Abstract
Polycyclic aromatic hydrocarbons (PAHs) are a diverse group of widespread environmental pollutants, some of which have been found to be estrogenic or antiestrogenic. Recent data have shown that hydroxylated PAH metabolites may be responsible for the estrogenic effects of some PAHs. The purpose of this study was to investigate the effects of several PAHs, as well as their monohydroxylated metabolites, on estrogen receptors (ERs), ERα and ERβ. Three parent PAHs and their monohydroxylated metabolites were each evaluated using transcriptional reporter assays in isogenic stable cell lines to measure receptor activation, competitive binding assays to determine ligand binding, and bioluminescence resonance energy transfer assays to assess dimerization. Finally, the estrogenic effects of the hydroxylated metabolites were confirmed by quantitative real-time PCR of estrogen-responsive target genes. Although the parent PAHs did not induce ERα or ERβ transcriptional activity, all of the monohydroxylated PAHs (1-OH naphthanol, 9-OH phenanthrene, 1-OH pyrene) selectively induced ERβ transcriptional activity at the concentrations tested, while not activating ERα. Additionally, the monohydroxylated PAHs were able to competively bind ERβ, induce ERβ homodimers, and regulate ERβ target genes. Although monohydroxylated PAHs appeared to have weak agonist activity to ERβ, our results showed that they can elicit a biologically active response from ERβ in human breast cancer cells and potentially interfere with ERβ signaling pathways.
Key Words: polycyclic aromatic hydrocarbons, estrogen receptors, monohydroxylated metabolites, dimerization, transcription, ligand binding.
Polycyclic aromatic hydrocarbons (PAHs) have been of increasing concern in the human health field due to their widespread dispersion in the environment and the adverse health effects associated with PAH exposure (Baird et al., 2005). Formed through the incomplete combustion of organic compounds, PAHs can be found in charbroiled foods, cigarette smoke, contaminated soil, vehicle exhaust, and in the atmosphere from the by-products of industrial processes. PAH exposure can have several adverse effects, including carcinogenesis and endocrine disruption.
Although PAHs are a diverse group of chemicals, most are metabolized by cytochrome P450s, a superfamily of enzymes that mediate the oxidation of lipophilic substrates (Anzenbacher, 2001; Bauer et al., 1995; Kim et al., 1998). The diol epoxide PAH metabolites are capable of inducing DNA damage (Baird et al., 2005), and many PAHs have been shown to be carcinogenic (Bauer et al., 1995; Kim et al., 1998). PAHs can also act as endocrine disrupting chemicals by interfering with normal estrogen signaling. Upon monohydroxylation, PAHs can induce estrogenic effects by directly interacting with estrogen receptors (ERs) (Arcaro et al., 1999; Fertuck et al., 2001a ,b). These data suggest that the estrogenic effects of PAHs are primarily mediated by the monohydroxylated PAH metabolites.
ERs, members of the nuclear receptor superfamily of transcription factors, exist in two distinct isoforms, α and β. Encoded by separate genes on different chromosomes, ERα and ERβ have both overlapping and unique biological functions. The DNA-binding domains share 96% homology, and ERs bind similar estrogen response elements (EREs) to regulate transcription of target genes. The ligand-binding domains (LBDs), containing the hormone-dependent activation function (AF-2) (Tora et al., 1989), have 55% identity and have similar, but not identical, ligand-binding pockets (Pike et al., 1999). Upon ligand binding, the receptors dimerize and bind DNA to initiate transcription of target genes that mediate distinct biological effects. In the presence of estrogen, ERα is a known driver of cell proliferation, especially in breast cancer cells, whereas ERβ has been shown to inhibit ERα-mediated cell proliferation (Hartman et al., 2006; Paruthiyil et al., 2004; Treeck et al., 2010).
Given the critical roles ERs play in regulating cell growth in response to estrogens, there has been significant effort put forth to understand and predict the impacts of xenoestrogens on ER singaling. However, most studies have been performed solely in the context of ERα, with a limited number of PAHs tested. Here we utilize several in vitro assays to assess the effects of three PAHs and their monohydroxylated metabolites, shown in Figure 1, on the transcriptional activation, ligand binding, and dimerization of both ERα and ERβ. Compounds were initially screened for transcriptional activation using a previously characterized pair of isogenic breast cancer cell lines with inducible expression of either ERα or ERβ and a stably integrated estrogen-responsive reporter (Shanle et al., 2011). These cell lines provide a sensitive tool to directly compare the transcriptional induction of ERα and ERβ. Next, bioluminescence resonance energy transfer (BRET) assays were performed to evaluate the dimerization status of ERs. BRET assays are able to monitor protein-protein interactions in a live, cell-based system (Powell and Xu, 2008; Tremblay et al., 1999). Fluorescence polarization experiments were utilized to generate competitive binding curves and determine half maximal inhibitory concentration (IC50) values. This provided a simple, yet specific way to determine whether the tested compound can compete with estrogen for binding to ER. Finally, compounds were evaluated for their ability to upregulate ERβ target genes via quantitative real-time PCR (qPCR).
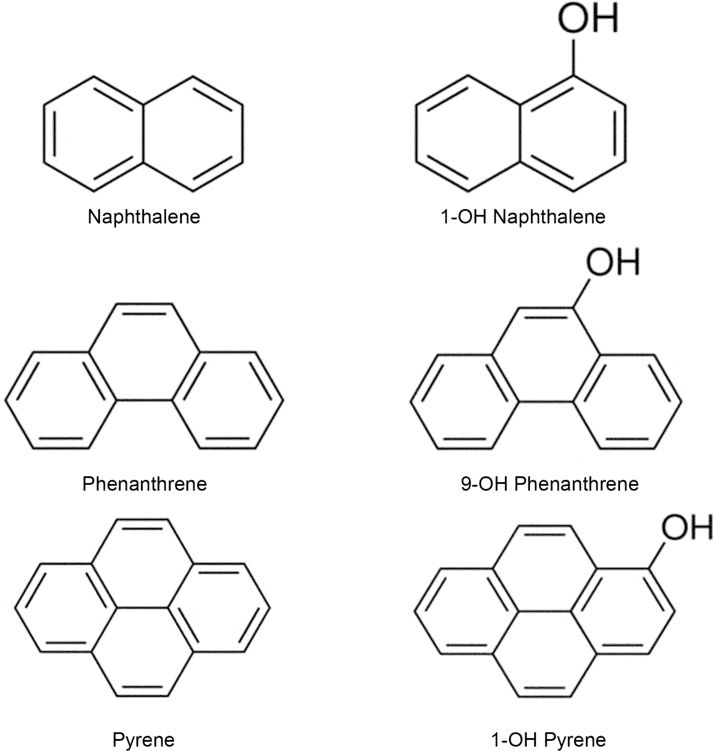
FIG. 1.
Chemical structures of select polycyclic aromatic compounds and monohydroxylated metabolites studied.
Naphthalene, phenanthrene, and pyrene were chosen as parent PAH compounds for study because they have been detected at high levels in contaminated environments (Arcaro et al., 1999), and they are considered by to be Priority Pollutants according to the U.S. Environmental Protection Agency. The hydroxylated metabolites were chosen due to their detection after metabolism of the parent compound (Cho et al., 2006; Rossbach et al., 2007). This is the first study to assess ER selective activity of these PAHs and their hydroxylated metabolites at the levels of transcriptional activity using isogenic reporter cell lines, ligand binding, and dimerization. The data demonstrate that monohydroxylated PAHs differentially interact with ERα and ERβ and exhibit stronger agonistic activity toward ERβ compared with ERα, suggesting that ERβ-mediated biological processes need to be evaluated to assess the outcomes of PAH exposure on humans.
MATERIALS AND METHODS
Chemicals. All PAH compounds were purchased from Sigma-Aldrich (St Louis, MO). Doxycycline (Dox) was obtained from Clontech (Mountain View, CA). ICI 182,780 was obtained from Tocris Bioscience (Ellisville, MO).
Cell culture and reporter assays. Cell culture media were obtained from Invitrogen (Carlsbad, CA). HEK293T cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Gibco Fetal Bovine Serum (FBS; Invitrogen) at 37°C and 5% CO2. Hs578T-ERαLuc and Hs578T-ERβLuc cells were previously created by Shanle et al. (2011) and were maintained in DMEM/F12 supplemented with L-glutamine and 10% Tet-system approved FBS (Clontech) at 37°C and 5% CO2.
Reporter assays were performed as previously reported (Shanle et al., 2011). Briefly, cells were seeded in triplicate at 104 cells/well on white 96-well tissue culture plates in phenol red-free DMEM/F12 supplemented with 5% charcoal-stripped FBS treated with 50ng/ml Dox. After 24h, media were removed and replaced with media treated with 50ng/ml Dox and vehicle (0.15% di- methyl sulfoxide [DMSO]) or PAH compounds diluted in DMSO. After 24h of treatment, the cells were washed with 30 µl of 1× PBS and lysed with 35 µl lysis buffer (100mM K2HPO4, 0.2% Triton X-100, pH 7.8). Thirty microliters of lysate were mixed 1:1 with luciferase substrate (Promega, Madison, WI), and luminescence was measured with a 700-nm filter on a Victor X5 microplate reader (PerkinElmer, Waltham, MA). Total protein was measured using the Bradford Method (Bio-Rad), and raw luciferase data were normalized to total protein. Approximate EC50 values were calculated using GraphPad Prism Software (Version 5.04; Graph-Pad Software Inc., San Diego, CA) and a three-parameter log versus response nonlinear regression.
BRET assays. The BRET assays were performed similarly to those previously reported (Powell and Xu, 2008). Briefly, HEK293T cells were transfected with BRET fusion plasmids (pCMX-ERα-RLuc and pCMX-ERα-YFP or pCMX-RLuc-ERβ and pCMX-YFP-ERβ). Twenty-four hours after transfection, cells were trypsinized and resuspended in triplicate in PBS at approximately 50,000 cells per well in a white 96-well plate. Cells were then incubated with vehicle (0.6% DMSO), 10nM E2, or monohydroxylated PAH compound for 1h at room temperature. Coelenterazine h (Promega) was added to PBS at a final concentration of 5µM. Emission measurements at 460nm and 535nm were immediately taken on a Victor X5 microplate reader (PerkinElmer). BRET ratios were calculated as previously described (Koterba and Rowan, 2006; Powell and Xu, 2008).
Competitive binding assays. Competitive binding assays were performed using the PolarScreen ERβ Competitive Binding Assay Kit, Green (Invitrogen) according to the manufacturer’s protocol. Recombinant human ERβ (20nM) and fluorescein-labeled estradiol were incubated for 4h with the monohydroxylated PAH compounds. Fluorescence polarization was measured using a Victor X5 microplate reader (PerkinElmer). Approximate IC50 values were determined by GraphPad Prism Software (Graph-Pad Software Inc.) from competitive binding curves.
Western blot analysis. Western blots were performed similarly to those previously reported (Shanle et al., 2011) with cells treated for 48h with vehicle (DMSO) or 10µM monohydroxylated PAH compound. Total protein was quantified using Bio-Rad Protein Assay (Bio-Rad), 35 μg of protein was resolved by SDS-PAGE, and membranes were incubated with 1:1000 anti-FLAG-M2 antibody (Sigma) overnight at 4°C. Membranes were then incubated with goat anti-rabbit HRP secondary antibody (Licor Biosciences, Lincoln, NE) for 1h at room temperature and visualized using SuperSignal West Pico Chemiluminescent Substrate (ThermoScientific, Waltham, MA) on autoradiography film. Membranes were then washed and incubated with 1:5000 anti-β-Actin (Sigma) for 1h at room temperature, then incubated with goat anti-mouse HRP secondary antibody (Licor Biosciences) for 1h at room temperature and visualized using SuperSignal West Pico Chemiluminescent Substrate (ThermoScientific) on autoradiography film.
qPCR analysis. Hs578T-ERβLuc cells were cultured in phenol red-free DMEM/F12 supplemented with 10% charcoal-stripped FBS for 3 days prior to experiment to remove any residual estrogens. Cells were seeded into 10-cm tissue culture plates in phenol red-free DMEM/F12 supplemented with 5% stripped serum and treated with 50ng/ml of Dox 24h prior to PAH treatment. Cells were then treated with 50ng/ml Dox plus 0.1% DMSO control, 10nM E2, 10µM 1-OH-naphthalene, 5µM 9-OH phenanthrene, or 5µM 1-OH pyrene for 24h. Total RNA was extracted using HP Total RNA Kit (VWR Scientific, West Chester, PA) according to the manufacturer’s protocol. One microgram of RNA was reverse transcribed using Superscript II RT according to the manufacturer’s protocol (Invitrogen), and qPCR was performed using TaqMan Prime Time custom designed assays (IDT, Coralville, IA), FastStart Universal Probe Master Mix (Roche Scientific, Basel, Switzerland), and a CFX96 instrument (Bio-Rad). Primer and probe sequences are shown in Table 1.
TABLE 1.
Primer and Probe Sequences
| RPL13A | Primer 1 | 5ʹ-TGT TTG ACG GCA TCC CAC-3ʹ |
| Primer 2 | 5ʹ-CTG TCA CTG CCT GGT ACT TC-3ʹ | |
| Probe | 5ʹ-CTT CAG ACG CAC GAC CTT GAG GG-3ʹ | |
| C3 | Primer 1 | 5ʹ-AAC TAC ATC ACA GAG CTG CG-3ʹ |
| Primer 2 | 5ʹ-AAG TCC TCA ACG TTC CAC AG-3ʹ | |
| Probe | 5ʹ-CGT TTC CCG AAG TGA GTT CCC AGA-3ʹ | |
| JAG1 | Primer 1 | 5ʹ-GGA CTA TGA GGG CAA GAA CTG-3ʹ |
| Primer 2 | 5ʹ-AAA TAT ACC GCA CCC CTT CAG-3ʹ | |
| Probe | 5ʹ-TCA CAC CTG AAA GAC CAC TGC CG-3ʹ | |
| NRIP1 | Primer 1 | 5ʹ-AGA TTC CCT GTC CTC CTT CA-3ʹ |
| Primer 2 | 5ʹ-GGA AGT GTT TGG ATT GTG AGC-3ʹ | |
| Probe | 5ʹ-TGT GCA TCT TCT GGC TGT GTT TCT CC-3ʹ |
Statistical analyses. Two-tailed Student’s t-tests were performed using GraphPad Prism version 5.04 for Windows, GraphPad Software (www. graphpad.com).
RESULTS
Monohydroxylated PAHs Selectively Activate ERβ in Reporter Cell Lines
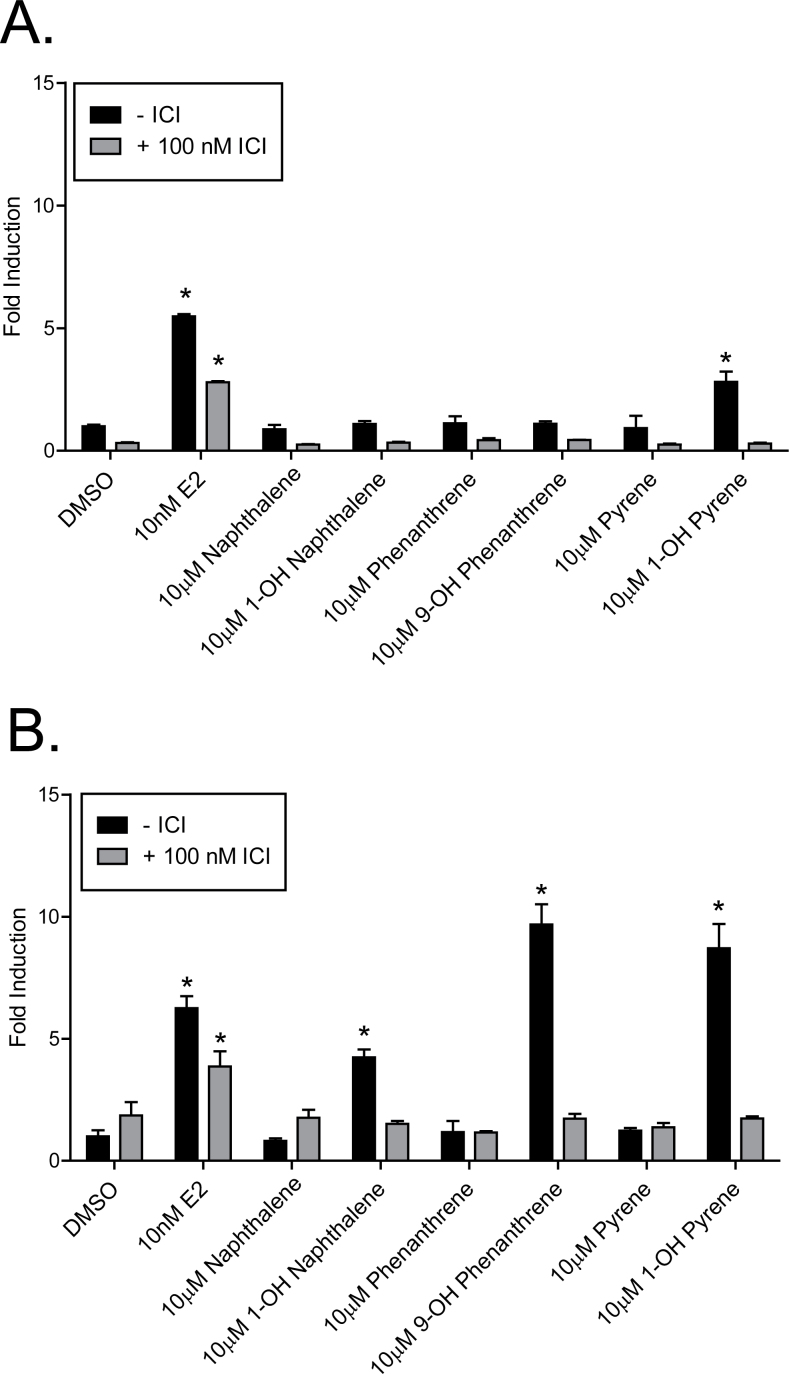
In order to test the hypothesis that hydroxylated PAHs may have estrogenic activity with differential effects on ERα and ERβ, we first utilized Hs578T-ERαLuc and Hs578T-ERβLuc reporter cells (Shanle et al., 2011). These cell lines have inducible expression of ERα or ERβ, respectively, and a stably integrated luciferase reporter just downstream of three tandem EREs. Previous work has shown that these cell lines are highly sensitive to estrogenic ligands and can be used to distinguish ER subtype selective ligands (Shanle et al., 2011). In this system, cells are first treated with Dox to induce expression of the receptor, followed by treatment with the corresponding compounds. In our initial experiments comparing the activation of ERα and ERβ, we observed that only hydroxylated PAHs conferred estrogenic activity at 10µM (Fig. 2). The monohydroxylated PAH compounds were able to induce the ERE-luciferase reporter activity primarily in the Hs578T-ERβLuc cells (Fig. 2B). In these cells, 1-OH naphthalene, 9-OH phenanthrene, and 1-OH pyrene induced a 4.2-, 9.7-, and 8.7-fold change over DMSO vehicle control, respectively (p < 0.01 in all cases). In contrast, only 1-OH pyrene induced the ERE-luciferase reporter activity in the Hs578T-ERαLuc cell line (p < 0.01), but not nearly to the same degree as that of 17β-estradiol (E2) (Fig. 2A). The ER antagonist ICI 182,780 blocked PAH-induced expression in all cases, reducing the luciferase signal to that of vehicle-treated cells. Reporter expression induced by 10nM E2 was not fully blocked by ICI 182,780 cotreatment because of the high concentration and potency of E2. No induction of reporter gene activity was seen in control experiments in which cells were not treated with Dox (Supplementary fig. 1), further confirming ER-mediated induction of the luciferase reporter.
FIG. 2.
Differential activation of ERα and ERβ by select monohydroxylated PAH compounds. (A) Hs578T-ERαLuc and (B) Hs578T-ERβLuc stable cell lines were treated in triplicate with 10µM of PAH compound in the presence or absence of 100nM ICI 182,780 for 24h. Data are expressed as fold induction of raw luciferase units per mg protein over the DMSO control ± SD. Experiments were repeated at least twice. *p < 0.01 compared with DMSO control.
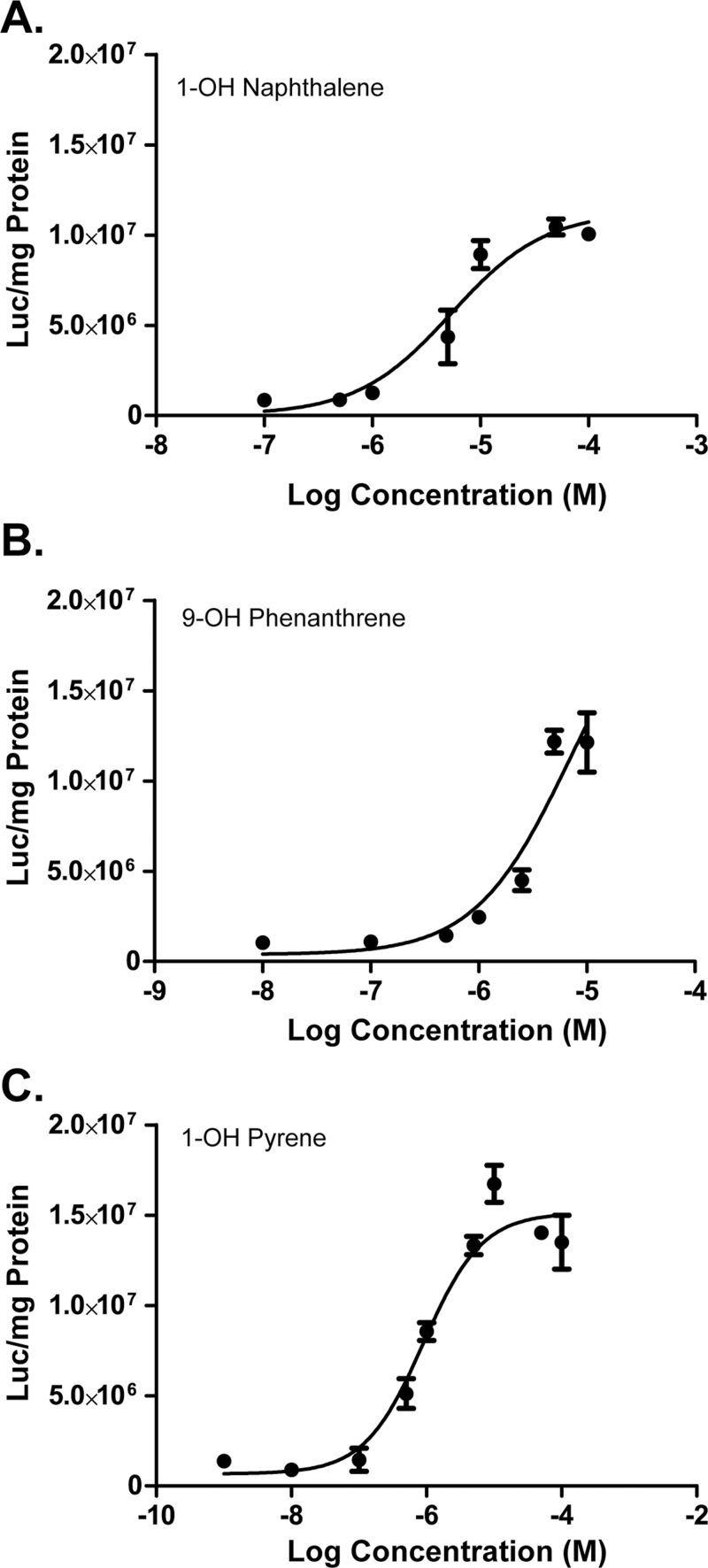
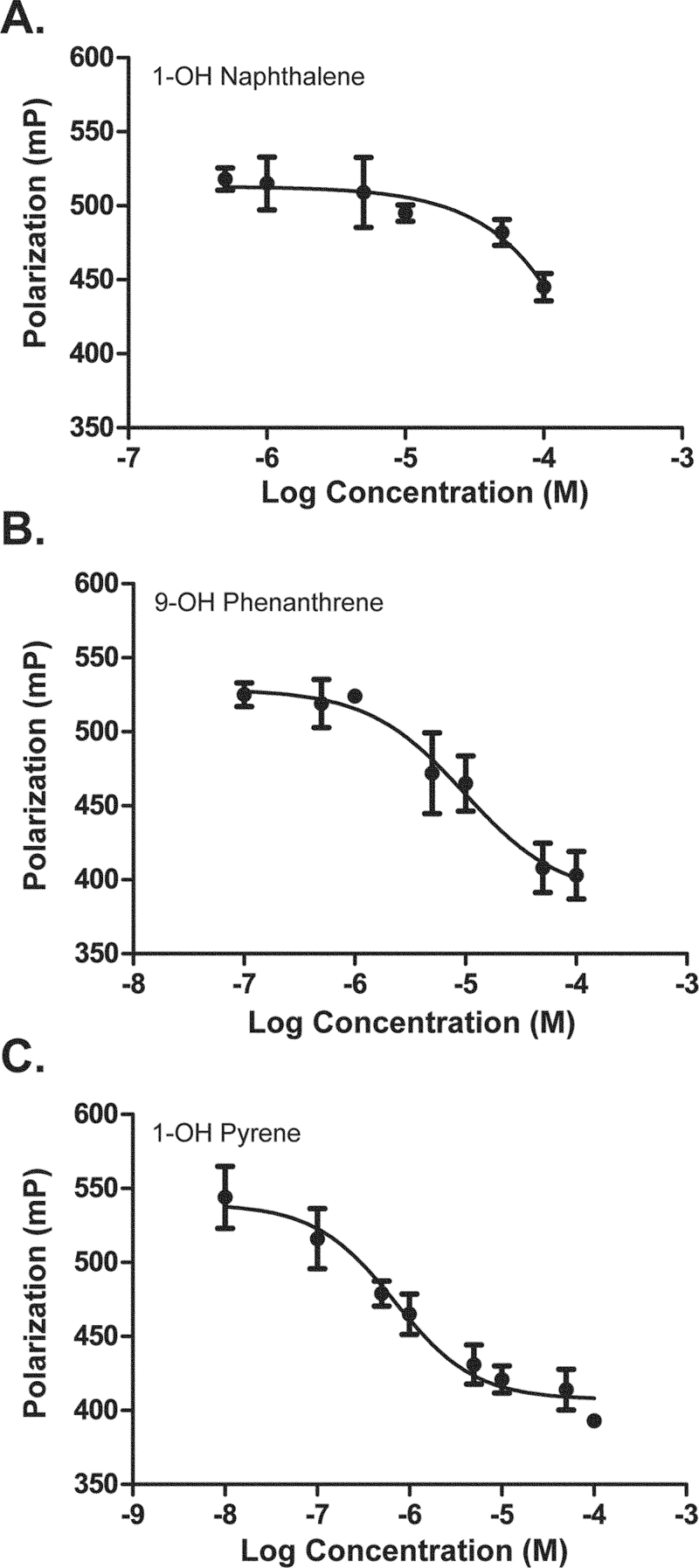
We next determined the dose-dependent effects of the hydroxylated PAHs in the Hs578T-ERβLuc cells (Fig. 3). The half maximal effective concentration (EC50) values for 1-OH naphthalene and 1-OH pyrene were found to be approximately 5.38 and 0.89µM, respectively. 9-OH Phenanthrene proved to be cytotoxic at concentrations greater than 10µM, and the dose-response curve did not adequately saturate; however, an approximate EC50 value was estimated to be ≥ 6.8µM.
FIG. 3.
Monohydroxylated PAHs activate ERβ in a dose-dependent manner. Hs578T-ERβLuc cells were treated with Dox for 24h followed by treatment with a range of concentrations of (A) 1-OH naphthalene, (B) 9-OH phenanthrene, or (C) 1-OH pyrene. The mean and SD shown are from triplicates of one representative experiment repeated twice.
Monohydroxylated PAHs Induce ERβ Dimers and Directly Bind the Receptor
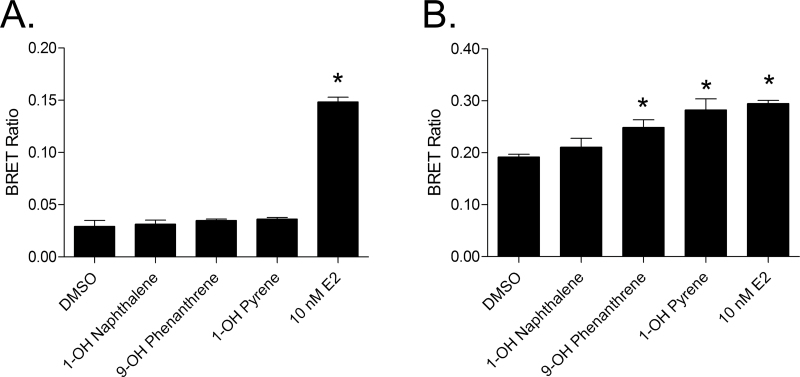
To further dissect the mechanism through which the monohydroxylated PAHs activate ERβ and confirm the selectivity of the compounds, ER dimerization induced by the compounds was assessed using BRET assays. BRET assays allow the determination of dimer formation in live cells by transfecting cells with an energy donor (ER fused to Renilla luciferase) and acceptor (ER fused to yellow fluorescent protein) (see Powell and Xu, 2008). Upon transfecting the cells with the fusion constructs for ERα or ERβ, 9-OH phenanthrene and 1-OH pyrene were shown to significantly induce ERβ homodimerization (p = 0.02 and 0.01, respectively) (Fig. 4B). In contrast, 1-OH naphthalene did not significantly induce ERβ dimerization as determined by the BRET assay (p = 0.35). Following the trend seen in the ERα ERE-reporter assay, the monohydroxylated PAH compounds were unable to induce ERα homodimers (Fig. 4A).
FIG. 4.
Monohydroxylated PAH compounds selectively induce ERβ/β homodimers. (A) BRET data for 293T cells transfected with CMX-ERα-RLuc and CMX-ERα-YFP, showing no ERα/α dimerization upon treatment with PAH compounds in triplicate. (B) BRET data for 293T cells transfected with CMX-RLuc-ERβ and CMX-YFP-ERβ, showing ERβ/β dimerization when treated with PAH compounds in triplicate. The experiment was performed three times. Error bars represent SEM. *p < 0.05 compared with DMSO control.
In order to confirm that ERβ dimerization and ERE-luciferase activity were directly induced by ligand binding, the ability of the monohydroxylated PAH compounds to displace fluorescein-labeled estradiol from human ERβ was assessed in a competitive binding assay (Fig. 5). The competition with E2 indicates that compounds directly bind to ERβ in the same ligand-binding pocket as E2. These competitive binding data yielded half maximal inhibitory concentration (IC50) values for 9-OH phenanthrene and 1-OH pyrene at 9.75 and 0.69µM, respectively. In support of the BRET results, 1-OH naphthalene showed a much lower affinity for ERβ as evidenced by Figure 5A, but it was still able to displace E2 at higher concentrations. The approximate IC50 value for 1-OH naphthalene was estimated at or greater than 0.48µM.
FIG. 5.
Monohydroxylated PAHs can bind ERβ in vitro. Competitive binding curves for monohydroxylated PAH compounds displacing fluorescein-labeled estradiol from human ERβ. Purified hERβ and fluorescein-labeled estradiol were incubated for 4h with serial dilutions in triplicate of (A) 1-OH naphthanol, (B) 9-OH phenanthrene, and (C) 1-OH pyrene. Error bars represent SD.
After determining that monohydroxylated PAHs bind ERβ, Western blots with FLAG antibody were used to determine the degradation status of the receptor (Supplementary fig. 2), as some ER ligands cause degradation of the receptor upon binding. These Western blots confirmed that ERβ was not degraded by the monohydroxylated PAHs within 48h of treatment.
Monohydroxylated PAHs Exhibit Estrogenic Activity on ERβ Target Genes
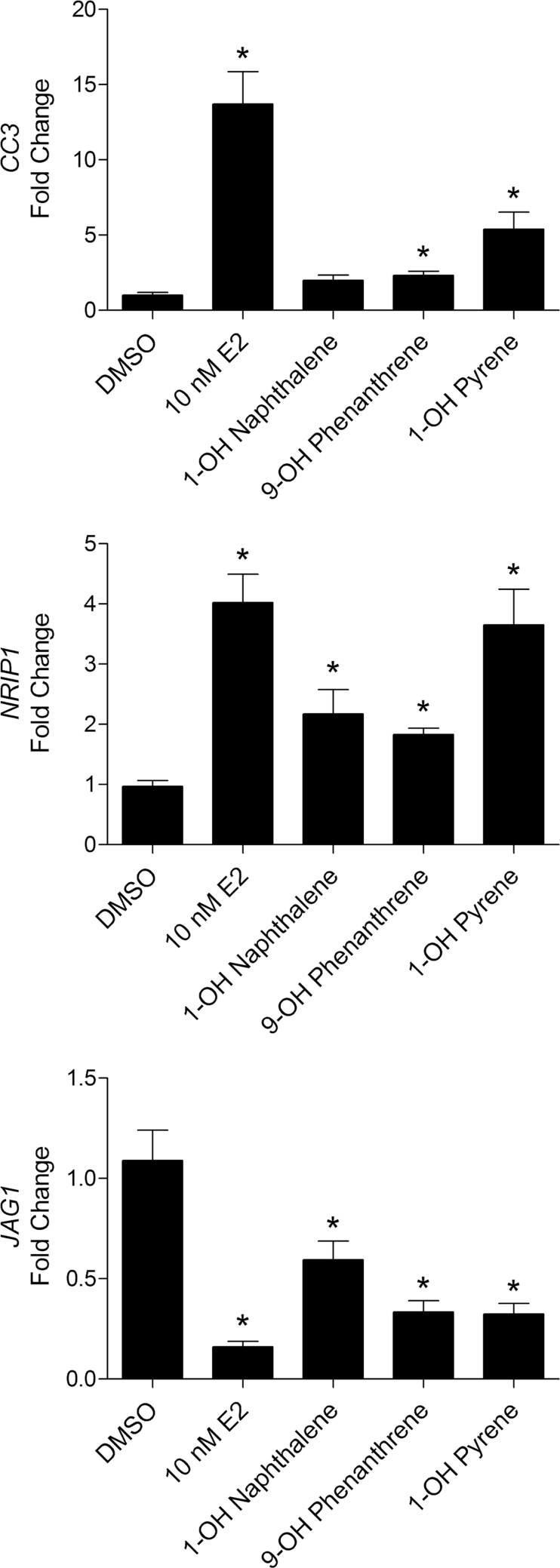
To further validate the reporter assay and BRET assay results, the regulation of endogenous ERβ target genes was assessed. Estrogen responsive target genes of ERβ were previously identified in Hs578T-ERβ cells (Secreto et al., 2007). Two upregulated target genes (CC3 and NRIP1) and one downregulated target gene (JAG1) were selected for analysis by qPCR (Fig. 6). At 10µM, 1-OH naphthalene was able to induce CC3 and NRIP1 expression 2.1- and 2.2-fold over DMSO, respectively. Although the increased expression of CC3 did not reach statistical significance (p = 0.06), NRIP1 was significantly upregulated by 1-OH naphthalene (p = 0.02). Treatment with 5µM 9-OH phenanthrene was able to significantly induce CC3 and NRIP1 expression 2.4-fold (p = 0.02) and 1.9-fold (p < 0.01) over DMSO, respectively. Similarly, 5µM 1-OH pyrene was able to significantly induce CC3 and NRIP1 expression 5.6-fold (p = 0.02) and 3.8-fold (p < 0.01) over DMSO, respectively. Additionally, all three monohydroxylated PAH compounds were able to downregulate the expression of JAG1, generating mean fold changes of 0.64 (p = 0.02), 0.36 (p < 0.01), and 0.32 (p < 0.01) over the DMSO control. It is important to note that although all compounds displayed some estrogenic activity on the target genes tested, the estrogenic response was not as robust as that of E2.
FIG. 6.
Monohydroxylated PAHs can regulate ERβ target genes similar to estradiol. Expression of ERβ target genes (CC3, NRIP1, and JAG1) was determined by measuring relative mRNA levels using qPCR. RNA was collected following treatment with 0.1% DMSO, 10nM E2, 10µM 1-OH naphthalene, 5µM 9-OH phenanthrene, or 5µM 1-OH pyrene for 24-h and 48-h treatment with 50ng/ml Dox. Data are expressed as fold induction compared with DMSO control. Error bars represent SEM. *p < 0.05 compared with DMSO control.
DISCUSSION
Numerous studies have investigated the relationship between PAHs, their hydroxylated metabolites, and potential interactions with the ERs, yet most have focused on ERα (reviewed by Santodonato, 1997). Hayakawa et al. (2007) reported estrogenic and antiestrogenic activity for multiple monohydroxylated derivatives of common PAHs in a yeast two-hybrid assay expressing ERα. Similar to our findings, they also reported that the parent PAH compounds lacked any estrogenic or antiestrogenic activity. Charles et al. (2000) also reported estrogenic activity for hydroxylated metabolites of the carcinogen benzo[a]pyrene (B[a]P) in MCF-7 cells, which primarily express ERα. Despite these previous findings, there have been relatively few studies comparing the effects of monohydroxylated PAHs on the differential activation and dimerization of ERα and ERβ.
Our results, consistent with prior studies, indicate that hydroxylated PAHs are the active estrogenic species and can differentially activate either ERα or ERβ. Although the compounds we tested exhibited no interaction with ERα, the interaction with ERβ is novel and significant. Inhibition of luciferase signal by the ER antagonist ICI 182,780, as well as the lack of luciferase signal in the absence of Dox, demonstrates that the results of the reporter assay are ERβ mediated. Competition with fluorescein-labeled estradiol indicates that these monohydroxylated PAH compounds directly bind to ERβ at the same ligand-binding pocket as E2. Fertuck et al. (2001a) investigated different parental PAH and metabolite compounds, and they similarly reported that hydroxylated PAHs were able to compete with estrogen and bind ERs with a slight preference for ERβ. Their data, consistent with our findings, suggest that hydroxylated PAHs may preferentially affect ERβ signaling. Given ERβ’s role in normal development and function in reproductive tissues as well as in the lungs, colon, prostate, and cardiovascular system, disruption of and interference with ERβ signaling could have implications in normal development, as well as in cancers and malfunctions of these tissues.
In addition to the reporter assay and competitive binding data, the BRET and qPCR data confirm that 9-OH phenanthrene and 1-OH pyrene induce a biologically active ERβ response in this system. Given our data, 1-OH naphthalene may not necessarily induce ERβ homodimers even at the high concentration tested (10µM). In support of these data, ligand-binding assays with 1-OH naphthalene demonstrate a relatively low binding affinity for ERβ. Despite the negative BRET data, qPCR for endogenous ERβ target genes suggest that 1-OH naphthalene is capable of inducing a slight biologically active ERβ response for some ERβ target genes although not to the same extent as E2. Collectively, the data obtained for 1-OH naphthalene demonstrate an important consideration of the in vitro assays used in this study: different assays have different sensitivities for detecting estrogenic activity and ER subtype selectivity. The ERβ homodimerization BRET assay typically shows a 1.5- to 2-fold induction with E2 treatment because of high ligand-independent dimerization (Powell and Xu, 2008). In addition, the BRET ratios ultimately depend on the conformational changes within the receptor fusion proteins, which allow for efficient energy transfer, and different ligands will induce different conformational changes, thereby affecting the BRET ratio output. Despite the lower fold changes for the ERβ homodimerization assay, BRET has been successfully used in a high-throughput manner to identify ER dimer selective ligands (Powell et al., 2010) and, in this study, demonstrated a significant induction of ERβ homodimerization by two other monohydroxylated PAHs, 1-OH pyrene and 9-OH phenanthrene.
Although each monohydroxylated PAH tested gave a similar pattern of results, the relative activity of each compound is quite different. Our data indicate that 1-OH naphthalene is the weakest ERβ agonist among the tested metabolites, as demonstrated by low reporter gene output, a lack of saturation in the dose-response reporter assays, low induction of ERβ dimerization, and a lower binding affinity for ERβ. In contrast, 1-OH pyrene and 9-OH phenanthrene appear to be fairly efficient ERβ agonists. Both ligands induced ERE-reporter gene activity similar to E2 and effectively displaced E2 from the ERβ ligand-binding pocket. Both compounds also significantly elicited ERβ homodimerization. 9-OH Phenanthrene generated data similar to 1-OH pyrene with the exception that it proved to be cytotoxic at concentrations greater than 10µM, resulting in difficulty to obtain accurate EC50 values. Despite the cytotoxicity of 9-OH phenanthrene at high concentrations, treatment with lower concentrations of 9-OH phenanthrene (5µM) stimulated the regulation of endogenous ERβ target genes in Hs578T-ERβLuc cells. These data suggest that some monohydroxylated PAHs can affect ERβ-mediated signaling prior to inducing general cytotoxicity.
Although our data did not indicate that any of the monohydroxylated PAHs tested had an effect on ERα, others have reported ERα estrogenic effects for these compounds. Hayakawa et al. (2007) reported that all three monohydroxylated PAHs exhibited little to no ERα estrogenic activity, but that 1-OH pyrene was able to compete with E2 for ERα binding. Additionally, Wiele et al. (2004) reported that 1-OH pyrene showed ERα estrogenic activity in colon extracts from a simulator of the human intestinal microbial ecosystem. Discrepancies across these studies may be due to the use of different assays and cell lines to assess the estrogenic activity.
Overall, these data suggest that common monohydroxylated PAHs can interact, positively or negatively, with ER signaling. We can conclude from our results and from other studies that hydroxylated PAHs are the active estrogenic species and can differentially bind ERα or ERβ, likely in a cell- and tissue-specific manner. Few studies assessing the physiological serum concentrations of monohydroxylated PAHs have been published, although monohydroxylated PAHs may be used as urine biomarkers to assess exposure to PAHs (Elovaara et al., 2006). It is therefore difficult to predict the concentrations of monohydroxylated PAHs that reach tissues such as the mammary gland, and the concentrations shown to be estrogenic in these studies may or may not be reached in the serum. Some estrogenic compounds in the diet, such as genistein found in soy products, can reach serum concentrations near the micromolar range (Cassidy et al., 2006). Ultimately, the physiological exposure to monohydroxylated PAHs will be a function of both exposure and metabolic activity, which will greatly vary among individuals. These in vitro studies, however, demonstrate the potential for monohydroxylated PAHs to impact ERβ-mediated signaling and provide a framework for assessing the impacts of other environmental chemicals on the dimerization and transcriptional activities of ERα and ERβ.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (R01-CA125387 to W.X.); the National Institute of Environmental Health and Sciences (T32 ES007015 to E.S.); the Department of Defense Breast Cancer Research Program (Era of Hope Scholar Award to W.X. and predoctoral training grant to E.S.); the Greater Milwaukee Foundation (Shaw Scientist Award to W.X.).
Supplementary Material
ACKNOWLEDGMENTS
There are no conflicts of interest.
REFERENCES
- Anzenbacher P., Anzenbacherová E. (2001). Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. 58, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro K. F., O’Keefe P. W., Yang Y., Clayton W., Gierthy J. F. (1999). Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology 133, 115–127 [DOI] [PubMed] [Google Scholar]
- Baird W. M., Hooven L. A., Mahadevan B. (2005). Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 45, 106–114 [DOI] [PubMed] [Google Scholar]
- Bauer E., Guo Z., Ueng Y. F., Bell L. C., Zeldin D., Guengerich F. P. (1995). Oxidation of benzo[a]pyrene by recombinant human cytochrome P450 enzymes. Chem. Res. Toxicol. 8, 136–142 [DOI] [PubMed] [Google Scholar]
- Cassidy A., Brown J. E., Hawdon A., Faughnan M. S., King L. J., Millward J., Zimmer-Nechemias L., Wolfe B., Setchell K. D. (2006). Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J. Nutr. 136, 45–51 [DOI] [PubMed] [Google Scholar]
- Charles G. D., Bartels M. J., Zacharewski T. R., Gollapudi B. B., Freshour N. L., Carney E. W. (2000). Activity of benzo[a]pyrene and its hydroxylated metabolites in an estrogen receptor-alpha reporter gene assay. Toxicol. Sci. 55, 320–326 [DOI] [PubMed] [Google Scholar]
- Cho T. M., Rose R. L., Hodgson E. (2006). In vitro metabolism of naphthalene by human liver microsomal cytochrome P450 enzymes. Drug Metab. Dispos. 34, 176–183 [DOI] [PubMed] [Google Scholar]
- Elovaara E., Mikkola J., Mäkelä M., Paldanius B., Priha E. (2006). Assessment of soil remediation workers’ exposure to polycyclic aromatic hydrocarbons (PAH): Biomonitoring of naphthols, phenanthrols, and 1-hydroxypyrene in urine. Toxicol. Lett. 162, 158–163 [DOI] [PubMed] [Google Scholar]
- Fertuck K. C., Kumar S., Sikka H. C., Matthews J. B., Zacharewski T. R. (2001a). Interaction of PAH-related compounds with the alpha and beta isoforms of the estrogen receptor. Toxicol. Lett. 121, 167–177 [DOI] [PubMed] [Google Scholar]
- Fertuck K. C., Matthews J. B., Zacharewski T. R. (2001b). Hydroxylated benzo[a]pyrene metabolites are responsible for in vitro estrogen receptor-mediated gene expression induced by benzo[a]pyrene, but do not elicit uterotrophic effects in vivo. Toxicol. Sci. 59, 231–240 [DOI] [PubMed] [Google Scholar]
- Hartman J., Lindberg K., Morani A., Inzunza J., Ström A., Gustafsson J. A. (2006). Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 66, 11207–11213 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Onoda Y., Tachikawa C., Hosoi S., Yoshida M., Chung S., Kizu R., Toriba A., Kameda T., Tang N. (2007). Estrogenic/antiestrogenic activities of polycyclic aromatic hydrocarbons and their monohydroxylated derivatives by yeast two-hybrid assay. J. Health Sci. 53, 562–570 [Google Scholar]
- Kim J. H., Stansbury K. H., Walker N. J., Trush M. A., Strickland P. T., Sutter T. R. (1998). Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis 19, 1847–1853 [DOI] [PubMed] [Google Scholar]
- Koterba K. L., Rowan B. G. (2006). Measuring ligand-dependent and ligand-independent interactions between nuclear receptors and associated proteins using Bioluminescence Resonance Energy Transfer (BRET). Nucl. Recept. Signal. 4, e021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruthiyil S., Parmar H., Kerekatte V., Cunha G. R., Firestone G. L., Leitman D. C. (2004). Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 64, 423–428 [DOI] [PubMed] [Google Scholar]
- Pike A. C., Brzozowski A. M., Hubbard R. E., Bonn T., Thorsell A. G., Engström O., Ljunggren J., Gustafsson J. A., Carlquist M. (1999). Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 18, 4608–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell E., Huang S. X., Xu Y., Rajski S. R., Wang Y., Peters N., Guo S., Xu H. E., Hoffmann F. M., Shen B, et al. (2010). Identification and characterization of a novel estrogenic ligand actinopolymorphol A. Biochem. Pharmacol. 80, 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell E., Xu W. (2008). Intermolecular interactions identify ligand-selective activity of estrogen receptor alpha/beta dimers. Proc. Natl. Acad. Sci. U.S.A. 105, 19012–19017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach B., Preuss R., Letzel S., Drexler H., Angerer J. (2007). Biological monitoring of occupational exposure to polycyclic aromatic hydrocarbons (PAH) by determination of monohydroxylated metabolites of phenanthrene and pyrene in urine. Int. Arch. Occup. Environ. Health 81, 221–229 [DOI] [PubMed] [Google Scholar]
- Santodonato J. (1997). Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: Relationship to carcinogenicity. Chemosphere 34, 835–848 [DOI] [PubMed] [Google Scholar]
- Secreto F. J., Monroe D. G., Dutta S., Ingle J. N., Spelsberg T. C. (2007). Estrogen receptor alpha/beta isoforms, but not betacx, modulate unique patterns of gene expression and cell proliferation in Hs578T cells. J. Cell. Biochem. 101, 1125–1147 [DOI] [PubMed] [Google Scholar]
- Shanle E. K., Hawse J. R., Xu W. (2011). Generation of stable reporter breast cancer cell lines for the identification of ER subtype selective ligands. Biochem. Pharmacol. 82, 1940–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. (1989). The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59, 477–487 [DOI] [PubMed] [Google Scholar]
- Treeck O., Lattrich C., Springwald A., Ortmann O. (2010). Estrogen receptor beta exerts growth-inhibitory effects on human mammary epithelial cells. Breast Cancer Res. Treat. 120, 557–565 [DOI] [PubMed] [Google Scholar]
- Tremblay A., Tremblay G. B., Labrie F., Giguère V. (1999). Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol. Cell 3, 513–519 [DOI] [PubMed] [Google Scholar]
- Wiele T. V. D., Vanhaecke L., Boeckaert C., Peru K., Headley J., Verstraete W., Siciliano S. (2005). Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ. Health Perspect 113, 6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.