weber F. Pathogenic viruses: smart manipulators of the interferon system. Curr. Top. Microbiol. Immunol. 2007;316:315–334. doi: 10.1007/978-3-540-71329-6_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeFilippis VR. Induction and evasion of the type I interferon response by cytomegaloviruses. Adv. Exp. Med. Biol. 2007;598:309–324. doi: 10.1007/978-0-387-71767-8_22. [DOI] [PubMed] [Google Scholar]
- 92.Unterholzner L, Bowie AG. The interplay between viruses and innate immune signaling: recent insights and therapeutic opportunities. Biochem. Pharmacol. 2008;75(3):589–602. doi: 10.1016/j.bcp.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 93.Brandt T, Heck MC, Vijaysri S, Jentarra GM, Cameron JM, Jacobs BL. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not induction of a protective immune response. Virology. 2005;333(2):263–270. doi: 10.1016/j.virol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Hakki M, Marshall EE, De Niro KL, Geballe AP. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. J. Virol. 2006;80(23):11817–11826. doi: 10.1128/JVI.00957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marshall EE, Bierle CJ, Brune W, Geballe AP. Essential role for either TRS1 or IRS1 in human cytomegalovirus replication. J. Virol. 2009;83(9):4112–4120. doi: 10.1128/JVI.02489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jentarra GM, Heck MC, Youn JW, et al. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: scarification vaccination. Vaccine. 2008;26(23):2860–2872. doi: 10.1016/j.vaccine.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vical Vical completes enrollment and reports positive interim data in CMV vaccine Phase 2 trial. www.medicalnewstoday.com/articles/129159.php.