Abstract
Zearalenone (ZEA) is a mycotoxin commonly found in contaminated livestock feed and human food with levels in the range of ppb and low ppm. It was hypothesized that ZEA, an endocrine disruptor, could affect puberty and early pregnancy. To test this hypothesis, newly weaned (3 weeks old) C57BL/6J female mice were exposed to 0, 0.002, 4, 10, and 40 ppm ZEA and 0.05 ppm diethylstilbestrol (positive control) in phytoestrogen-free AIN-93G diet. Females exposed to 10 and 40 ppm ZEA diets showed earlier onset of vaginal opening. Those treated with 40 ppm ZEA diet also had earlier first copulation plug and irregular estrous cyclicity. At 8 weeks old, all females were mated with untreated stud males on AIN-93G diet during mating. Treatment resumed upon identification of a vaginal plug on gestation day 0.5 (D0.5). Embryo implantation was assessed on D4.5. Exposure to 40 ppm ZEA diet resulted in reduced percentage of plugged mice with implantation sites, distended uterine appearance, and retained expression of progesterone receptor in D4.5 uterine epithelium. To determine the exposure timing and mechanisms of disrupted embryo implantation, four groups of females were fed with 0 or 40 ppm ZEA diets during premating (weaning to mating) and postmating (D0.5–D4.5), respectively. Premating exposure to 40 ppm ZEA diet reduced fertilization rate, whereas postmating exposure to 40 ppm ZEA diet delayed embryo transport and preimplantation embryo development, which subsequently affected embryo implantation. These data demonstrate that postweaning exposure to dietary ZEA can promote premature onset of puberty and disrupt early pregnancy events.
Key Word: zearalenone, vaginal opening, fertilization, embryo transport, embryo development, embryo implantation.
Zearalenone (ZEA) is a mycotoxin produced by several Fusariam species (Zinedine et al., 2007). It is commonly found in livestock feed and human food such as corn, rice, oats, and wheat. Quantifiable ZEA was detected in 15% of 13,075 food samples and 9877 unprocessed grain samples collected during 2005 and 2010 in Europe. Among them, detectable levels in processed food groups for human consumption were in the ppb range with the highest at 823 ppb, and those in unprocessed grains with the highest reaching 3 ppm (EFSA, 2011). Worldwide, ZEA contamination levels in food are usually in the range of ppb and low ppm, with the highest reported reaching 600 ppm (EFSA, 2011; Price et al., 1993; Sangare-Tigori et al., 2006; Zinedine et al., 2007). The tolerable daily intake (TDI) of ZEA established by the Panel on Contaminants in the Food Chain in Europe is 0.25 µg/kg body weight (EFSA, 2011).
ZEA is mainly metabolized in the liver to α- and β-zearalenol by 3α- and 3β-hydroxysteroid dehydrogenases, respectively. Alpha-zearalenol is the dominant ZEA derivative in pigs (Kuiper-Goodman et al., 1987), humans (Mirocha et al., 1981), rats, and mice (Bravin et al., 2009). ZEA and its metabolites are then glucuronidated by uridine diphospho-glucuronosyltransferase (EFSA, 2011; Pfeiffer et al., 2010; Zinedine et al., 2007). Cytochrome P450 may also be involved in ZEA metabolism (EFSA, 2011). ZEA and its metabolites have estrogenic effects due to their structural similarity with 17β-estradiol (E2) (Gromadzka et al., 2009). The order of estrogenicity is E2 > α-zearalenol > ZEA > β-zearalenol based on gene induction assay (Frizzell et al., 2011), uterotrophic assay (Ueno and Tashiro, 1981), and estrogen receptor binding assay (Kuiper-Goodman et al., 1987). Different metabolic pathways and varied estrogenicity of ZEA and its metabolites may contribute to the variable species susceptibility to ZEA exposure (Malekinejad et al., 2006).
ZEA has been associated with certain pathological conditions in females and shown to have adverse effects on embryo and/or female reproductive function in different species. In humans, dietary exposure to ZEA has been associated with precocious pubertal development (Deng et al., 2012; Massart and Saggese, 2010). ZEA and α-zearalenol were also detected in the serum of patients with endometrial cancer (Gajecki et al., 2004). In pigs, gestational exposure to ZEA caused blastocyst degeneration (Long et al., 1992) and fetal loss (Long and Diekman, 1984); exposure of sexually immature gilts to ZEA resulted in hyperestrogenism, including stimulated uterine cell proliferation (Gajecka et al., 2012), and affected the development and maturation of ovarian follicles (Zwierzchowski et al., 2005). Immature gilts seem to be more predisposed to ZEA insult than other age groups of swine (Jakimiuk et al., 2009). In ewes, premating exposure to ZEA for 10 days reduced ovulation and fertilization, but the same doses and length of treatment starting 5 days postmating did not affect pregnancy rate or cause embryonic loss (Smith et al., 1990). In rats, neonatal exposure to ZEA caused persistent anovulatory estrous in adulthood (Kumagai and Shimizu, 1982); two-generation exposure (during mating and gestation only) to dietary ZEA (10mg/kg/day) decreased pregnancy rate and increased embryo resorption in the uterus (Becci et al., 1982). In mice, gestational exposure (gestational day [D]15–D18) or prepubertal exposure (postnatal day [PND]15–PND18) to ZEA (10mg/kg/day, sc) for 4 days accelerated the onset of vaginal opening (VO), extended the estrous phase, and prolonged the period of anovulatory ovary (Nikaido et al., 2004, 2005), and neonatal exposure (PND1–PND10) to ZEA (5–30 µg/mouse) caused delayed VO, persistent estrous, and sterility (Ito and Ohtsubo, 1994).
Despite extensive reports on the adverse effects of ZEA on female fertility (EFSA, 2011), it has not been systemically studied with regard to potential effects of postweaning dietary ZEA exposure on early pregnancy events in vivo. Because only minimal amounts of ZEA and its metabolites could be transmitted to the milk (Prelusky et al., 1990), most mammals, including humans, start to be exposed to ZEA directly from food, which is the main route for ZEA exposure, once they are weaned from mother’s milk prior to puberty. The goal of this study was to test the hypothesis that postweaning exposure to ZEA could affect female puberty and early pregnancy events in newly weaned C57BL/6J females fed with diets containing environmental relevant levels of ZEA. Various parameters, including the timing of VO and first copulation plug, estrous cycle, fertilization, embryo transport, embryo development, and embryo implantation, were determined. The results could help risk assessment of ZEA and increase our understanding of the mechanisms of ZEA action on female fertility.
MATERIALS AND METHODS
Animals.
C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) to establish a colony in the Coverdell Rodent Vivarium at the University of Georgia. All mice were housed in polypropylene cages with free access to a casein-based phytoestrogen-free AIN-93G diet (Bio-Serv, Frenchtown, NJ) and water in polypropylene water bottles. The animal facility was maintained on a 12-h light/dark cycle (0600h to 1800h) at 23±1°C with 30–50% relative humidity. All methods used in this study were approved by the Animal Subjects Programs of the University of Georgia and conform to National Institutes of Health guidelines and public law.
Diet preparation.
0, 0.0005, 1, 2.5, and 10mg of ZEA (Fermentek, Israel) and 0.0125mg of diethylstilbestrol (DES; Sigma) were first dissolved in 70ml 100% ethanol (Alcohol 200 Proof; Decon Lab Inc.) and then diluted with 30ml ddH2O to make 100ml 70% ethanol solutions. Each solution was mixed with 250g AIN-93G powder in a glass bowl to prepare 0, 0.002, 4, 10, and 40 ppm ZEA diets and 0.05 ppm DES diet. Each diet was well mixed, squeezed by hand into pellets (about 2cm in diameter and 6cm in length), and dried for 48h at room temperature in a dark hood. The diets were prepared fresh every 2 weeks and kept at 4°C in a dark room until use. The rationale for the dose selection: 0.002 ppm ZEA diet was about the dose of 0.25 µg/kg/day TDI of ZEA in Europe (EFSA, 2011); 4, 10, and 40 ppm ZEA were based on the levels reported in contaminated foods (Zinedine et al., 2007). Taking into account the average food consumption and body weight, these doses can be expressed approximately as 0.00025, 0.5, 1.25, and 5mg/kg/day, respectively. The positive control 0.05 ppm dietary DES in diet was shown to affect pregnancy outcome (Lamb et al., 1985).
Treatments.
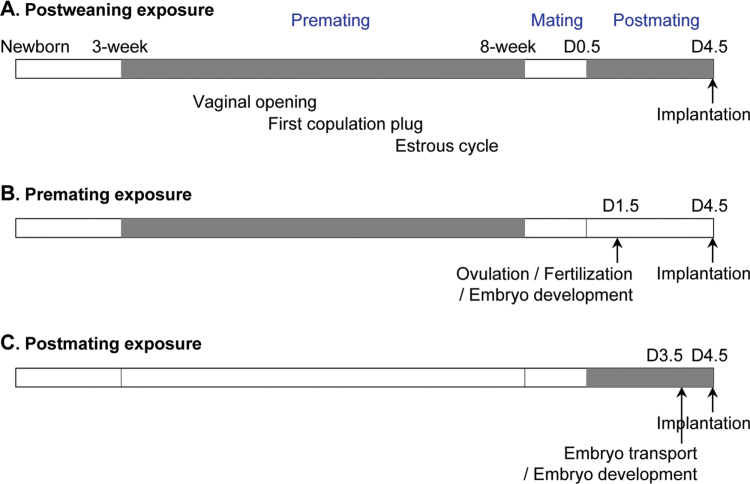
Postweaning exposure (Fig. 1A): Newly weaned female pups (3 weeks old) were randomly assigned into six groups (0, 0.002, 4, 10, and 40 ppm ZEA and 0.05 ppm DES) with littermates separated into different groups. At 8 weeks old, they were mated with young stud C57BL/6J untreated males (for all matings, mice were put together during daytime between 1100h and 1700h). The mice were on control AIN-93G diet during mating. Once a vaginal plug was detected on D0.5, the original exposure regimen was resumed. Mice were assessed on D4.5 at 1100h to determine embryo implantation using blue dye reaction (Ye et al., 2005). If no implantation site was observed, one side of uterine horn and oviduct was flushed with 1× PBS to determine the presence, location, and morphology of embryos to indicate pregnancy status. Part of the other uterine horn was flash-frozen for immunohistochemistry, and the rest was fixed in 10% formalin solution. During the treatment, body weight, food consumption, and water consumption were measured weekly. Premating exposure (Fig. 1B): Newly weaned C57BL/6J female mice were exposed to 0 or 40 ppm ZEA diets. The treatment ended when they were mated with untreated stud males at 8 weeks old. One set of mice on the 40 ppm ZEA-treated group was assessed on D4.5 to determine embryo implantation following the same procedure described in postweaning exposure. Another set of mice were analyzed on D1.5 to determine the presence and morphology of oocytes and embryos in the oviduct. To minimize the potential recovery from ZEA treatment, only the females plugged on the first night of mating were included in the study and analyzed on D1.5 in the 40 ppm ZEA-treated group. Both oviducts were flushed. The oocytes showing no pronuclei or polar body were considered to be unfertilized oocytes (Viveiros et al., 2003). Postmating exposure (Fig. 1C): Untreated 8-week-old C57BL/6J female mice were mated with untreated stud males. Once plugged, the females were exposed to 0 or 40 ppm ZEA diets. One set of mice on the 40 ppm ZEA-treated group was evaluated on D4.5 to determine embryo implantation. Another set of females was analyzed on D3.5 at 1100h. Both uterine horns and oviducts were flushed with 1× PBS to determine the presence, location, and morphology of embryos. Embryo development stage was categorized into morula, early blastocyst with small blastocoel (Baczkowski et al., 2004), and blastocyst. Pregnancy rate was defined as the percentage of plugged mice with embryo(s) and/or implantation site(s) in the reproductive tract detected on D1.5–D4.5. The number of mice in each group was indicated in the Results section and legends of figures.
Fig. 1.
Treatment regimens. Grey, treatment; white, no treatment; D0.5, D1.5, D3.5, and D4.5, gestation day 0.5, 1.5, 3.5, and 4.5, respectively. (A) Postweaning exposure. VO, first copulation plug, and estrous cycle were examined during premating exposure. Vaginal plug was checked every morning during mating. The morning of plug identification was designated as D0.5. Implantation was detected on D4.5. (B) Premating exposure. Oviducts were flushed on D1.5 for oocytes/embryos to determine ovulation, fertilization, and early embryo development. Implantation was detected on D4.5. (C) Postmating exposure. Oviducts and uterine horns were flushed on D3.5 to assess embryo number, embryo transport, and preimplantation embryo development. Implantation was detected on D4.5.
VO, first copulation plug, and estrous stages.
VO was checked daily from PND22 until PND40. Once VO was detected, the female was mated with a stud male to determine the timing of the first copulation plug. A vaginal smear was collected daily between 1500h and 1600h for 21 days starting from 6 weeks of age to determine estrous stages. A complete cycle would include diestrus/metestrus, proestrus, and estrous stages (Caligioni, 2009). A total of five mice were included in each of the 0, 10, and 40 ppm ZEA-treated groups.
Histology.
Fixed D4.5 uterine horns were embedded in paraffin and cross-sectioned (5 µm). Sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin as previously described (Diao et al., 2011a).
Immunohistochemistry.
Frozen uterine cross sections (10 µm) were immunostained to detect progesterone receptor (PR) expression as previously described (Xiao et al., 2011).
Statistical analyses.
ANOVA-type models were fitted using SigmaStat 3.5 for continuous outcomes. For the data that passed the normality and equal variance tests, such as food consumption, water consumption, and duration days of estrous, one-way ANOVA followed by Student-Neuman-Keuls (SNK) multiple comparison test was used. For the data that failed the normality and equal variance tests, such as age at VO, age at first copulation plug, interval between VO and first copulation plug, number of complete estrous cycles, number of implantation sites, and number of embryos and oocytes, ANOVA on ranks followed by Dunn’s method, which does not give precise p values but > 0.05 or < 0.05, was used. Postweaning body weights were analyzed with a two-way Repeated-Measures ANOVA (with time and dose as independent factors) followed by SNK multiple comparison test. Mean ± SD was reported for the continuous outcomes. Chi-squared test and Fisher’s exact test with p value adjusted based on Holm’s method for comparing multiple groups were used as appropriate for percentage of plugged mice with implantation sites, fertilization rate, embryo location, and embryo development. The significant level was set at p < 0.05.
RESULTS
Postweaning Exposure to ZEA Diet Accelerated the Timing of VO and First Copulation Plug
Postweaning dietary exposure to ZEA (Fig. 1A) at 0.002–40 ppm did not affect food consumption, water consumption, or body weight of the treated females. However, the females treated with 0.05 ppm DES diet had significantly increased water consumption starting from the third week of treatment and significantly increased body weight after 5 weeks of treatment (data not shown).
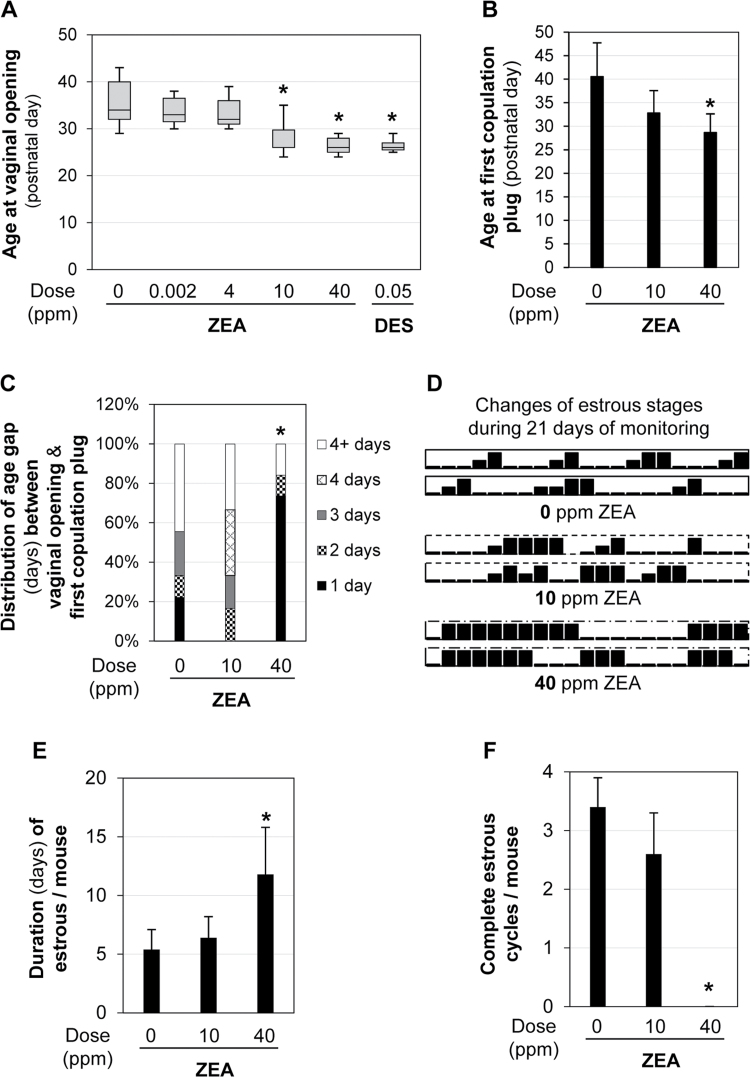
VO is an indication of puberty onset (Safranski et al., 1993). The mean age of VO in the control group (0 ppm ZEA) was 33.5±2.1 days old (N = 50). Those in 2 ppb ZEA (32.5±1.5 days old, N = 11) and 4 ppm ZEA (32.6±2.1 days old, N = 12) groups were comparable to the control. However, those in the 10 and 40 ppm ZEA-treated groups had significantly younger ages of VO compared with the control at age 27.1±2.2 (N = 30, p < 0.05) and 25.6±0.7 (N = 30, p < 0.05) days old, respectively. There was no significant difference in the age of VO between the 10 and 40 ppm ZEA-treated groups. The age of VO in the positive control 0.05 ppm DES-treated group (25.9±0.8 days old, N = 15) was comparable to that in both 10 and 40 ppm ZEA-treated groups but significantly younger than the remaining groups (Fig. 2A). The time of VO in both 40 ppm ZEA group and 0.05 ppm DES groups was only 3 days after the start of the treatments.
Fig. 2.
Postweaning exposure to dietary ZEA on puberty and estrous cycle. (A) The average age at VO in each group. N = 11–50. Error bars: SD. *p < 0.05 compared with 0 ppm ZEA control group. (B) The average age at first copulation plug. Error bars: SD. *p < 0.05 compared with 0 ppm ZEA-treated group. (C) Distribution of age gap (days) between VO and first copulation plug. *p = 0.0166, percentage of mice with 1-day gap. (B and C) N = 9 (0 ppm ZEA), N = 6 (10 ppm ZEA), and N = 19 (40 ppm ZEA). (D–F) Estrous cyclicity of five 6-week-old mice each in 0, 10, and 40 ppm ZEA groups for 3 weeks. (D) Estrous cyclicity of two representative mice in 0, 10, and 40 ppm ZEA-treated groups during 21 days of monitoring. High bar, estrous stage; median bar, proestrus; low bar, diestrus/metestrus. (E) Duration (days) of estrous per mouse during the 21 days of monitoring in 0, 10, and 40 ppm ZEA-treated groups. *p = 0.0008 compared with 0 ppm ZEA-treated group. (F) The number of complete estrous cycles per mouse in each group during the 21 days of monitoring. *p < 0.05 compared with 0 ppm ZEA-treated group. (E and F) N = 5 in each group. Error bars: SD.
The first copulation plug is another indication of puberty onset (Safranski et al., 1993). It was detected at age 40.6±7.2 days old in the control group (N = 9), 32.8±4.8 (N = 6, p > 0.05 compared with the control) days old in the 10 ppm ZEA-treated group, and 28.7±3.9 (N = 19, p < 0.05 compared with the control) days old in the 40 ppm ZEA-treated group. (Fig. 2B). Although the average age of the first copulation plug was reduced by ~8 days in the 10 ppm ZEA-treated group compared with the control, the difference was not statistically significant.
The average age intervals between VO and first copulation plug were 6.2±6.3 (N = 9), 6.2±4.8 (N = 6), and 2.7±3.9 (N = 19) days for 0, 10, and 40 ppm ZEA-treated groups, respectively. This interval was significantly decreased in the 40 ppm ZEA-treated group (p < 0.05) compared with the control. In addition, a significantly higher percentage of mice had only one-day gap in the 40 ppm ZEA-treated group (14/19 = 73.7%, p = 0.0166) than in the control (2/9 = 22.2%) (Fig. 2C).
Postweaning Exposure to 40 ppm ZEA Diet Disrupted Estrous Cyclicity
Daily recording for 21 days revealed that the duration (days) in estrous stage per mouse was significantly increased in the 40 ppm ZEA-treated group (11.8±4.0 days, p = 0.008) but not that in the 10 ppm ZEA-treated group (6.4±1.8 days) compared with the control group (5.4±1.7 days) (Figs. 2D and E). In addition, the females in 40 ppm ZEA-treated group lacked a clear proestrus stage (Fig. 2D), thus no complete estrous cycle during those 21 days monitored (Fig. 2F). These data indicate that postweaning exposure to 40 ppm ZEA diet can disrupt estrous cyclicity.
Postweaning Exposure to 40 ppm ZEA Disrupted Embryo Implantation
The postweaning exposure regimen included three segments: premating (3–8 weeks), mating (from cohabitation to identification of a vaginal plug), and postmating (D0.5–D4.5) (Fig. 1A). During the mating segment, both males and females were on the control AIN-93G diet to avoid any potential contribution from the males for any adverse implantation outcome. The duration of mating segment was comparable among all the groups (data not shown), and the average was about 2.86 days.
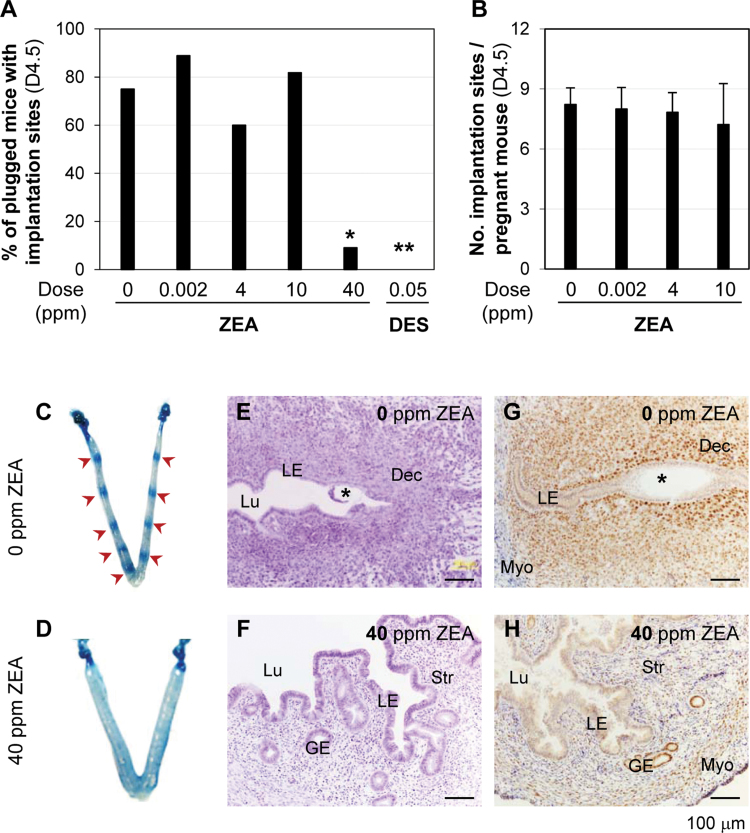
Comparable percentages of plugged mice with implantation sites on D4.5 were observed among 0 ppm (9/12, the number of mice with implantation sites detected on D4.5 over the number of plugged mice detected on D0.5), 0.002 ppm (8/9), 4 ppm (6/10), and 10 ppm (9/11) ZEA-treated groups (Fig. 3A). A significantly decreased percentage of plugged mice with implantation sites was observed in 40 ppm ZEA-treated group (1/11, p = 0.0112) and the positive control 0.05 ppm DES-treated group (0/11, p = 0.0015) (Fig. 3A). There was no significant difference in the average number of implantation sites per pregnant mouse among 0 ppm (8.2±0.8), 0.002 ppm (8.0±1.0), 4 ppm (7.8±1.0), and 10 ppm (7.2±2.0) ZEA-treated groups (Fig. 3B) although there was a decreasing trend with the increase of ZEA doses. None of the females without implantation sites in these four groups had embryos in the reproductive tract, indicating that they were not pregnant, and all the pregnant ones had embryo implantation. All the uteri without implantation sites in the 40 ppm ZEA-treated group (10/11, three with embryos) and 0.05 ppm DES-treated group (11/11, one with embryos) had a distended appearance that was not present in the control (Figs. 3C and D and data not shown).
Fig. 3.
Effects of postweaning ZEA exposure on embryo implantation detected on D4.5. (A) The percentage of plugged mice with implantation sites in each group. N = 9–12. *p = 0.0112; **p = 0.0015 compared with 0 ppm ZEA control group. (B) Average number of implantation sites per pregnant mouse. N = 6–9. Error bars: SD. (C) A representative D4.5 uterus in control group. Red arrows, implantation sites. (D) A representative D4.5 uterus in 40 ppm ZEA-treated group. (E) Histology of an implantation site in C. (F) Histology of the uterus in D. (G) Immunohistochemistry of PR in a section from an implantation site in C. (H) Immunohistochemistry of PR in a section from D, E, & F. Hematoxylin and eosin staining of fixed uterine section (5 µm). (G and H) Brown staining indicating PR expression in frozen uterine sections (10 µm). No specific staining in the negative control (data not shown). Black star, embryo; LE, uterine luminal epithelium; Lu, uterine lumen; Dec, decidual zone; Str, stroma; GE, glandular epithelium; Myo, myometrium. Scale bar: 100 µm. Figure available in color online.
In the postweaning 40 ppm ZEA-treated group, 5 of the 11 treated mice had plugs detected in the first morning after cohabitation, one of which had seven implantation sites and the remaining four had neither implantation sites nor embryos in the reproductive tract detected on D4.5. The pregnancy rate was 1/5 = 20%, which was marginally different from that in the control group (5/6 = 83.3%, p = 0.080). The remaining six treated mice had plugs detected after the third morning (between the fourth and eighth mornings). None had implantation sites, but three of them had embryos in the reproductive tract. The pregnancy rate was 3/6 = 50%, which was comparable with that in the control group that had plugs detected after the third morning (4/6 = 66.7%, p = 1.000). Among these three females with embryos, one had two hatched blastocysts flushed from one uterine horn, one had a hatched blastocyst and a fragmented embryo with intact zona pellucida flushed from one oviduct, and the third one had two hatched blastocysts and one hatching blastocyst flushed from one oviduct (data not shown). These results indicate that postweaning exposure to 40 ppm ZEA diet affects embryo implantation and might also affect embryo development and embryo transport.
Postweaning Exposure to 40 ppm ZEA Altered Uterine Histology and PR Expression in D4.5 Uterus
Uterine histology was examined in D4.5 fixed uterine tissues. In the control group, a section of an implantation site showed that implantation had occurred and decidualization was obvious (Fig. 3E). However, the uteri with distended appearance in both 40 ppm ZEA (Fig. 3F) and 0.05 ppm DES-treated groups (data not shown) had enlarged uterine lumen and tall uterine epithelium (Fig. 3F).
PR expression was detected in D4.5 uterus. In the D4.5 control uteri, PR had disappeared from the uterine luminal epithelium (LE) and was highly expressed in the stromal compartment, especially the primary decidual zone (Fig. 3G). In the D4.5 uteri without implantation sites from 40 ppm ZEA and 0.05 ppm DES-treated groups, PR remained expressed in the LE and highly expressed in the glandular epithelium, but it was relatively low in the stromal compartment (Fig. 3H and data not shown). Both uterine histology and PR expression confirm estrogenic effect and failed implantation in the 40 ppm ZEA-treated and positive control 0.05 ppm DES-treated groups (Figs. 3E–H and data not shown).
DES (0.05 ppm) was included as a positive control in the postweaning study to determine any potential effects of ZEA on VO (Fig. 2A) and embryo implantation (Fig. 3A). Because postweaning study had established the positive effects of ZEA on promoting VO and blocking embryo implantation, which were similar as the effects of DES, DES was not included in the following premating study and postmating study.
Premating Exposure to 40 ppm ZEA Reduced Fertilization Rate and Disrupted Early Embryo Development
Postweaning ZEA exposure (Fig. 1A) included premating and postmating exposure (D0.5–D4.5). Multiple early pregnancy events, such as ovulation, fertilization, embryo development, and embryo transport, can subsequently affect embryo implantation. Hence, further experiments were done to determine the critical exposure period(s) that could affect embryo implantation and the potential mechanisms that could account for the disrupted embryo implantation upon postweaning exposure to 40 ppm ZEA diet (Fig. 3). Newly weaned females were exposed to 40 ppm ZEA diet until mating day (8 weeks old) and then placed on the control diet (premating) (Fig. 1B), or the mice were on control diet until a vaginal plug was identified and then placed on 40 ppm ZEA diet (postmating) (Fig. 1C), and the early pregnancy events were analyzed. The control group (0 ppm ZEA) implantation data from D4.5 postweaning study (Figs. 1A and 3) were also used in these premating and postmating studies.
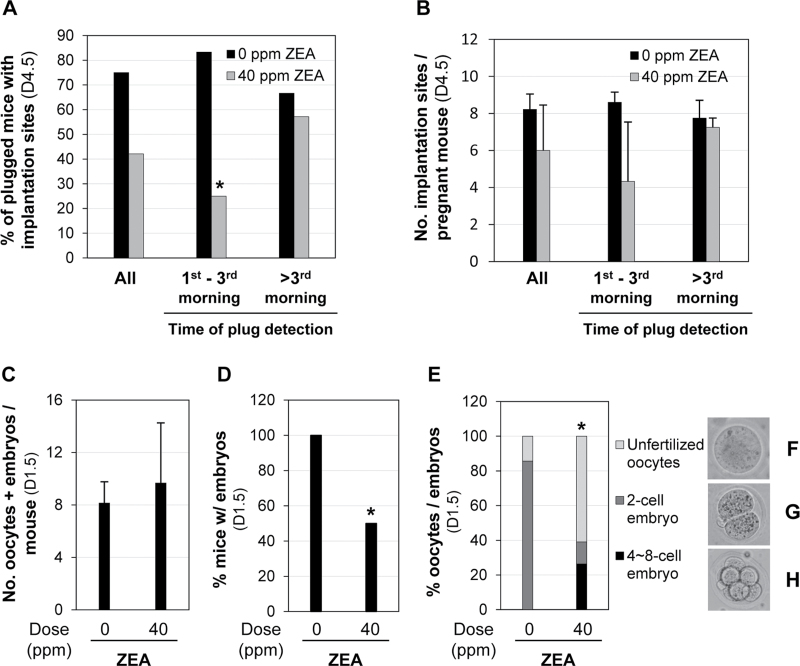
Premating exposure to 40 ppm ZEA did not seem to have a significant effect on the percentage of plugged mice with implantation sites detected on D4.5 (8/19 = 42% vs. 9/12 = 75% in the control, p = 0.1378). However, an interesting observation was made between the time a vaginal plug was detected and the percentage of plugged mice with implantation sites detected on D4.5. Out of the 19 plugged females in the 40 ppm ZEA-treated group, eight were detected in the first morning after cohabitation with two of them having implantation sites on D4.5 (2/8 = 25.0%), four were detected in the second and third mornings with one having implantation sites (1/4 = 25.0%), and the remaining seven were detected after the third morning (between the 5th and 12th mornings) with four of them having implantation sites (4/7 = 57.1%). If the data from the mice with plugs detected between first and third mornings were combined, 3 out of 12 plugged mice (25.0%) had implantation sites on D4.5, which was significantly lower than that in the control group (5/6 = 83.3%, p = 0.043). However, no significant difference was observed in the mice with plugs detected after the third morning (40 ppm ZEA-treated group: 4/7 = 57.1%; control group: 4/6 = 66.7%, p = 1.000) (Fig. 4A). Although no significant difference was observed between 0 and 40 ppm ZEA-treated groups in the average number of implantation sites per pregnant mouse on D4.5, a trend of increased number of implantation sites was observed in the 40 ppm ZEA-treated group if the plugs were detected after the third morning (Fig. 4B). These data (Figs. 4A and B) suggest that premating ZEA treatment affects embryo implantation, but the effect diminishes after the treatment ends.
Fig. 4.
Effects of premating exposure to 40 ppm ZEA diet on embryo implantation detected on D4.5 and oocytes/embryos detected on D1.5. (A) The percentage of plugged mice with implantation sites detected on D4.5. *p = 0.043. (B) The average number of implantation sites per pregnant mouse detected on D4.5. Error bars: SD. (A and B) All, combined data for each group; first to third (1st–3rd) morning and after third (>3rd) morning, the time when a vaginal plug was detected after cohabitation. (C) Average number of oocytes and embryos per mouse. N = 6 and N = 13 for 0 and 40 ppm ZEA-treated groups, respectively. Error bars: SD. (D) Percentage of mice with embryos on D1.5. *p = 0.047. N = 7 and N = 14 for 0 and 40 ppm ZEA-treated groups, respectively. (E) Relative percentage of unfertilized oocytes, two-cell stage embryos, and four- to eight-cell stage embryos from mice with embryos (0 ppm ZEA, N = 49; 40 ppm ZEA, N = 87). *p < 0.0001, percentage of unfertilized oocytes compared with 0 ppm ZEA-treated group. (F, G, and H) Representative images of an unfertilized oocyte (F), a two-cell stage embryo (G), and a four- to eight-cell stage embryo (H), respectively.
To determine the potential cause for the reduced percentage of mice with implantation sites in the 40 ppm ZEA-treated group (Fig. 4A), another set of newly weaned females underwent premating exposure to 0 and 40 ppm ZEA diets. Only the mice with a plug detected in the first morning after cohabitation with untreated stud males were included in the study to minimize the potential recovery from premating ZEA treatment. On D1.5, the percentage of mice having ovulated, indicated by the presence of unfertilized oocytes and/or embryos detected in the oviduct, was similar between 0 ppm ZEA-treated group (7/8 = 87.5%) and 40 ppm ZEA-treated group (14/16 = 87.5%, p = 1.0). The total number of both unfertilized oocytes and embryos was comparable between 0 ppm ZEA-treated group (8.2±1.6, N = 6, excluding the first mouse, which had four 2-cell embryos but the number of unfertilized oocytes was not recorded) and 40 ppm ZEA-treated group (9.7±4.6, N = 13, excluding the first mouse, which had unfertilized oocytes only but the number was not recorded; p = 0.478) (Fig. 4C). These data demonstrate that premating exposure to 40 ppm ZEA diet did not affect the number of oocytes that were ovulated. However, the percentage of ovulated mice with embryos was significantly reduced in the 40 ppm ZEA-treated group (7/14 = 50% vs. 7/7 = 100% in the control; p = 0.047) (Fig. 4D). Among the mice with embryos, the percentage of unfertilized oocytes was significantly higher in the 40 ppm ZEA-treated group (53/87 = 60.9% vs. 7/49 = 14.3% in the control; p < 0.0001) (Figs. 4E and F). Interestingly, all embryos in the control group (42/42) were at two-cell stage (Fig. 4G), but 67.5% (23/34, p < 0.0001) of the embryos in the 40 ppm ZEA-treated group were at four- to eight-cell stages (Fig. 4H). Collectively, these data demonstrated that premating exposure to 40 ppm ZEA diet did not affect the percentage of mice that ovulated or the number of oocytes ovulated (Fig. 4C). However, it adversely affected fertilization (Figs. 4D and E) and possibly early embryo development (Figs. 4E–H), which could subsequently affect embryo implantation (Fig. 4A).
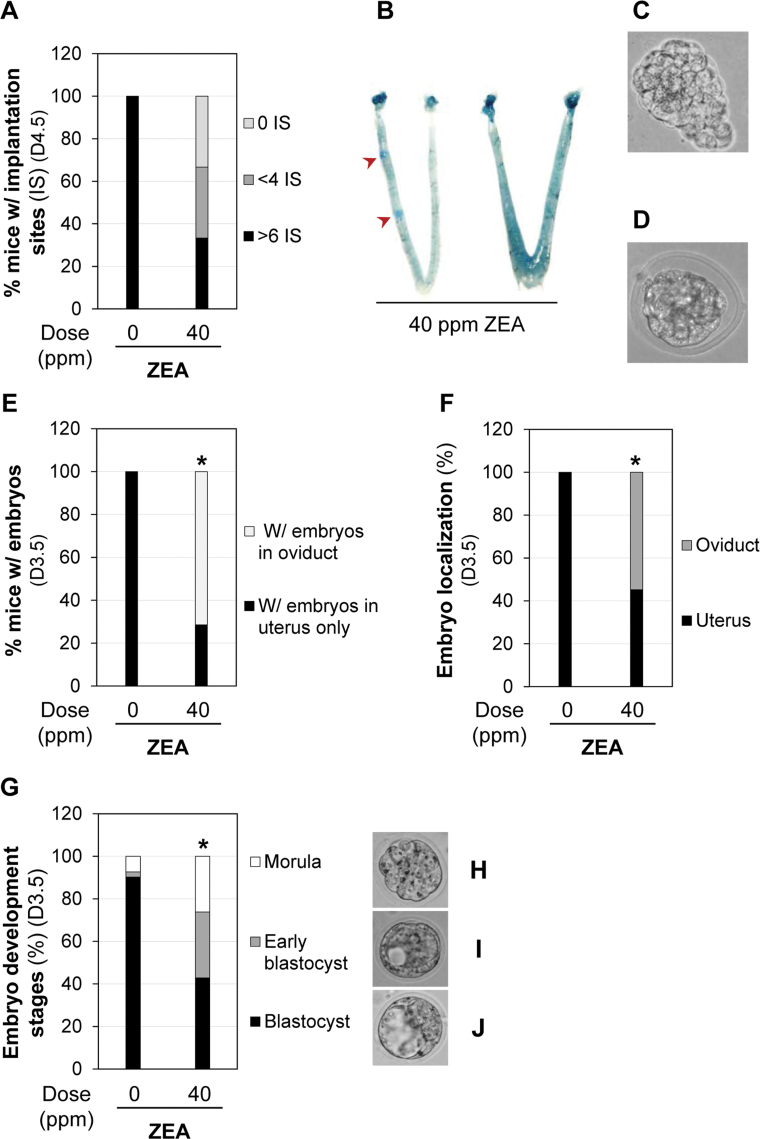
Postmating Exposure to 40 ppm ZEA Delayed Embryo Transport and Development
Postmating exposure (D0.5–D4.5) to 40 ppm ZEA diet did not affect the pregnancy rate (9/10 vs. 9/12 in the control; p = 0.59) detected on D4.5. This was expected because postmating exposure did not affect ovulation and fertilization, both of which normally occur during the dark cycle before 0500h of the mating night in mice (Nagy et al., 2003), before ZEA exposure. All nine pregnant mice in the control group had more than six implantation sites (Figs. 3C and 5A). Among the nine pregnant mice in the 40 ppm ZEA-treated group, three had more than six implantation sites; three had less than four implantation sites (Fig. 5B), with some showing faint blue bands, an indication of delayed implantation (Diao et al., 2011b); and three had no implantation sites, indicating that postmating exposure to 40 ppm ZEA diet also adversely affected embryo implantation. In the three mice without implantation sites, 11 embryos were flushed from the oviducts; in the three mice with < four implantation sites, 5 embryos were flushed from the oviducts of two mice, and the reproductive tract of one mouse was not flushed at the time of dissection. Among these 16 embryos recovered from the oviducts, 7 appeared normal D4.5 embryos without zona pellucida (Fig. 5C), but the other 9 embryos were underdeveloped and still surrounded by the zona pellucida (Fig. 5D), suggesting that embryo transport and embryo development were also affected by postmating exposure to 40 ppm ZEA diet.
Fig. 5.
Effects of postmating exposure to 40 ppm ZEA on embryo implantation, embryo transport, and embryo development. (A) Percentage of pregnant mice with different numbers of implantation sites (IS). N = 9 for both 0 and 40 ppm ZEA-treated groups. (B) Two representative D4.5 uteri of pregnant mice in 40 ppm ZEA-treated group showing delayed implantation and no implantation, respectively. Red arrowheads, faint blue bands indicating delayed implantation compared with control in Figure 3C. (C) A representative image of a normal-looking embryo recovered from a D4.5 oviduct exposed to 40 ppm ZEA. (D) A representative image of an underdeveloped embryo with retained zona pellucida recovered from a D4.5 oviduct exposed to 40 ppm ZEA. (E) Percentage of pregnant females with embryos localized in the oviduct or in the uterus only. N = 7 for both 0 and 40 ppm ZEA-treated groups. *p = 0.021. (F) Percentage of embryos in the uterus or the oviduct. *p < 0.0001. (G) Percentage of embryos in morula stage, early blastocyst, and blastocyst stages. *p < 0.0001. (F and G) N = 41 from seven mice in 0 ppm ZEA-treated group; N = 42 from seven mice in 40 ppm ZEA-treated group. (H, I, and J) Representative images of embryos at morula (H), early blastocyst (I), and blastocyst (J) stages from 40 ppm ZEA-treated group. Figure available in color online.
To further demonstrate the effects of postmating exposure to ZEA on embryo transport and preimplantation embryo development, the location and morphology of embryos were examined in the D3.5 reproductive tract. There was no significant difference in the pregnancy rate (based on the presence of embryos) between the control group (7/8) and 40 ppm ZEA-treated group (7/11, p = 0.34) and the number of embryos per pregnant mouse (41/7 = 5.9 vs. 42/7 = 6.0). All the embryos from the control group were flushed from the uterus. Among the seven pregnant mice in the 40 ppm ZEA-treated group, three had embryos in the oviduct only, two had embryos in both oviduct and uterus, and the remaining two had embryos in the uterus only. The percentage of pregnant mice with embryos in the oviduct was significantly increased in the 40 ppm ZEA-treated group (p = 0.021) (Fig. 5E). Among all flushed embryos in this group, 54.8% were from the oviduct (p < 0.0001) (Fig. 5F), indicating delayed embryo transport upon postmating 40 ppm ZEA treatment.
The stage of development was also examined for embryos flushed from uteri and oviducts on D3.5 (Fig. 5G). Among the 41 embryos recovered in the control group, 3 were at the morula stage (7.3%) (Fig. 5H), 1 was an early blastocyst (2.4%) (Fig. 5I), and 37 were expanded blastocysts (90.2%) (Fig. 5J). Among the 42 embryos recovered from the 40 ppm ZEA-treated group, 11 were at the morula stage (26.2%) (Fig. 5H), 9 were early blastocysts (30.9%) (Fig. 5I), and 18 were expanded blastocysts (42.9%) (Fig. 5J). These ratios were significantly different from those in the control (p < 0.0001; 2×3 Fisher’s exact test). The percentage of early blastocyst and morula was significantly higher in the 40 ppm ZEA-treated group than that in the control (57.1 vs. 9.7%, p < 0.0001), indicating delayed embryo development. These data demonstrated that postmating exposure to 40 ppm ZEA diet delayed embryo transport and embryo development, subsequently affecting embryo implantation (Figs. 5A and B).
DISCUSSION
Postweaning exposure to ZEA accelerates VO (Fig. 2A). VO is an early sign of puberty that can be affected by endocrine disruptors in rodents (Ojeda et al., 1980; Rollerova et al., 2011; Stoker et al., 2004, 2010; Walters et al., 1993). ZEA is an endocrine disruptor with estrogenic effects (Frizzell et al., 2011; Kuiper et al., 1998; Le Guevel and Pakdel, 2001; Yamasaki et al., 2002). Late gestational exposure (D15–D18) to ZEA (0.5 or 10mg/kg/day, sc injection) accelerated VO in CD-1 mouse offspring for more than 1 day (Nikaido et al., 2004). Our study indicates that postweaning exposure to 40 ppm ZEA diet for an average of 3–4 days can accelerate VO age by an average of about 8 days (Fig. 2A). Different exposure periods, doses, exposure routes, and mouse strains could all potentially contribute to the varied effects seen in the above two studies. VO is under neuroendocrine control (Hamm et al., 2004; Ojeda et al., 1980). However, the molecular mechanisms for VO and the effects of endocrine disruptors, e.g., ZEA, on VO, are still largely unknown.
Postweaning exposure to ZEA also disrupts estrous cyclicity (Figs. 2D–F). Many endocrine disruptors can affect estrous cyclicity, such as bisphenol A (Monje et al., 2010), chlorotriazine simazine (Zorrilla et al., 2010), estradiol valerate (Seidman et al., 2009), and genistein (Jefferson et al., 2007). Although it has not been investigated about the molecular mechanism of how the ZEA treatment regimen in this study affects estrous cyclicity, based on the literature, it is possible that ZEA and its derivatives may affect estrous cyclicity via hypothalamus-pituitary-ovarian axis. ZEA could alter the concentration of neuronal progestin receptors, which are neuroendocrine integrators (Levine et al., 2001), in ventromedial hypothalamus (Turcotte et al., 2005). Neonatal exposure to ZEA early in development alters postpubertal pituitary response to gonadotropin-releasing hormone in rats (Faber and Hughes, 1991). Zeranol (α-zearalanol), a synthetic derivative of ZEA and a growth promoter in livestock production (Leffers et al., 2001), can increase pituitary volume in Suffolk wethers (Carroll et al., 2007). ZEA could also alter levels of gonadotropins and sex steroids (Collins et al., 2006).
Postweaning exposure to ZEA could also disrupt embryo implantation. Postweaning ZEA exposure seemed to yield two sets of data on embryo implantation: those with plugs detected in the first morning after cohabitation appeared to mimic the premating data from overnight mating, and those with plugs detected after the third morning had phenotypes more similar to those from postmating exposure. The late set of data suggests recovery from premating exposure during extended mating interval. Premating and postmating exposure regimens helped dissect the contributing factors, including reduced fertilization, delayed embryo transport, and delayed preimplantation embryo development, for the disrupted embryo implantation observed upon postweaning exposure to 40 ppm ZEA diet.
Premating exposure to 40 ppm ZEA diet significantly reduces the percentage of plugged mice with implantation sites if the mice are mated within 3 days of cohabitation (Fig. 4A). A reduced fertilization rate (Fig. 4D) could be a main mechanism. Fertilization involves both oocytes and sperms. Our pilot experiment indicated that untreated females mated with males treated with 40 ppm ZEA diet for 3 weeks produced comparable litter size as those untreated females mated with untreated males (data not shown). This result suggests that the sperms, which were only exposed to residual ZEA and its metabolites in the ZEA-treated female reproductive tract between mating and fertilization, is not a significant contributing factor for the reduced fertilization rate. This observation led us to speculate that the oocyte quality and/or the oviductal environment might be affected by 40 ppm dietary ZEA treatment. Studies have shown that over half of primordial follicles formed by postnatal day 6 are eliminated by the time of puberty (Tingen et al., 2009). The accelerated onset of puberty by ~8 days in 40 ppm ZEA-treated group (Fig. 2A) may disrupt this normal follicle elimination process and lead to the development and ovulation of immature or poor quality oocytes that were not competent to be fertilized (Figs. 4D and E). Alternatively, ZEA may directly affect oocyte quality. Reports indicate that ZEA and its derivatives can inhibit oocyte maturation and induce chromatin abnormalities in cultured oocytes (Malekinejad et al., 2007; Minervini et al., 2001). Because fertilization occurs in the oviduct, it is also possible that the estrogenic ZEA may alter the oviductal environment that is less conducible for fertilization.
Postmating exposure to 40 ppm ZEA diet delays embryo transport (Figs. 5E and F). This adverse effect of ZEA on embryo transport in the oviduct is most likely attributed to its estrogenicity. It has been documented that estrogen and estrogenic chemicals can delay oviductal oocyte or embryo transport in cows (Wijayagunawardane et al., 1998), rabbits (El-Banna and Sacher, 1977), mice, guinea pigs, and hamsters (Greenwald, 1967; Xiao et al., 2011), but accelerate oviductal oocyte or embryo transport in rats (Akira et al., 1993; Greenwald, 1967; Ortiz et al., 1979). The molecular mechanism of estrogen and estrogenic compounds on oviductal transport is largely unknown.
Postmating exposure to 40 ppm ZEA diet also delays preimplantation embryo development (Fig. 5G). Delayed embryo development seemed to be associated with delayed embryo transport. Correlation analysis showed that the embryos retained in the oviduct were less developed than those in the uterus of 40 ppm ZEA-treated mice (p = 0.0003). This seeming correlation may reflect the varied sensitivity of individual mice to ZEA treatment, e.g., both embryo transport and embryo development are more affected in mice that are potentially more sensitive to ZEA exposure. Because estrogen can influence the expression of oviductal glycoprotein(s) that could interact with the gametes and early embryo and could affect litter size (Bhatt et al., 2004; Niu et al., 2006), it is possible that the estrogenic ZEA may affect preimplantation development via altered oviductal environment.
Our data appeared to show that premating ZEA exposure accelerated early embryo development (Fig. 4E), whereas postmating ZEA exposure delayed preimplantation embryo development (Fig. 5G). Here is one possible explanation: Premating ZEA exposure to 40 ppm ZEA diet increased duration of estrous and disrupted estrous cyclicity (Figs. 2D–F), thus influenced the mating time. Because the females in the premating study (Fig. 4E) were put with stud males between 1100h and 1700h and removed the next morning, it was possible that the ZEA-treated females were plugged much earlier than the control females, which normally mated during the night. We did one quick pilot experiment to support this hypothesis. We treated 24 adult female mice (2–4 months old) with 40 ppm ZEA diet for ~2 weeks. The females were cohabitated with fertile males ~1100h, and a vaginal plug was detected in nine females within 2.5h. Seven of these nine plugged females were dissected on D1.5 (real D2.0); three females had four- to eight-cell stage embryos, and the other four females did not have embryos in the oviduct. This pilot experiment supports that dys-synchronized estrous cycle caused by ZEA treatment could lead to early mating time. Subsequently, ovulation, fertilization, and early embryonic cell division occurred earlier and led to seemingly accelerated embryo development (Fig. 4E).
In summary, peripubertal and early pregnancy are two sensitive periods that can be influenced by ZEA exposure, which affects not only puberty and estrous cyclicity but also early pregnancy events, including fertilization, embryo development, embryo transport, and embryo implantation.
FUNDING
National Institutes of Health (R15HD066301, R01HD 065939 to X.Y.).
ACKNOWLEDGMENTS
The authors thank Dr Zhen Fu at the College of Veterinary Medicine, University of Georgia (UGA) for the access to the imaging system; Ms Kali King and Ms Allison Ellsworth at UGA for proofreading the manuscript; Mr Zhoumeng Lin at UGA for suggestions on statistical analyses; the Office of the Vice President for Research, the Graduate School, Interdisciplinary Toxicology Program, and Department of Physiology and Pharmacology at UGA.
REFERENCES
- Akira S., Sanbuissho A., Lin Y. C., Araki T. (1993). Acceleration of embryo transport in superovulated adult rats. Life Sci. 53, 1243–1251 [DOI] [PubMed] [Google Scholar]
- Baczkowski T., Kurzawa R., Głabowski W. (2004). Methods of embryo scoring in in vitro fertilization. Reprod. Biol. 4, 5–22 [PubMed] [Google Scholar]
- Becci P. J., Johnson W. D., Hess F. G., Gallo M. A., Parent R. A., Taylor J. M. (1982). Combined two-generation reproduction-teratogenesis study of zearalenone in the rat. J. Appl. Toxicol. 2, 201–206 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Kadam K., Saxena A., Natraj U. (2004). Fertilization, embryonic development and oviductal environment: Role of estrogen induced oviductal glycoprotein. Indian J. Exp. Biol. 42, 1043–1055 [PubMed] [Google Scholar]
- Bravin F., Duca R. C., Balaguer P., Delaforge M. (2009). In vitro cytochrome p450 formation of a mono-hydroxylated metabolite of zearalenone exhibiting estrogenic activities: Possible occurrence of this metabolite in vivo. Int. J. Mol. Sci. 10, 1824–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C. S. (2009). Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. A., Walker M. A., Hartsfield S. M., McArthur N. H., Welsh T. H., Jr (2007). Visual documentation of ovine pituitary gland development with magnetic resonance imaging following zeranol treatment. Lab. Anim. 41, 120–127 [DOI] [PubMed] [Google Scholar]
- Collins T. F., Sprando R. L., Black T. N., Olejnik N., Eppley R. M., Alam H. Z., Rorie J., Ruggles D. I. (2006). Effects of zearalenone on in utero development in rats. Food Chem. Toxicol. 44, 1455–1465 [DOI] [PubMed] [Google Scholar]
- Deng F., Tao F. B., Liu D. Y., Xu Y. Y., Hao J. H., Sun Y., Su P. Y. (2012). Effects of growth environments and two environmental endocrine disruptors on children with idiopathic precocious puberty. Eur. J. Endocrinol. 166, 803–809 [DOI] [PubMed] [Google Scholar]
- Diao H., Aplin J. D., Xiao S., Chun J., Li Z., Chen S., Ye X. (2011a). Altered spatiotemporal expression of collagen types I, III, IV, and VI in Lpar3-deficient peri-implantation mouse uterus. Biol. Reprod. 84, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Paria B. C., Xiao S., Ye X. (2011b). Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil. Steril. 95, 2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2011). Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 9, 2197 [Google Scholar]
- El-Banna A. A., Sacher B. (1977). A study on steroid hormone receptors in the rabbit oviduct and uterus during the first few days after coitus and during egg transport. Biol. Reprod. 17, 1–8 [DOI] [PubMed] [Google Scholar]
- Faber K. A., Hughes C. L., Jr (1991). The effect of neonatal exposure to diethylstilbestrol, genistein, and zearalenone on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Biol. Reprod. 45, 649–653 [DOI] [PubMed] [Google Scholar]
- Frizzell C., Ndossi D., Verhaegen S., Dahl E., Eriksen G., Sørlie M., Ropstad E., Muller M., Elliott C. T., Connolly L. (2011). Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 206, 210–217 [DOI] [PubMed] [Google Scholar]
- Gajecki M., Przybyłowicz M., Zielonka L., Zwierzchowski W., Obremski K., Skorska-Wyszyńska E., Gajecka M., Polak M., Jakimiuk E. (2004). Preliminary results of monitoring research on zearalenone presence in blood of women with neoplastic lesions in reproductive system. Pol. J. Vet. Sci. 7, 153–156 [PubMed] [Google Scholar]
- Gajęcka M., Rybarczyk L., Jakimiuk E., Zielonka Ł., Obremski K., Zwierzchowski W., Gajęcki M. (2012). The effect of experimental long-term exposure to low-dose zearalenone on uterine histology in sexually immature gilts. Exp. Toxicol. Pathol. 64, 537–542 [DOI] [PubMed] [Google Scholar]
- Goldman J. M., Murr A. S., Cooper R. L. (2007). The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B. Dev. Reprod. Toxicol. 80, 84–97 [DOI] [PubMed] [Google Scholar]
- Greenwald G. S. (1967). Species differences in egg transport in response to exogenous estrogen. Anat. Rec. 157, 163–172 [DOI] [PubMed] [Google Scholar]
- Gromadzka K., Waśkiewicz A., Goliński P., Swietlik J. (2009). Occurrence of estrogenic mycotoxin - Zearalenone in aqueous environmental samples with various NOM content. Water Res. 43, 1051–1059 [DOI] [PubMed] [Google Scholar]
- Hamm M. L., Bhat G. K., Thompson W. E., Mann D. R. (2004). Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol. Reprod. 71, 66–72 [DOI] [PubMed] [Google Scholar]
- Ito Y., Ohtsubo K. (1994). Effects of neonatal administration of zearalenone on the reproductive physiology of female mice. J. Vet. Med. Sci. 56, 1155–1159 [DOI] [PubMed] [Google Scholar]
- Jakimiuk E., Gajecka M., Jana B., Brzuzan P., Zielonka Ł., Skorska-Wyszyńska E., Gajecki M. (2009). Factors determining sensitivity of prepubertal gilts to hormonal influence of zearalenone. Pol. J. Vet. Sci. 12, 149–158 [PubMed] [Google Scholar]
- Jefferson W. N., Padilla-Banks E., Newbold R. R. (2007). Disruption of the female reproductive system by the phytoestrogen genistein. Reprod. Toxicol. 23, 308–316 [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T., van der Burg B., Gustafsson J. A. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139, 4252–4263 [DOI] [PubMed] [Google Scholar]
- Kuiper-Goodman T., Scott P. M., Watanabe H. (1987). Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 7, 253–306 [DOI] [PubMed] [Google Scholar]
- Kumagai S., Shimizu T. (1982). Neonatal exposure to zearalenone causes persistent anovulatory estrus in the rat. Arch. Toxicol. 50, 279–286 [DOI] [PubMed] [Google Scholar]
- Lamb J. C., Jameson C. W., Choudhury H., Gulati D. K. (1985). Fertility assessment by continuous breeding - Evaluation of diethylstilbestrol and a comparison of results from 2 laboratories. J. Am. Coll. Toxicol. 4, 173–184 [Google Scholar]
- Le Guevel R., Pakdel F. (2001). Assessment of oestrogenic potency of chemicals used as growth promoter by in-vitro methods. Hum. Reprod. 16, 1030–1036 [DOI] [PubMed] [Google Scholar]
- Leffers H., Naseby M., Vendelbo B., Skakkebaek N. E., Jørgensen M. (2001). Oestrogenic potencies of zeranol, oestradiol, diethylstilboestrol, bisphenol-A and genistein: Implications for exposure assessment of potential endocrine disrupters. Hum. Reprod. 16, 1037–1045 [DOI] [PubMed] [Google Scholar]
- Levine J. E., Chappell P. E., Schneider J. S., Sleiter N. C., Szabo M. (2001). Progesterone receptors as neuroendocrine integrators. Front. Neuroendocrinol. 22, 69–106 [DOI] [PubMed] [Google Scholar]
- Long G. G., Diekman M. A. (1984). Effect of purified zearalenone on early gestation in gilts. J. Anim. Sci. 59, 1662–1670 [DOI] [PubMed] [Google Scholar]
- Long G. G., Turek J., Diekman M. A., Scheidt A. B. (1992). Effect of zearalenone on days 7 to 10 post-mating on blastocyst development and endometrial morphology in sows. Vet. Pathol. 29, 60–67 [DOI] [PubMed] [Google Scholar]
- Malekinejad H., Maas-Bakker R., Fink-Gremmels J. (2006). Species differences in the hepatic biotransformation of zearalenone. Vet. J. 172, 96–102 [DOI] [PubMed] [Google Scholar]
- Malekinejad H., Schoevers E. J., Daemen I. J., Zijlstra C., Colenbrander B., Fink-Gremmels J., Roelen B. A. (2007). Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol. Reprod. 77, 840–847 [DOI] [PubMed] [Google Scholar]
- Massart F., Saggese G. (2010). Oestrogenic mycotoxin exposures and precocious pubertal development. Int. J. Androl. 33, 369–376 [DOI] [PubMed] [Google Scholar]
- Minervini F., Dell’Aquila M. E., Maritato F., Minoia P., Visconti A. (2001). Toxic effects of the mycotoxin zearalenone and its derivatives on in vitro maturation of bovine oocytes and 17 beta-estradiol levels in mural granulosa cell cultures. Toxicol. In Vitro 15, 489–495 [DOI] [PubMed] [Google Scholar]
- Mirocha C. J., Pathre S. V., Robison T. S. (1981). Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet. Toxicol. 19, 25–30 [DOI] [PubMed] [Google Scholar]
- Monje L., Varayoud J., Muñoz-de-Toro M., Luque E. H., Ramos J. G. (2010). Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor alpha expression in nuclei controlling estrous cyclicity. Reprod. Toxicol. 30, 625–634 [DOI] [PubMed] [Google Scholar]
- Nagy A., Gertsenstein M., Vintersten K., Behringer R. (2003). Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed. 48–55 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [Google Scholar]
- Nikaido Y., Danbara N., Tsujita-Kyutoku M., Yuri T., Uehara N., Tsubura A. (2005). Effects of prepubertal exposure to xenoestrogen on development of estrogen target organs in female CD-1 mice. In Vivo 19, 487–494 [PubMed] [Google Scholar]
- Nikaido Y., Yoshizawa K., Danbara N., Tsujita-Kyutoku M., Yuri T., Uehara N., Tsubura A. (2004). Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod. Toxicol. 18, 803–811 [DOI] [PubMed] [Google Scholar]
- Niu B. Y., Xiong Y. Z., Li F. E., Jiang S. W., Deng C. Y., Ding S. H., Guo W. H., Lei M. G., Zheng R., Zuo B., et al. (2006). Oviduct-specific glycoprotein 1 locus is associated with litter size and weight of ovaries in pigs. Asian Australas. J. Anim. Sci. 19, 632–637 [Google Scholar]
- Ojeda S. R., Advis J. P., Andrews W. W. (1980). Neuroendocrine control of the onset of puberty in the rat. Fed. Proc. 39, 2365–2371 [PubMed] [Google Scholar]
- Ortiz M. E., Villalón M., Croxatto H. B. (1979). Ovum transport and fertility following postovulatory treatment with estradiol in rats. Biol. Reprod. 21, 1163–1167 [DOI] [PubMed] [Google Scholar]
- Pfeiffer E., Hildebrand A., Mikula H., Metzler M. (2010). Glucuronidation of zearalenone, zeranol and four metabolites in vitro: Formation of glucuronides by various microsomes and human UDP-glucuronosyltransferase isoforms. Mol. Nutr. Food Res. 54, 1468–1476 [DOI] [PubMed] [Google Scholar]
- Prelusky D. B., Scott P. M., Trenholm H. L., Lawrence G. A. (1990). Minimal transmission of zearalenone to milk of dairy cows. J. Environ. Sci. Health. B. 25, 87–103 [DOI] [PubMed] [Google Scholar]
- Price W. D., Lovell R. A., McChesney D. G. (1993). Naturally occurring toxins in feedstuffs: Center for Veterinary Medicine Perspective. J. Anim. Sci. 71, 2556–2562 [DOI] [PubMed] [Google Scholar]
- Rollerova E., Wsolova L., Urbancikova M. (2011). Neonatal exposure to herbicide acetochlor alters pubertal development in female wistar rats. Toxicol. Mech. Methods 21, 406–417 [DOI] [PubMed] [Google Scholar]
- Safranski T. J., Lamberson W. R., Keisler D. H. (1993). Correlations among three measures of puberty in mice and relationships with estradiol concentration and ovulation. Biol. Reprod. 48, 669–673 [DOI] [PubMed] [Google Scholar]
- Sangare-Tigori B., Moukha S., Kouadio H. J., Betbeder A. M., Dano D. S., Creppy E. E. (2006). Co-occurrence of aflatoxin B1, fumonisin B1, ochratoxin A and zearalenone in cereals and peanuts from Côte d’Ivoire. Food Addit. Contam. 23, 1000–1007 [DOI] [PubMed] [Google Scholar]
- Seidman D. S., Itsekson A., Alesker M., Zolti M., Carp H., Wolman I. (2009). Estradiol valerate as a possible endocrine reproductive disruptor: Evidence from an in vivo rat model. Fertil. Steril. 91 (4 Suppl), 1510–1512 [DOI] [PubMed] [Google Scholar]
- Smith J. F., di Menna M. E., McGowan L. T. (1990). Reproductive performance of Coopworth ewes following oral doses of zearalenone before and after mating. J. Reprod. Fertil. 89, 99–106 [DOI] [PubMed] [Google Scholar]
- Stoker T. E., Gibson E. K., Zorrilla L. M. (2010). Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol. Sci. 117, 45–53 [DOI] [PubMed] [Google Scholar]
- Stoker T. E., Laws S. C., Crofton K. M., Hedge J. M., Ferrell J. M., Cooper R. L. (2004). Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol. Sci. 78, 144–155 [DOI] [PubMed] [Google Scholar]
- Tingen C. M., Bristol-Gould S. K., Kiesewetter S. E., Wellington J. T., Shea L., Woodruff T. K. (2009). Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol. Reprod. 81, 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte J. C., Hunt P. J., Blaustein J. D. (2005). Estrogenic effects of zearalenone on the expression of progestin receptors and sexual behavior in female rats. Horm. Behav. 47, 178–184 [DOI] [PubMed] [Google Scholar]
- Ueno Y., Tashiro F. (1981). alpha-Zearalenol, a major hepatic metabolite in rats of zearalenone, an estrogenic mycotoxin of Fusarium species. J. Biochem. 89, 563–571 [DOI] [PubMed] [Google Scholar]
- Viveiros M. M., O’Brien M., Wigglesworth K., Eppig J. J. (2003). Characterization of protein kinase C-delta in mouse oocytes throughout meiotic maturation and following egg activation. Biol. Reprod. 69, 1494–1499 [DOI] [PubMed] [Google Scholar]
- Walters L. M., Rourke A. W., Eroschenko V. P. (1993). Purified methoxychlor stimulates the reproductive tract in immature female mice. Reprod. Toxicol. 7, 599–606 [DOI] [PubMed] [Google Scholar]
- Wijayagunawardane M. P., Miyamoto A., Cerbito W. A., Acosta T. J., Takagi M., Sato K. (1998). Local distributions of oviductal estradiol, progesterone, prostaglandins, oxytocin and endothelin-1 in the cyclic cow. Theriogenology 49, 607–618 [DOI] [PubMed] [Google Scholar]
- Xiao S., Diao H., Smith M. A., Song X., Ye X. (2011). Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 32, 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K., Takeyoshi M., Yakabe Y., Sawaki M., Imatanaka N., Takatsuki M. (2002). Comparison of reporter gene assay and immature rat uterotrophic assay of twenty-three chemicals. Toxicology 170, 21–30 [DOI] [PubMed] [Google Scholar]
- Ye X., Hama K., Contos J. J., Anliker B., Inoue A., Skinner M. K., Suzuki H., Amano T., Kennedy G., Arai H., et al. (2005). LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinedine A., Soriano J. M., Moltó J. C., Mañes J. (2007). Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 45, 1–18 [DOI] [PubMed] [Google Scholar]
- Zorrilla L. M., Gibson E. K., Stoker T. E. (2010). The effects of simazine, a chlorotriazine herbicide, on pubertal development in the female Wistar rat. Reprod. Toxicol. 29, 393–400 [DOI] [PubMed] [Google Scholar]
- Zwierzchowski W., Przybyłowicz M., Obremski K., Zielonka L., Skorska-Wyszyńska E., Gajecka M., Polak M., Jakimiuk E., Jana B., Rybarczyk L., et al. (2005). Level of zearalenone in blood serum and lesions in ovarian follicles of sexually immature gilts in the course of zearalenone micotoxicosis. Pol. J. Vet. Sci. 8, 209–218 [PubMed] [Google Scholar]