Abstract
The neuropeptide neurotensin (NT) is closely associated with dopaminergic and glutamatergic systems in the rat brain. Central injection of NT into the nucleus accumbens (NAcc) or peripheral administration of NT receptor agonists, reduces many of the behavioral effects of psychostimulants. However, the role of endogenous NT in the behavioral effects of psychostimulants (e.g. DA agonists and NMDA receptor antagonists) remains unclear. Using a NTR antagonist, SR142948A, the current studies were designed to examine the role of endogenous NT in DA receptor agonist- and NMDA receptor antagonist-induced disruption of prepulse inhibition of the acoustic startle response (PPI), locomotor hyperactivity and brain-region specific c-fos mRNA expression. Adult male rats received a single i.p. injection of SR142948A or vehicle followed by d-amphetamine, apomorphine or dizocilpine challenge. SR142948A had no effect on baseline PPI, but dose-dependently attenuated D-amphetamine- and dizocilpine-induced PPI disruption and enhanced apomorphine-induced PPI disruption. SR142948A did not significantly affect either baseline locomotor activity or stimulant-induced hyperlocomotion. Systemic SR142948A administration prevented c-fos mRNA induction in mesolimbic terminal fields (prefrontal cortex, lateral septum, NAcc, ventral subiculum) induced by all three psychostimulants implicating the VTA as the site for NT modulation of stimulant-induced PPI disruption. Further characterization of the NT system may be valuable to find clinical useful compounds for schizophrenia and drug addiction.
Keywords: Prepulse inhibition, Locomotion, d-amphetamine, Apomorphine, Dizocilpine, c-fos, Neurotensin
1. Introduction
Neurotensin (NT) is a tridecapeptide isolated by Carraway and Leeman in 1973 (Carraway and Leeman, 1973). The NT system is closely associated with brain dopamine (DA) systems (for review see (Binder et al., 2001b)) and implicated in the mechanisms of reward and addiction as well as the pathophysiology of schizophrenia and mechanism of action of antipsychotic drugs (APDs) (Cáceda et al., 2006). Peripherally administered NT receptor (NTR) agonists have consistently been shown to decrease the behavioral effects of psychostimulant compounds (Feifel et al., 1999; Boules et al., 2001; Shilling et al., 2003; Boules et al., 2007, 2010; Li et al., 2010). These findings are hypothesized to be caused by antidopaminergic effects of NT in the nucleus accumbens (NAcc) because direct injection of NT into the nucleus accumbens (NAcc) (Ervin et al., 1981; Kalivas et al., 1984; Steinberg et al., 1994; Feifel et al., 1997), but not the ventral tegmental area (VTA) or prefrontal cortex (PFC) (Kalivas et al., 1983; Feifel and Reza, 1999b), mimics the behavioral effects of peripherally administered NTR agonists.
In addition, release of endogenous NT is necessary for some of the behavioral effects of psychostimulant drugs. In transgenic mice lacking the NT gene, amphetamine-induced hyperlocomotion is not altered but amphetamine-induced-disruption of prepulse inhibition of the startle response (PPI) is markedly disrupted (Kinkead et al., 2005). These effects do not appear to be mediated via an action of NT at the NTR1 because the effects of d-amphetamine and dizocilpine (MK801) on PPI do not differ between NTR1 knockout mice and wildtype controls (Feifel et al., 2010). In addition, administration of the small molecule NTR antagonists, while having no effect on appetitive or aversive conditioning (Grimond-Billa et al., 2008; Norman et al., 2010) or the acute locomotor effects of psychostimulants (Poncelet et al., 1994; Gully et al., 1995; Casti et al., 2004; Panayi et al., 2005), but see (Wagstaff et al., 1994; Betancur et al., 1998; Marie-Claire et al., 2008), blocks the development of behavioral sensitization (Costa et al., 2001; Casti et al., 2004; Panayi et al., 2005; Costa et al., 2007), and apomorphine-induced turning and yawning (Poncelet et al., 1994; Gully et al., 1995).
Reduction of behavioral response to stimulants is a well known model for APD action. The current studies were designed to scrutinize the role of endogenous NT in the acute behavioral effects of three psychostimulants: the indirect DA agonist d-amphetamine, the direct DA agonist apomorphine and the glutamatergic antagonist dizocilpine. All three compounds induce locomotor hyperactivity and disrupt PPI, effects that are antagonized by atypical APDs. Indirect DA agonists such as methamphetamine and d-amphetamine increase NT-like immunoreactivity (Letter et al., 1987; Merchant et al., 1988) and mRNA expression (Merchant et al., 1994) in dorsal and ventral striatum, most likely via the effects of DA at D1 receptors (Merchant et al., 1988). Similarly, extracellular NT release in the prefrontal cortex (PFC) is increased by DA receptor agonists (Bean et al., 1990; During et al., 1992). In contrast, antagonism of NMDA receptors with dizocilpine has no effect on NT mRNA expression (Hanson et al., 1995) but completely prevents the increased NT release induced by D1-stimulation (Wagstaff et al., 1997).
c-fos is an immediate early gene frequently used as a marker for neuronal activity. Increased c-fos products have been reported in DA mesolimbic regions (PFC, cingulate, lateral septum (LS), NAcc, thalamus, subiculum and VTA), known to be involved in regulation of sensorimotor gating and locomotion, after stimulant administration: d-amphetamine (Dalia and Wallace, 1995; Jaber et al., 1995; Wang et al., 1995; Asin et al., 1996; Vanderschuren and Kalivas, 2000; Wirtshafter, 2000; Uslaner et al., 2001; Miyamoto et al., 2004), apomorphine (Cole et al., 1992; Dilts et al., 1993; Paul et al., 1995) and dizocilpine (Dragunow and Faull, 1990; Gass et al., 1992, 1993; Nakki et al., 1996; Bozas et al., 1997; Panegyres and Hughes, 1997; Gao et al., 1998; Fujimura et al., 2000; Szakacs et al., 2003).
Based on previous knock out and NTR antagonists experiments we hypothesized that blockade of NT neurotransmission with the NTR antagonist SR142948A (Gully et al., 1997) will enhance psychostimulant-induced PPI disruption and expression of c-fos in the mesolimbic system, without modifying psychostimulant induced-hyperlocomotion.
2. Experimental procedures
2.1. Animals and housing
Adult male Sprague Dawley rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) were used for all anatomical and behavioral studies. All animals were housed in an environmentally controlled animal facility with food and water available ad libitum and a regular light cycle (lights on 7 am; lights off 7 pm). All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee (IACUC) in compliance with NIH (http://grants.nih.gov/grants/olaw/olaw.htm) recommendations based on National Research Council guidelines [NRC, Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, National Academies Press, Washington, DC, 2003].
2.2. Compounds
SR142948A (2-[[5-(2,6-dimethoxyphenyl)-1-(4-(N-(3-dimethylaminopropyl)-N-methylcarbamoyl)-2-isopropylphenyl)-1H-pyrazole3-carbonyl]amino] adamantane-2-carboxylic acid, hydrochloride, Sanofi Research, Toulouse, France) was microsuspended in 0.9% saline+ several drops of Tween 20 (Gully et al., 1997). Apomorphine, d-amphetamine sulfate and dizocilpine (all from Sigma, St. Louis, MO) were dissolved in 0.9% saline. All compounds were administered in a fixed volume of 1.0 ml/kg.
2.3. PPI assessment
PPI was performed between 11:00 and 16:00 h in a San Diego Instruments (San Diego, CA) startle chamber. Startle amplitude was measured by converting the vibrations of a Plexiglas cylinder (resting platform) caused by the rat startle response into analog signals by a piezoelectric unit. These signals were then digitized, represented as arbitrary startle units and stored in a personal computer. The testing session began with 5 min of acclimatization to the startle chamber in the presence of 65 dB background white noise. Testing consisted of nine 120 dB pulses alone and 18 pulses preceded (100 ms) by a prepulse of 4, 8, or 12 dB above background. Pulses were presented in a pseudorandom order with an average of 15 s between trials. Change in PPI was calculated using the formula:%PPI = 100–(startle amplitude with prepulse × 100/startle amplitude with pulse alone).
2.4. Effects of SR142948A on PPI
Adult male Sprague–Dawley rats (n=55) received single i.p. injections of SR142948A (0.01–1000 μg/kg) or vehicle 1 h before PPI testing. Each rat was tested individually 4 separate times with 6 to 14 days between testing. Doses of SR142948A were administered in a pseudorandom order with no rat receiving the same dose twice. Rat weights varied from 250 to 400 g from the first to the last test session.
The time course of the effects of SR142948A on PPI was examined at the 100 μg/kg dose. Adult male Sprague–Dawley rats (n=55; 200–250 g) received a single i.p. injection of SR142948A 1, 4, 8 or 12 h before PPI testing.
2.5. Effects of SR142948A on dopamine agonists and NMDA receptor antagonist-induced disruption of PPI of the acoustic startle reflex
Animals received single injections of either SR142948A (1.0 or 100 μg/kg, i.p.) or vehicle 1 h before testing (Binder et al., 2001a; Norman et al., 2010). Apomorphine (0.1 or 0.5 mg/kg, s.c.) or 0.9% saline was administered immediately before PPI testing. d-amphetamine sulfate (0.2, 2.0 or 4.0 mg/kg, i.p.) or 0.9% saline was administered 10 min before PPI testing. Dizocilpine (0.1 mg/kg s.c.) or 0.9% saline was administered 10 min before PPI testing. N=13–15 for each treatment group, except vehicle-saline N=6 and SR142948A-saline N = 7. All animals were tested only once.
2.6. Locomotor assessment
Animals received SR142948A (100 μg/kg, i.p.) or vehicle 1 h before behavioral testing. The one hour interval was chosen based on previous studies in our laboratory (Cáceda et al., 2005). Rats were divided into different groups (n = 7–12) and used to test the effects of d-amphetamine (2 mg/kg, i.p., 10 min before testing), apomorphine (0.5 mg/kg, s.c., immediately before testing) and dizocilpine (0.1 mg/kg, s.c., 10 min before testing) on locomotor behavior.
Activity measurements were evaluated by placing rats in an open field consisting of a clear Plexiglas box (40 × 40 × 40 cm) with a black floor in standard room light. Activity was recorded at 5-min intervals for 1 h and quantified by a computer-operated tracking system of 16 photo beams per side (TruScan System, Coulbourn Instruments, Allentown, PA). The distance traveled in each 5-minute interval was measured as the total of all vectored X–Y coordinate changes. In addition, data was collected for the number of entries in the vertical plane to reflect two-leg rearing.
2.7. c-fos mRNA in situ hybridization
Immediately after locomotor testing (2 h after SR142948A administration), animals were killed by decapitation and brains removed and stored at −80 °C. c-fos mRNA expression was assessed by in situ hybridization in limbic regions.
A template plasmid containing a Hind III/Sma I fragment of the rat c-fos gene provided kindly by Dr. Thomas Curran, University of Colorado, was linearized with Sma I and used to generate an antisense 35S labeled riboprobe with nucleotides, 35S-UTP, and T7 RNA polymerase (T7/T3 MAXIscript™, Ambion, Austin, TX). A sense 35S-labeled riboprobe was generated using T3 RNA polymerase and a Hind III linearized template. Unincorporated nucleotides were removed from the reactions using Quick Spin™ Columns (Roche). The 35S-labeled probes were then diluted to 1 × 106 cpm/100 μl in hybridization buffer (62.5% formamide, 12.5% dextran sulfate, 0.375 M NaCl, 2.5% Denhardt's solution, 12.5 mM Tris, 1.25 mM EDTA; ph 8.0) and stored at −20 °C until use. The protocol for in situ hybridization was adapted from Simmons et al. (1989). Briefly, slide mounted tissue (20 μm) was fixed in 4% paraformaldehyde for 5 min, then underwent a proteinase K digestion followed by acetylation in acetic anhydride to block positive charges in the tissue induced by proteinase K. The sections were rinsed in 2 × SSC buffer (NaCl/citrate) and quickly dehydrated in ascending ethanol concentrations. After drying at room temperature, 100 μl (1 × 106 cpm) of riboprobe mixture was added to each slide. The slides were then covered with parafilm and incubated overnight at 60 °C. The following day, the parafilm was removed and the slides were rinsed in 4 × SSC before RNAase digestion (1:500 dilution of 10 mg/ml RNAse A) to remove nonspecifically bound riboprobe. The slides were washed, gradually desalted, and incubated at 60 °C for 1 h in order to decrease the background signal. Slides were then rapidly dehydrated in ethanol (containing salt and DTT), drained, dried at room temperature and exposed to Biomax MR film (Kodak).
2.8. Film quantification
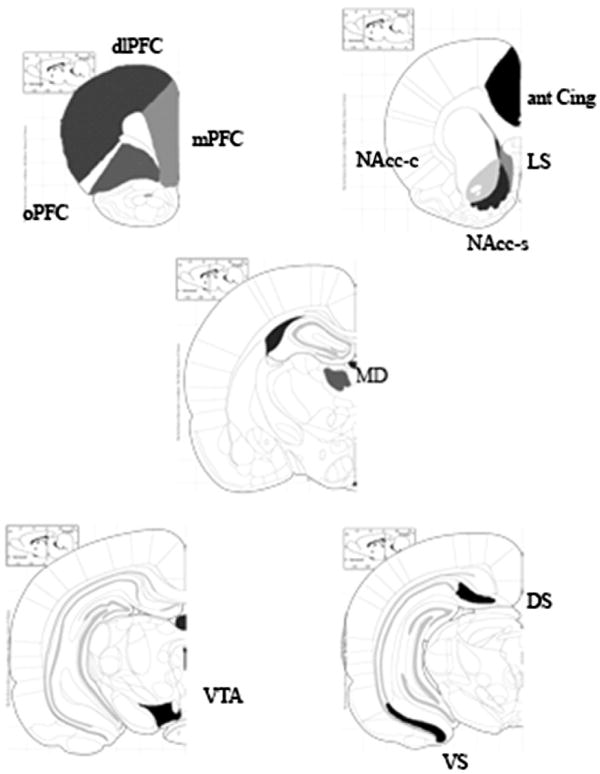
Quantitative autoradiography was performed by computerized densitometry with Analytical Imaging Station (AIS, Imaging Research Inc., Ontario, Canada). Optical densities were calibrated with coexposed 14C-standards revealing brain substance-like quench coefficients for iodinated isotopes (Amersham Biosciences, Piscataway, NJ) and converted to nCi/mg protein. Measurements were obtained bilaterally from PFC, cingulate, LS, NAcc, mediodorsal nucleus of the thalamus (MD), VTA and subiculum as shown in Fig. 1 (Paxinos and Watson, 1986).
Fig. 1.
Regions selected to measure the effects of systemic administration of SR142948A on c-fos mRNA expression induced by d-amphetamine, apomorphine and dizocilpine. (dlPFC: dorsolateral prefrontal cortex, mPFC: oPFC: orbitofrontal cortex, ant Cing: anterior cingulated, LS: lateral septum, NAcc-s: nucleus accumbens shell, NAcc-c: nucleus accumbens core, MD: mediodorsal thalamus, VTA: ventral tegmental area, VS: ventral subiculum, DS: dorsal subiculum) (Paxinos and Watson, 1986).
2.9. Data analysis
Full descriptive statistics were computed for each variable within each group. Two-way ANOVA (pretreatment dose × time) was used to analyze SR142948A time course and dose effects on PPI. General linear model (GLM) ANOVA was used to assess the effects of SR142948A pretreatment, drug (d-amphetamine, apomorphine and dizocilpine) and prepulse on PPI. Two way ANOVA was used to analyze SR142948A pretreatment and drug (d-amphetamine, apomorphine and dizocilpine) on locomotion, rearing and induction of c-fos mRNA expression. Post-hoc comparisons were calculated with Dunnett's test. Significance was set at p<0.05.
3. Results
3.1. Effects of SR142948A administration on PPI
There was no significant effect of SR142948A administration on pulse alone startle amplitude at any dose, or time point examined. The acute dose response effects (0.01 to 1000 μg/kg) of SR142948A on PPI were examined. GLM ANOVA demonstrated a significant effect of prepulse intensity on PPI (p<0.001). There were no significant treatment effects or significant interactions between prepulse intensity and treatment. Two-way ANOVA (prepulse intensity × time after injection) of SR142948A (100 μg/kg) revealed a significant prepulse intensity effect (p<0.001), but no significant effect of time.
3.2. SR142948A administration effect on PPI disruption by d-amphetamine
Only d-amphetamine at the highest dose (4 mg/kg) significantly increased pulse alone startle amplitude (p<0.001). SR142948A significantly reduced d-amphetamine-induced elevation of the startle amplitude (p<0.01). GLM ANOVA (pretreatment × d-amphetamine × -prepulse intensity) demonstrated a significant effect prepulse intensity (p<0.001).
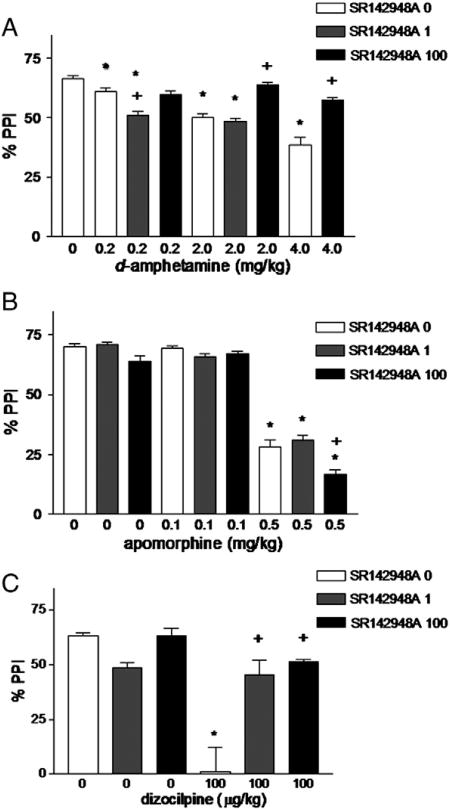
There were no significant interactions between prepulse intensity and any treatment, therefore data from all prepulse intensities were combined and the effects of the two drugs, i.e. SR142948A pretreatment and d-amphetamine, apomorphine and dizocilpine dose (see below), analyzed by two-way ANOVA (Fig. 2A). There was a significant effect of d-amphetamine (p<0.001) and SR142948A (p<0.001). d-amphetamine (2.0 and 4.0 mg/kg) significantly decreased PPI. SR142948A, at 1 μg/kg but not 100 μg/kg, further potentiated PPI reduction by the dose of d-amphetamine dose (0.2 mg/kg). SR142948A, at 100 μg/kg, prevented PPI reduction by the higher doses of d-amphetamine (2 and 4 mg/kg).
Fig. 2.
Effect of systemic administration of SR142948A on disruption of prepulse inhibition by d-amphetamine (A), apomorphine (B), and dizocilpine (C). *p<0.01 compared to control; +p<0.001 compared to same dose of d-amphetamine or apomorphine without SR142948A pretreatment; n=6–15 in each group.
3.3. SR142948A administration enhances PPI disruption by apomorphine
Two-way ANOVA (apomorphine dose × SR142948A dose) showed no significant effect of apomorphine or SR142948A on pulse alone startle amplitude. No apomorphine × SR142948A interaction was observed. GLM ANOVA (pretreatment×apomorphine dose × prepulse intensity) demonstrated a significant overall effect of prepulse intensity (p<0.0001). There was a significant effect of SR142948A (p<0.0001) and apomorphine dose (p<0.0001). Apomorphine (0.1 mg/kg) had no significant effect on PPI. Apomorphine (0.5 mg/kg) significantly disrupted PPI compared to control. Pretreatment with SR142948A (100 μg/kg) potentiated the disruptive effect of apomorphine (0.5 mg/kg). See Fig. 2B.
3.4. SR142948A administration antagonizes PPI disruption by dizocilpine
There were no significant effects of SR142948A, dizocilpine, or SR142948A × dizocilpine interaction on pulse alone startle amplitude. GLM ANOVA (pretreatment × dizocilpine dose × prepulse intensity) demonstrated a significant effect of prepulse intensity. There was a significant effect of SR142948A (p=0.002), dizocilpine dose (p<0.001). The PPI disruptive effect of dizocilpine was significantly decreased by pretreatment with SR142948A (1 and 100 μg/kg). See Fig. 2C.
3.5. SR142948A administration does not modify hyperlocomotion induced by d-amphetamine, dizocilpine or apomorphine
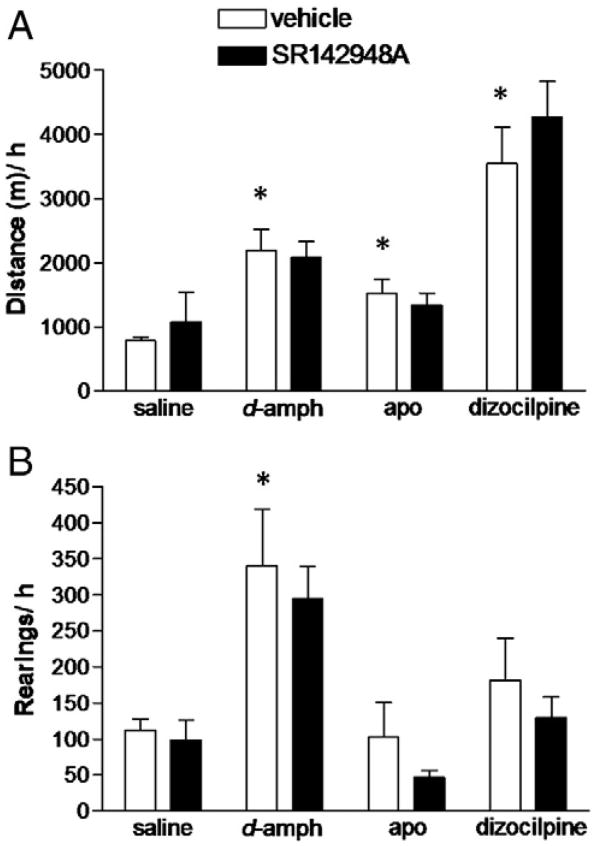
In a separate set of animals, the effect of pretreatment with SR142948A on d-amphetamine-, apomorphine- and dizocilpine-induced hyperlocomotion and rearing was tested (Fig. 3). Two-way ANOVA (pretreatment × drug) showed significant effect of drug (p<0.001) on locomotion. All three drugs (amphetamine, apomorphine and dizocilpine) increased locomotion. Post hoc analysis revealed that pretreatment with SR142948A did not significantly alter hyperlocomotion induced by d-amphetamine (2 mg/kg), apomorphine (0.5 mg/kg) or dizocilpine (0.1 mg/kg). Two-way ANOVA indicated a significant effect of drug on rearing (p<0.001). Post hoc analysis revealed an effect solely of d-amphetamine (2 mg/kg) increasing rearing which was unaffected by SR142948A pretreatment.
Fig. 3.
Effect of systemic administration of SR142948A on A) hyperlocomotion and B) rearing, induced by d-amphetamine and dizocilpine, or apomorphine. Pretreatment with SR142948A (100 μ/kg) had no effect on hyperlocomotion induced by d-amphetamine (2 mg/kg) and dizocilpine (0.1 mg/kg), or apomorphine (0.5 mg/kg). *p<0.05, **p<0.01 (D-amph: d-amphetamine; apo: apomorphine); n=7–12 in each group.
3.6. Effects of SR142948A pretreatment on d-amphetamine-, dizocilpine-, or apomorphine-induced c-fos mRNA expression
SR142948A alone had no significant effect on c-fos mRNA expression. Psychotomimetic drug-induction of c-fos mRNA expression was observed in several limbic regions including the PFC, anterior cingulate cortex, LS, NAcc, MD, VTA and ventral subiculum (Table 1). Pretreatment with SR142948A significantly reduced d-amphetamine-, apomorphine- and dizocilpine-induction of c-fos mRNA expression in the PFC, reduced d-amphetamine- and apomorphine-induction of c-fos mRNA expression in the MD, and reduced d-amphetamine-induction of c-fos mRNA expression in the NAcc.
Table 1.
C-fos expression following challenge with amphetamine, apomorphine or dizocilpine, after pretreatment with vehicle or SR142948A.
| Region | Saline | Amphetamine | Apomorphine | Dizocilpine | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Vehicle | SR 142948A | Vehicle | SR 142948A | Vehicle | SR 142948A | Vehicle | SR 142948A | |
|
|
|
|
|
|
|
|
|
|
| N=7 | N=7 | N=9 | N=10 | N=8 | N=7 | N=8 | N=10 | |
| Medial PFC | 38.4±7.6 | 48.4 ±13.2 | 95.6± 9.0a | 63.2±7.6b | 93.0±9.0a | 65.7±9.0b | 78.4±13.2 | 74.1±7.6 |
| Dorsolateral PFC | 5.7±1.6 | 4.9±2.8 | 11.4±1.9a | 13.2±1.6a | 17.2±1.9a | 14.4±1.9a | 21.8±2.8a | 10.7±1.6 |
| Orbital PFC | 42.7±10.2 | 46.0±16.7 | 104.6±11.7a | 74.5±10.2a, b | 112.5±11.7a | 85.5±11.8a, b | 86.7±16.7a | 66.0±10.2b |
| Anterior cingulate | 37.5±6.7 | 42.2±9.5 | 79.5±6.7a | 61.9±6.7 | 55.1±7.8 | 52.7±6.7 | 62.8±9.3 | 66.1±5.8 |
| Posterior cingulate | 57.5±11.4 | 58.3±15.2 | 105.0±12.4a | 87.9±11.5a | 105.4±12.4a | 102.3±12.4a | 75.7±15.2 | 71.5±11.51 |
| Lateral septum | 28.9±6.8 | 38.4±11.7 | 62.3±6.8a | 45.2±6.8 | 57.2±7.9a | 46.1±6.8 | 60.1±9.5a | 74.6±5.9a |
| Nucleus accumbens shell | 10.11±3.0 | 12.3±5.8 | 36.2±4.0a | 21.3±3.9b | 25.2±3.6a | 15.2±3.9 | 18.8±4.5 | 23.5±3.4 |
| Nucleus accumbens core | 4.1±1.8 | 10.6 ±3.8 | 27.2±1.8a | 11.3±11.8b | 10.8±2.3 | 6.8±1.8 | 10.2±2.9 | 18.0±1.4 |
| Mediodorsal thalamus | 33.2±7.5 | 36.1±9.9 | 110.2±7.8a | 84±7.9a, b | 102.5±7.8a | 74.3±7.9a, b | 86.7 ±8.9a | 107.5±7.0a |
| Ventral tegmental area | 24.1±6.7 | 33.2±10.9 | 79.6±6.7a | 62.9±6.7a | 55.7±6.7 | 47.4±6.6 | 49.6±9.5 | 52.9±5.7 |
| Ventral subiculum | 37.5±5.4 | 42.3±8.8 | 79.5±5.3a | 61.9±6.1a | 55.1±5.3a | 52.7±5.9 | 62.8±8.8a | 66.1±5.4a |
| Dorsal subiculum | 15.2±5.4 | 15.1±8.8 | 53.6±7.3a | 42.6±6.4 | 46.5±7.3a | 22.6±7.3 | 27.6±8.8 | 23.0±5.4 |
Values represent nCi/mg protein; data shown as mean±standard error.
p<0.05 compared to saline × vehicle group.
p<0.05 compared to vehicle within same drug
4. Discussion
4.1. Summary of results
Our results do not support our original hypothesis. The three psychostimulants used in these studies all disrupted PPI, increased locomotor activity, and induced c-fos mRNA expression in limbic brain regions. Pretreatment with SR142948A did not modify baseline PPI or locomotion, however, it antagonized d-amphetamine (at medium and high doses) — and dizocilpine-induced PPI disruption and potentiated apomorphine-induced PPI disruption with no effect on baseline or stimulant-induced hyperlocomotion.
4.2. SR142948A administration induced selective antagonism of d-amphetamine- and dizocilpine-induced PPI disruption
As previously described, systemic SR142948A administration did not significantly affect baseline PPI in adult male rats (Cáceda et al., 2005). The antagonism of dizocilpine- and d-amphetamine- (at higher doses) induced PPI disruption by systemic administration of SR142948A was unexpected because of the large literature showing an APD-like effect when NT neurotransmission is increased, particularly NTR1, in the NAcc (Ervin et al., 1981; Robledo et al., 1993; Feifel et al., 1997; Cáceda et al., 2005), as well as after systemic treatment with NTR1 and NTR2 agonists (Feifel et al., 1999; Shilling et al., 2003; Boules et al., 2010). Paradoxically, this effect is lost or even reversed with chronic administration of NT or NT agonists, most likely though desensitization (Hertel et al., 2001; Norman et al., 2008). It is possible that blockade of excitatory NT neurotransmission, particularly at the VTA, given its rich NT innervation and NTR density, may decrease DA release in mesolimbic terminal fields (Fatigati et al., 2000; Geisler and Zahm, 2006). This hypothesis is supported by intra-VTA NT injections induced hyperlocomotion (Kalivas et al., 1982; Bauco and Rompre, 2003) with no effect on baseline PPI (Feifel and Reza, 1999b). Concordant with this view is the observed biphasic action of NT after icv administration, low doses decrease locomotor activity and higher ones increase it. This is thought to be related to the predominant stimulation of NTRs in the NAcc at lower doses and VTA at higher ones, associated with NT diffusion to the VTA and stimulation of NTRs (Nouel et al., 1990). Moreover, SR142948A blockade of both NTR1 and NTR2 likely alters the NTR1/NTR2 balance, as well as neurotransmission via other unidentified NTRs. This is supported by in vitro data suggesting that SR142948A exerts opposite effects on the NTR1 and NTR2, acting as an antagonist and agonist, respectively (Vita et al., 1998).
4.3. Differential regulation of sensorimotor gating and locomotion
Manipulation of other neurotransmitter systems also affects PPI and locomotion distinctly (Sills, 1999; Schwienbacher et al., 2002). Even though these behaviors are regulated by the same anatomical structures (i.e. NAcc and its output to the ventral pallidum-MD and ventral pallidum-pedunculopontine nucleus circuits (Koch, 1999; Mogenson et al., 1993; Pennartz et al., 1994; Swerdlow et al., 2001)), it is very plausible that their actual neural substrates involve different neuronal subpopulations within the NAcc, with discrete connectivity that are differentially affected by SR142948A administration (Pennartz et al., 1994).
4.4. d-amphetamine versus apomorphine
Systemic administration of SR142948A displayed partially similar effects to increased NT neurotransmission via NTR1 and NTR2 (Feifel et al., 1999; Shilling et al., 2003; Boules et al., 2010), resembling the action of atypical APDs such as clozapine. However, the ability of both typical and atypical APDs to counteract behavioral effects of apomorphine differs from SR142948A. Curiously, manipulations of NT (Feifel et al., 1999) and other neuropeptide systems, including oxytocin (Feifel and Reza, 1999a), μ opioids (Swerdlow et al., 1991), and CCK (Feifel et al., 2001) also antagonize d-amphetamine- but not apomorphine-induced PPI disruption. In contrast, SR142948A administration had a more complex dose-dependent interaction with d-amphetamine and actually enhanced apomorphine-induced PPI disruption. Overall, these results seem paradoxical and difficult to interpret. They may be related to a differential effect of SR142948A on DA release (d-amphetamine and dizocilpine) versus postsynaptic DA receptor stimulation (apomorphine).
4.5. Effects of SR142948A pretreatment on psychostimulant-induced c-fos mRNA expression on several limbic regions
As previously reported (Alonso et al., 1999; Binder et al., 2004; Fadel et al., 2006), pretreatment with SR142948A did not alter baseline c-fos mRNA expression, but prevented d-amphetamine-induced c-fos mRNA expression induction in the mPFC, orbital PFC (oPFC), NAcc and MD, apomorphine-induced expression in the mPFC, oPFC and MD, and dizocilpine-induced expression in the oPFC. This is in part, concordant with two previous reports that evaluated the effect of blockade of NT neurotransmission on DA agonist-induced c-fos mRNA expression. Alonso et al. (1999) observed that both SR 48692 (a selective NTR1 antagonist) and SR142948A reduced c-fos mRNA induction in the NAcc, CPu, globus and ventral pallidum following concurrent administration of D1 and D2 agonists. Recently, Fadel et al. (2006) reported that SR48692 prevented d-amphetamine-induced c-fos mRNA induction in the medial striatum but not in the NAcc (Fadel et al., 2006). The ability of SR142948A acute systemic administration to prevent c-fos mRNA activation by psychotomimetic drugs in mesolimbic terminal fields could be related to NTR blockade either on: 1) VTA cell bodies, decreasing NT excitatory input and subsequently VTA DAergic firing and DA release on terminal fields, or 2) presynaptic mesolimbic terminal regions, interfering with DA release or 3) postsynaptically in mesolimbic terminal fields altering DA-glutamate interactions. The first possibility is more likely, considering the excitatory nature of NTergic innervation in the VTA (Shi and Bunney, 1992). This would support the hypothesis of the VTA as the anatomical site for antagonism of stimulant-induced PPI disruption by SR142948A administration.
The three drugs tested (d-amphetamine, apomorphine and dizocilpine) decreased PPI, induced hyperlocomotion and increased c-fos expression in the oPFC, LS, MD and ventral subiculum. However, the behavioral effects following SR142948A pretreatment were not correlated with the pattern of c-fos mRNA activation. Interestingly, c-fos mRNA was induced in the oPFC with all three psychostimulants and this effect was prevented by SR142948A administration. One potential explanation is that these drugs disrupt sensorimotor gating through different mechanisms that converge on the PFC. This is in agreement with known prefrontal glutamatergic projections to the NAcc that are considered necessary for the action of stimulants (Harvey and Lacey, 1997; Beurrier and Malenka, 2002; Hjelmstad, 2004) and have been hypothesized to be involved in the pathogenesis of schizophrenia (Brady and O'Donnell, 2004; Carlsson, 2006).
APDs and stimulants induce c-fos mRNA and NT/NN mRNA expression in slightly different anatomical patterns (Merchant et al., 1994). However, the effects of both APDs and stimulants on the NT system are partially mediated by c-fos (Merchant, 1994). Blockade of c-fos mRNA expression prevented haloperidol NT/NN mRNA induction in the rat dorsolateral striatum, but not in the NAcc shell (Merchant et al., 1994). In contrast, evidence that c-fos mediates at least some NT effects based upon the use of NTR agonists and antagonists points to NT circuits as intermediaries of APD-mediated c-fos induction in the NAcc and the dorsolateral striatum (Fadel et al., 2001).
4.6. Limitations
In addition to the antagonistic effects of SR142948A on NTR1 and NTR2, it may have an agonistic effect on NTR2 and other NTRs. SR142948A was administered systemically, which inherently limits any accurate information on its neuro-anatomical site of action. C-fos expression data does not always exhibit a causal relationship with behavioral results. Experimental approaches to overcome anatomical and pharmacological non-specificities would include local suppression of NTR1 or NTR2 neurotransmission with viral vectors or optogenetic technology. Furthermore, it is important to consider that the c-fos data was obtained after locomotion testing. To our knowledge there is no evidence that this testing alters brain c-fos expression but this remains a possibility.
In summary, systemic administration of SR142948A had a complex effect on PPI disruption caused by two DA agonists and a NMDA receptor antagonist, whereas it had no effect on baseline or stimulant increased locomotion. Additionally, SR142948A pretreatment decreased drug-induced c-fos activation in several mesolimbic regions. The data suggest the VTA as the site for the antagonism of stimulant-induced PPI disruption. Further experiments are clearly warranted to further investigate this hypothesis. Further evaluation of the NT system might lead to development of compounds useful for schizophrenia and other mental illnesses.
Acknowledgments
Role of funding source: Funding for this study was provided by NIMH Grant NIH MH39415; the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We thank Dr. Jorge L. Gamboa for hid critical review of the manuscript.
Abbreviations
- APD
antipsychotic drug
- NT
neurotensin
- PPI
prepulse inhibition of acoustic startle response
- NAcc
nucleus accumbens
- NTR
NT receptor
- VTA
ventral tegmental area
Footnotes
Contributors: Drs. Cáceda, Binder and Kinkead performed the experiments, analyzed the data and performed all statistical analyses. Drs. Cáceda and Kinkead drafted the manuscript. Drs. Cáceda, Binder, Nemeroff and Kinkead all participated in study concept and experimental design. Drs. Binder and Nemeroff provided critical revision of the manuscript for important intellectual content. Drs. Kinkead and Nemeroff provided funding and study supervision.
Conflict of interest: Elisabeth B. Binder, Ph.D. receives funding from NIMH, PharmaNeuro Boost and the Behrens-Weise Foundation.
Ricardo Cáceda, M.D., Ph.D. receives funding from NIMH, NIDA, NIAAA, the Arsht Foundation and is partial owner of MyHealthWin LLC.
Becky Kinkead: Funding from NIMH.
Charles B. Nemeroff, M.D., Ph.D.
Research/Grants: National Institutes of Health (NIH) Agency for Healthcare Research and Quality (AHRQ)
Speakers Bureau: None
Consulting: Xhale, Takeda
Stockholder: CeNeRx BioPharma, NovaDel Pharma, Inc., PharmaNeuroBoost, Revaax Pharma, Xhale
Other Financial Interests: CeNeRx BioPharma, PharmaNeuroBoost
Patents: Method and devices for transdermal delivery of lithium (US 6,375,990B1), Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027B2)
Scientific Advisory Boards: American Foundation for Suicide Prevention (AFSP), CeNeRx BioPharma, National Alliance for Research on Schizophrenia and Depression (NARSAD), NovaDel Pharma, Inc., PharmaNeuroBoost, Anxiety Disorders Association of America (ADAA)
Board of Directors: AFSP, NovaDel Pharma, Inc.
References
- Alonso R, Gnanadicom H, Frechin N, Fournier M, Le Fur G, Soubrie P. Blockade of neurotensin receptors suppresses the dopamine D1/D2 synergism on immediate early gene expression in the rat brain. Eur J Neurosci. 1999;11:967–974. doi: 10.1046/j.1460-9568.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D, Nikkel A. Amphetamine induces Fos-like immunoreactivity in the striatum of primates. Brain Res. 1996;719:138–142. doi: 10.1016/0006-8993(96)00140-0. [DOI] [PubMed] [Google Scholar]
- Bauco P, Rompre PP. Central neurotensin receptor activation produces differential behavioral responses in Fischer and Lewis rats. Psychopharmacology. 2003;168:253–261. doi: 10.1007/s00213-003-1436-8. [DOI] [PubMed] [Google Scholar]
- Bean AJ, During MJ, Roth RH. Effects of dopamine autoreceptor stimulation on the release of colocalized transmitters: in vivo release of dopamine and neurotensin from rat prefrontal cortex. Neurosci Lett. 1990;108:143–148. doi: 10.1016/0304-3940(90)90721-k. [DOI] [PubMed] [Google Scholar]
- Betancur C, Cabrera R, de Kloet ER, Pélaprat D, Rostène W. Role of endogenous neurotensin in the behavioral and neuroendocrine effects of cocaine. Neuropsychopharmacology. 1998;19:322–332. doi: 10.1016/S0893-133X(98)00028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J Neurosci. 2002;22:5817–5822. doi: 10.1523/JNEUROSCI.22-14-05817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Kilts CD, Nemeroff CB. Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: evidence from animal models of sensorimotor gating. J Neurosci. 2001a;21:601–608. doi: 10.1523/JNEUROSCI.21-02-00601.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MO, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001b;53:453–486. [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin receptor antagonist SR 142948A alters Fos expression and extrapyramidal side effect profile of typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2004;29:2200–2207. doi: 10.1038/sj.npp.1300546. [DOI] [PubMed] [Google Scholar]
- Boules M, Warrington L, Fauq A, McCormick D, Richelson E. A novel neurotensin analog blocks cocaine- and d-amphetamine-induced hyperactivity. Eur J Pharmacol. 2001;426:73–76. doi: 10.1016/s0014-2999(01)01197-9. [DOI] [PubMed] [Google Scholar]
- Boules M, Shaw A, Fredrickson P, Richelson E. Neurotensin agonists: potential in the treatment of schizophrenia. CNS Drugs. 2007;21:13–23. doi: 10.2165/00023210-200721010-00002. [DOI] [PubMed] [Google Scholar]
- Boules M, Liang Y, Briody S, Miura T, Fauq I, et al. NT79: a novel neurotensin analog with selective behavioral effects. Brain Res. 2010;1308:35–46. doi: 10.1016/j.brainres.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozas E, Tritos N, Phillipidis H, Stylianopoulou F. At least three neurotransmitter systems mediate a stress-induced increase in c-fos mRNA in different rat brain areas. Cell Mol Neurobiol. 1997;17:157–169. doi: 10.1023/a:1026309727518. [DOI] [PubMed] [Google Scholar]
- Brady AM, O'Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceda R, Kinkead B, Owens MJ, Nemeroff CB. Virally mediated increase in neurotensin 1 receptor in the nucleus accumbens decreases the behavioral effects of mesolimbic system activation by amphetamine and dizocilpine. J Neurosci. 2005;110:1–7. doi: 10.1523/JNEUROSCI.4282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceda R, Kinkead B, Owen MJ, Nemeroff CB. Neurotensin role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39(Suppl 1):S10–S14. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- Carraway RE, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Casti P, Marchese G, Casu G, Ruiu S, Pani L. Blockade of neurotensin receptors affects differently hypo-locomotion and catalepsy induced by haloperidol in mice. Neuropharmacology. 2004;47:128–135. doi: 10.1016/j.neuropharm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM. D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- Costa FG, Frussa-Filho R, Felicio LF. The neurotensin receptor antagonist, SR48692, attenuates the expression of amphetamine-induced behavioural sensitisation in mice. Eur J Pharmacol. 2001;428:97–103. doi: 10.1016/s0014-2999(01)01271-7. [DOI] [PubMed] [Google Scholar]
- Costa FG, Frussa-Filho R, Canteras NS, Valera AG, Felicio LF. Blockade of neurotensin receptors during amphetamine discontinuation indicates individual variability. Neuropeptides. 2007;41:83–91. doi: 10.1016/j.npep.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dalia A, Wallace LJ. Amphetamine induction of c-fos in the nucleus accumbens is not inhibited by glutamate antagonists. Brain Res. 1995;694:299–307. doi: 10.1016/0006-8993(95)00794-q. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Jr, Helton TE, McGinty JF. Selective induction of Fos and FRA immunoreactivity within the mesolimbic and mesostriatal dopamine terminal fields. Synapse. 1993;13:251–263. doi: 10.1002/syn.890130308. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull RL. MK-801 induces c-fos protein in thalamic and neocortical neurons of rat brain. Neurosci Lett. 1990;111:39–45. doi: 10.1016/0304-3940(90)90341-6. [DOI] [PubMed] [Google Scholar]
- During MJ, Bean AJ, Roth RH. Effects of CNS stimulants on the in vivo release of the colocalized transmitters, dopamine and neurotensin, from rat prefrontal cortex. Neurosci Lett. 1992;140:129–133. doi: 10.1016/0304-3940(92)90698-7. [DOI] [PubMed] [Google Scholar]
- Ervin GN, Birkemo LS, Nemeroff CB, Prange AJ., Jr Neurotensin blocks certain amphetamine-induced behaviours. Nature. 1981;291:73–76. doi: 10.1038/291073a0. [DOI] [PubMed] [Google Scholar]
- Fadel J, Dobner PR, Deutch AY. The neurotensin antagonist SR 48692 attenuates haloperidol-induced striatal Fos expression in the rat. Neurosci Lett. 2001;303:17–20. doi: 10.1016/s0304-3940(01)01708-6. [DOI] [PubMed] [Google Scholar]
- Fadel J, Dobner PR, Deutch AY. Amphetamine-elicited striatal Fos expression is attenuated in neurotensin null mutant mice. Neurosci Lett. 2006;402:97–101. doi: 10.1016/j.neulet.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Fatigati MD, Anderson RM, Rompré P. Effects of prefrontal cortex microinjection of neurotensin-(8–13) on midbrain dopamine and non-dopamine cell firing. Brain Res. 2000;876:196–200. doi: 10.1016/s0006-8993(00)02654-8. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology. 1999a;141:93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL. Effects of neurotensin administered into the ventral tegmental area on prepulse inhibition of startle. Behav Brain Res. 1999b;106:189–193. doi: 10.1016/s0166-4328(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minor KL, Dulawa S, Swerdlow NR. The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Res. 1997;760:80–84. doi: 10.1016/s0006-8993(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. J Pharmacol Exp Ther. 1999;288:710–713. [PubMed] [Google Scholar]
- Feifel D, Priebe K, Shilling PD. Startle and sensorimotor gating in rats lacking CCK-A receptors. Neuropsychopharmacology. 2001;24:663–670. doi: 10.1016/S0893-133X(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Feifel D, Pang Z, Shilling PD, Melendez G, Schreiber R, Button D. Sensorimotor gating in neurotensin-1 receptor null mice. Neuropharmacology. 2010;58:173–178. doi: 10.1016/j.neuropharm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Hashimoto K, Yamagami K. Effects of antipsychotic drugs on neurotoxicity, expression of fos-like protein and c-fos mRNA in the retrosplenial cortex after administration of dizocilpine. Eur J Pharmacol. 2000;398:1–10. doi: 10.1016/s0014-2999(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Gao XM, Hashimoto T, Tamminga CA. Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse. 1998;29:14–28. doi: 10.1002/(SICI)1098-2396(199805)29:1<14::AID-SYN2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gass P, Spranger M, Herdegen T, Bravo R, Kock P, et al. Induction of FOS and JUN proteins after focal ischemia in the rat: differential effect of the N-methyl-D-aspartate receptor antagonist MK-801. Acta Neuropathol. 1992;84:545–553. doi: 10.1007/BF00304474. [DOI] [PubMed] [Google Scholar]
- Gass P, Herdegen T, Bravo R, Kiessling M. Induction and suppression of immediate early genes in specific rat brain regions by the non-competitive N-methyl-d-aspartate receptor antagonist MK-801. Neuroscience. 1993;53:749–758. doi: 10.1016/0306-4522(93)90621-l. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Neurotensin afferents of the ventral tegmental area in the rat: [1] re-examination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur J Neurosci. 2006;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- Grimond-Billa SK, Norman C, G WB, Cassaday HJ. Selectively increased trace conditioning under the neurotensin agonist PD 149163 in an aversive procedure in which SR 142948A was without intrinsic effect. J Psychopharmacol. 2008;22:290–299. doi: 10.1177/0269881106081528. [DOI] [PubMed] [Google Scholar]
- Gully D, Jeanjean F, Poncelet M, Steinberg R, Soubrie P, et al. Neuropharmacological profile of non-peptide neurotensin antagonists. Fundam Clin Pharmacol. 1995;9:513–521. doi: 10.1111/j.1472-8206.1995.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Gully D, Labeeuw B, Boigegrain R, Oury-Donat F, Bachy A, et al. Biochemical and pharmacological activities of SR 142948A, a new potent neurotensin receptor antagonist. J Pharmacol Exp Ther. 1997;280:802–812. [PubMed] [Google Scholar]
- Hanson GR, Midgley LP, Bush LG, Gibb JW. Response of extrapyramidal and limbic neurotensin systems to phencyclidine treatment. Eur J Pharmacol. 1995;278:167–173. doi: 10.1016/0014-2999(95)00127-7. [DOI] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel P, Byskov L, Didriksen M, Arnt J. Induction of tolerance to the suppressant effect of the neurotensin analogue NT69L on amphetamine-induced hyperactivity. Eur J Pharmacol. 2001;422:77–81. doi: 10.1016/s0014-2999(01)01076-7. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO. Dopamine excites nucleus accumbens neurons through the differential modulation of glutamate and GABA release. J Neurosci. 2004;24:8621–8628. doi: 10.1523/JNEUROSCI.3280-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Cador M, Dumartin B, Normand E, Stinus L, Bloch B. Acute and chronic amphetamine treatments differently regulate neuropeptide messenger RNA levels and Fos immunoreactivity in rat striatal neurons. Neuroscience. 1995;65:1041–1050. doi: 10.1016/0306-4522(94)00537-f. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ., Jr Neuroanatomical site specific modulation of spontaneous motor activity by neurotensin. Eur J Pharmacol. 1982;78:471–474. doi: 10.1016/0014-2999(82)90491-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Burgess SK, Nemeroff CB, Prange AJ., Jr Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience. 1983;8:495–505. doi: 10.1016/0306-4522(83)90195-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ., Jr Neurotensin microinjection into the nucleus accumbens antagonizes dopamine-induced increase in locomotion and rearing. Neuroscience. 1984;11:919–930. doi: 10.1016/0306-4522(84)90203-3. [DOI] [PubMed] [Google Scholar]
- Kinkead B, Dobner PR, Egnatashvili V, Murray T, Deitemeyer N, Nemeroff CB. Neurotensin-deficient mice have deficits in prepulse inhibition: restoration by clozapine but not haloperidol, olanzapine or quetiapine. J Pharmacol Exp Ther. 2005;315:256–264. doi: 10.1124/jpet.105.087437. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Letter AA, Merchant K, Gibb JW, Hanson GR. Effect of methamphetamine on neurotensin concentrations in rat brain regions. J Pharmacol Exp Ther. 1987;241:443–447. [PubMed] [Google Scholar]
- Li Z, Boules M, Williams K, Peris J, Richelson E. The novel neurotensin analog NT69L blocks phencyclidine (PCP)-induced increases in locomotor activity and PCP-induced increases in monoamine and amino acids levels in the medial prefrontal cortex. Brain Res. 2010;1311:28–36. doi: 10.1016/j.brainres.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie-Claire C, Palminteri S, Romualdi P, Noble F. Effects of the selective neurotensin antagonist SR 142948A on 3,4-methylenedioxymethamphetamineinduced behaviours in mice. Neuropharmacology. 2008;54:1107–1111. doi: 10.1016/j.neuropharm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Merchant KM. c-fos antisense oligonucleotide specifically attenuates haloperidol-induced increases in neurotensin/neuromedin N mRNA expression in rat dorsal striatum. Mol Cell Neurosci. 1994;5:336–344. doi: 10.1006/mcne.1994.1040. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Letter AA, Gibb JW, Hanson GR. Changes in the limbic neurotensin systems induced by dopaminergic drugs. Eur J Pharmacol. 1988;153:1–9. doi: 10.1016/0014-2999(88)90581-x. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Hanson GR, Dorsa DM. Induction of neurotensin and c-fos mRNA in distinct subregions of rat neostriatum after acute methamphetamine: comparison with acute haloperidol effects. J Pharmacol Exp Ther. 1994;269:806–812. [PubMed] [Google Scholar]
- Miyamoto S, Snouwaert JN, Koller BH, Moy SS, Lieberman JA, Duncan GE. Amphetamine-induced Fos is reduced in limbic cortical regions but not in the caudate or accumbens in a genetic model of NMDA receptor hypofunction. Neuropsychopharmacology. 2004;29:2180–2188. doi: 10.1038/sj.npp.1300548. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Brudzinsky SM, Wu M, Yang CR, Yim CCY. From motivation to action: a review of dopaminergic regulation of limbic-nucleus accumbens-ventral pallidum-pedunculopontine nucleus circuitries involved in limbic-motor integration. In: Kalivas PW, Barnes CD, editors. Limbic Motor Circuits and Neuropsychiatry. CRC Press; Boca Raton, FL: 1993. pp. 193–236. [Google Scholar]
- Nakki R, Sharp FR, Sagar SM, Honkaniemi J. Effects of phencyclidine on immediate early gene expression in the brain. J Neurosci Res. 1996;45:13–27. doi: 10.1002/(SICI)1097-4547(19960701)45:1<13::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Norman C, Beckett SR, Spicer CH, Ashton D, Langlois X, Bennett GW. Effects of chronic infusion of neurotensin and a neurotensin NT1 selective analogue PD149163 on amphetamine-induced hyperlocomotion. J Psychopharmacol. 2008;22:300–307. doi: 10.1177/0269881107083838. [DOI] [PubMed] [Google Scholar]
- Norman C, Grimond-Billa SK, Bennett GW, Cassaday HJ. A neurotensin agonist and antagonist decrease and increase activity, respectively, but do not preclude discrete cue conditioning. J Psychopharmacol. 2010;24:373–381. doi: 10.1177/0269881108097721. [DOI] [PubMed] [Google Scholar]
- Nouel D, Dubuc I, Kitabgi P, Costentin J. Centrally administered [D-Trp11] neurotensin, as well as neurotensin protected from inactivation by thiorphan, modifies locomotion in rats in a biphasic manner. Peptides. 1990;11:551–555. doi: 10.1016/0196-9781(90)90058-d. [DOI] [PubMed] [Google Scholar]
- Panayi F, Colussi-Mas J, Lambas-Senas L, Renaud B, Scarna H, Berod A. Endogenous neurotensin in the ventral tegmental area contributes to amphetamine behavioral sensitization. Neuropsychopharmacology. 2005;30:871–879. doi: 10.1038/sj.npp.1300638. [DOI] [PubMed] [Google Scholar]
- Panegyres PK, Hughes J. Activation of c-fos mRNA in the brain by the kappa-opioid receptor agonist enadoline and the NMDA receptor antagonist dizocilpine. Eur J Pharmacol. 1997;328:31–36. doi: 10.1016/s0014-2999(97)83023-3. [DOI] [PubMed] [Google Scholar]
- Paul ML, Currie RW, Robertson HA. Priming of a D1 dopamine receptor behavioural response is dissociated from striatal immediate-early gene activity. Neuroscience. 1995;66:347–359. doi: 10.1016/0306-4522(94)00582-p. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Souilhac J, Gueudet C, Terranova JP, Gully D, et al. Effects of SR 48692, a selective non-peptide neurotensin receptor antagonist, on two dopamine-dependent behavioural responses in mice and rats. Psychopharmacology. 1994;116:237–241. doi: 10.1007/BF02245067. [DOI] [PubMed] [Google Scholar]
- Robledo P, Maldonado R, Koob GF. Neurotensin injected into the nucleus accumbens blocks the psychostimulant effects of cocaine but does not attenuate cocaine self-administration in the rat. Brain Res. 1993;622:105–112. doi: 10.1016/0006-8993(93)90808-z. [DOI] [PubMed] [Google Scholar]
- Schwienbacher I, Fendt M, Hauber W, Koch M. Dopamine D1 receptors and adenosine A1 receptors in the rat nucleus accumbens regulate motor activity but not prepulse inhibition. Eur J Pharmacol. 2002;444:161–169. doi: 10.1016/s0014-2999(02)01622-9. [DOI] [PubMed] [Google Scholar]
- Shi WX, Bunney BS. Actions of neurotensin: a review of the electrophysiological studies. Ann N Y Acad Sci. 1992;668:129–145. doi: 10.1111/j.1749-6632.1992.tb27345.x. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Richelson E, Feifel D. The effects of systemic NT69L, a neurotensin agonist, on baseline and drug-disrupted prepulse inhibition. Behav Brain Res. 2003;143:7–14. doi: 10.1016/s0166-4328(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Sills TL. Amphetamine dose dependently disrupts prepulse inhibitionof the acoustic startle response in rats within a narrow time window. Brain Res Bull. 1999;48:445–448. doi: 10.1016/s0361-9230(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- Steinberg R, Brun P, Fournier M, Souilhac J, Rodier D, et al. SR 48692, a non-peptide neurotensin receptor antagonist differentially affects neurotensin-induced behaviour and changes in dopaminergic transmission. Neuroscience. 1994;59:921–929. doi: 10.1016/0306-4522(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Geyer MA. Opiate-dopamine interactions in the neural substrates of acoustic startle gating in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:415–426. doi: 10.1016/0278-5846(91)90072-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Szakacs R, Weiczner R, Mihaly A, Krisztin-Peva B, Zador Z, Zador E. Non-competitive NMDA receptor antagonists moderate seizure-induced c-fos expression in the rat cerebral cortex. Brain Res Bull. 2003;59:485–493. doi: 10.1016/s0361-9230(02)00965-6. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vita N, Oury-Donat F, Chalon P, Guillemot M, Kaghad M, et al. Neurotensin is an antagonist of the human neurotensin NT2 receptor expressed in Chinese hamster ovary cells. Eur J Pharmacol. 1998;360:265–272. doi: 10.1016/s0014-2999(98)00678-5. [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Bush LG, Gibb JW, Hanson GR. Endogenous neurotensin antagonizes methamphetamine-enhanced dopaminergic activity. Brain Res. 1994;665:237–244. doi: 10.1016/0006-8993(94)91343-9. [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Gibb JW, Hanson GR. Role of dopamine D1- and NMDA receptors in regulating neurotensin release in the striatum and nucleus accumbens. Brain Res. 1997;748:241–244. doi: 10.1016/s0006-8993(96)01380-7. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D. A comparison of the patterns of striatal Fos-like immunoreactivity induced by various dopamine agonists in rats. Neurosci Lett. 2000;289:99–102. doi: 10.1016/s0304-3940(00)01269-6. [DOI] [PubMed] [Google Scholar]