Abstract
Compact fluorescent lamps contain small quantities of mercury, whose release can lead to human exposures of potential concern in special cases involving multiple lamps, confined spaces, or young children. The exposure scenarios typically involve solid lamp debris that slowly releases elemental mercury vapor to indoor spaces. Here we propose and demonstrate a reactive barrier approach for the suppression of that mercury release, and demonstrate the concept using uncoated amorphous nano-selenium as the reactive component. Multi-layer structures containing an impregnated reactive layer and a mercury vapor barrier are fabricated, characterized, and evaluated in three exposure prevention scenarios: carpeted break sites, disposal/recycling bags, and boxes as used for retail sales, shipping and collection. The reactive barriers achieve significant suppression of mercury release to indoor spaces in each of the three scenarios. The nano-selenium barriers also exhibit a unique indicator function that can reveal the location of Hg-contamination by local reaction-induced change in optical properties. The article also presents results on equilibrium Hg vapor pressure above lamp debris, mathematical modeling of reaction and transport processes within reactive barriers, and landfill stability of nano-selenium and its reaction products.
Introduction
The market for compact fluorescent lamps (CFLs) continues to grow rapidly, and the environmental health and safety (EHS) issues associated with their mercury content have begun to generate discussion in the press and the scientific literature [1–6]. Two recent studies in this journal have specifically addressed the EHS aspects of mercury in CFLs [2,4]. Eckelman et al. [2] present an analysis of the net effects of CFL use on environmental mercury emissions, which comprise environmental release upon breakage and the avoidance of Hg emission from coal combustion associated with electricity savings from replacement of less-efficient incandescent bulbs. That study reports a net reduction in environmental Hg emissions from CFL use in most regions, but also large region-to-region differences that reflect the variable fraction of coal in the regional power mix. Johnson et al. [4] report on the timing and extent of Hg-vapor release from CFLs following accidental breakage, and evaluated a panel of 28 sorbents for their ability to capture Hg-vapor under conditions relevant to CFL breakage. Another relevant study in the recent literature is a review by Corrazza et al. on fabrication techniques designed to minimize lamp Hg content through intelligent dosing schemes [1]. These recent studies and references therein provide useful background on the forms, release, and health effects of mercury in lamps, which will not be repeated here.
The present article focuses on a new technology to reduce direct human exposure to elemental Hg vapor over the life-cycle of fluorescent lamps. Although mercury amounts in the new generation of lamps are small (typically less than 5 mg in new CFLs and less than 10 mg in new tube-type lamps [1]), the number of lamps in use is very large, and accidental releases can occur in the immediate presence of consumers or workers during use, transport, and collection. The recent case-study and testing program in the state of Maine illustrates the current consumer risk perception as well as real uncertainties surrounding clean-up procedures [7]. The information to date suggests the following scenarios as worthy targets for the development of exposure management technologies:
Break sites on carpets and porous substrates
Lamp debris on hard surfaces can be removed by hand cleaning, but complete removal from porous substrates requires vacuuming, which is not recommended as conventional domestic equipment can become contaminated and spread mercury [7]. Broken lamps spill phosphor powder into the carpet fiber bed, which continues to evolve Hg-vapor over hours to days [4].
Receptacles for single spent or broken lamps
Several agencies recommend disposal of lamp debris in sealed polyethylene bags, but the Maine study reports significant loss of Hg vapor through such bags [7]. If CFL debris is placed in polymer bags and placed in indoor waste receptacles, the release of mercury to indoor spaces will continue to occur.
Multi-lamp shipping, collection, and recycling boxes
Both wholesale delivery and recycling involve multiple lamps in single containers, typically boxes, and lamp breakage during shipping in these containers can occur. These scenarios may involve small numbers of lamps in consumer “mail back” programs, or large numbers of lamps in wholesale shipping or late-stage recycling steps (after local consolidation), where the total Hg amounts are large and there is an increased potential for biologically significant exposure to retail or transport workers. The first collection point in CFL recycling chains is often a retail store without specialized equipment or ventilation for Hg management. Lamps are often thrown into large boxes where the potential exists for significant numbers of broken lamps to coexist in a confined space outfitted with one or more portals where humans may encounter Hg-vapor at high concentration.
One would like to have practical technologies to reduce or eliminate Hg-vapor exposure in each of these scenarios. A recent study [4] evaluated Hg sorbents under conditions relevant to CFL breaks, but did not address how the sorbents should be formulated or their mode of use. Here we develop a reactive barrier concept for suppressing Hg vapor release in the target scenarios above and demonstrate the concept using the highest activity sorbent from the Johnson et al. study [4], which is uncoated amorphous nano-selenium (nSe).
Materials and Methods
Materials
A commercial 13 W (60 W incandescent equivalent) mini-spiral CFL was chosen as the standard lamp for this study. Amorphous uncoated nSe was synthesized in colloidal suspension by reduction of sodium selenite (Na2SeO3, Alfa Aesar) by glutathione (GSH, reduced form, TCI America) using sodium hydroxide for pH control as described in Johnson et al. [4].
Reactive barriers
The active portion of the nSe reactive barriers were fabricated by immersing an 11″×11″ cellulose-based kitchen towel (P&G) in freshly synthesized colloidal nSe solution followed by air drying for at least 24 hours. The towel capacity was measured to be 0.03 g-water/cm2-superficial area, which combined with the nSe colloidal concentration of 0.2 mg/ml gives an 11″×11″ layer containing approximately 5 mg of elemental selenium. The active layers were combined with barrier layers and in some cases third, protective layers comprising cloth, paper, or cardboard depending on the application (vida infra). The reactive barriers were characterized by FE-SEM and by USEPA method EPA-1311 Toxic Characteristic Leaching Procedure (TCLP) testing with analysis for both Hg and Se at an independent certified facility (New England Testing Laboratory, Providence, RI). The TCLP testing included fresh reactive barriers, used reactive barriers, the standard 13 W lamp, and a series of spent (non-functioning) lamps obtained from the Brown University internal recycling program.
Equilibrium Hg vapor pressure determination
Freshly fractured CFL debris was poured into new 500 ml Pyrex Erlenmeyer flasks, which were then sealed with PTFE-wrapped rubber stoppers containing gas inlet and outlet ports. The system was sealed and the vapor space sampled over time with a 1 ml chromatography syringe. Mercury vapor concentrations were determined by injection of the 1 ml gas sample into a previously calibrated atomic fluorescence vapor-phase mercury analyzer (10.525 Sir Galahad model from PS Analytical (Kent, UK)). To study the effect of partial Hg desorption, the flask was flushed with air at 1 L/min using a Teflon-lined pump and the outlet gas flow split with 20% directed to the mercury analyzer to determine the total amount of mercury removed. After 1 and 2 mg of mercury were removed, the gas venting was stopped and the syringe sampling experiment repeated to track the approach to the equilibrium vapor pressure. To accelerate the removal of an additional mg of mercury (for a total of 3 mg of mercury), heating tape was wrapped around the flask exterior and raised to 100 °C. In comparison, the boiling point of mercury is 357 °C, and fluorescent lamp recycling retort temperatures operate at a minimum of 500 °C. The flask was returned to room temperature and ventilated before it was resealed and the syringe sampling experiment repeated.
Reactive barrier testing
The sorbent-loaded barriers were tested in three different configurations involving carpets, bags, and boxes. In all cases the standard CFLs were catastrophically fractured and the gas phase sampled through PTFE tubing and PTFE-lined pumps for real-time analysis by atomic fluorescence. For the carpet experiments, CFLs were broken on an internal standard 18″ × 12″ Berber carpet patch in a 15′ × 20′ × 30′ laboratory space. Pieces larger than 1 cm were removed to simulate hand clean-up, and then 0.21 L/min of gas was sampled directly above the break epicenter at a height of 2±0.5 cm. These time-resolved measurements were carried out with a variety of reactive barriers and reactive barrier components and with no treatment as a control experiment.
For the testing of disposal/recycling bags, CFLs were placed inside reactive barrier lined polyethylene bags, sealed, placed in a 2L flexible PTFE enclosure, and broken under a continuous 1 L/min gas flow similar to the release experiments of Johnson et al. [4]. For the recycle box experiments, CFLs were placed in either sorbent lined or unlined boxes and placed in a 5 gallon polyethylene pail (M&M Industries, Chattanooga, TN) as a secondary container and test chamber. This container is DOT and UN approved for solid wastes, is outfitted with a vapor tight gasket, and is commonly used by bulb return recycling programs. A ¼″ hole was made in the lid and 30 cm of PTFE tubing was threaded through to create an air sampling port. The air inside the test chamber was sampled at 0.21 L/min and analyzed to determine Hg-vapor release from the lined boxes to the test chamber.
Results and Discussion
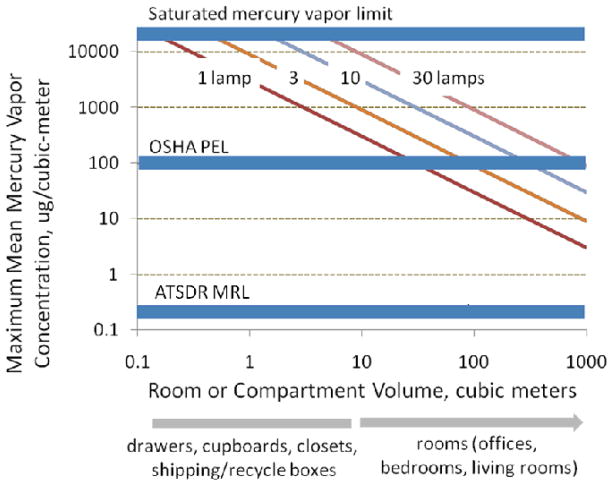
Before considering Hg capture in reactive barriers, it is important to characterize the anticipated vapor concentrations. The amount of mercury in a single CFL is small (3–5 mg) and thus in many cases a mass balance limits the possibilities for biologically relevant exposure. Figure 1 presents stoichiometric limits as a function of room/container size and number of broken bulbs for comparison to regulatory and advisory limits established for human health protection. Breaking a single CFL in a room of 30 m3 or larger cannot produce mean Hg-vapor concentrations above the Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) due to mass limitations alone. This is a very common exposure scenario that poses no significant occupational risk. The maximum achievable exposures increase when multiple bulbs are involved, and/or in smaller confined spaces like closets or small offices. In contrast, all the scenarios in Figure 1 give maximum vapor concentrations above the Agency for Toxic Substances and Disease Registry (ATSDR) chronic minimal risk level (MRL) of 0.2 μg/m3 [8]. There is the potential for significant exposure according to this criterion, but the actual exposures will be a complex function of evaporation rates, adsorptive sinks, ventilation, and patterns of human activity.
Figure 1.
Stoichiometric limits governing the maximum mean mercury levels in air as a function of room/container size and number of broken lamps. These theoretical limits are based on 3 mg Hg per lamp and complete volatilization with no ventilation or adsorptive sinks.
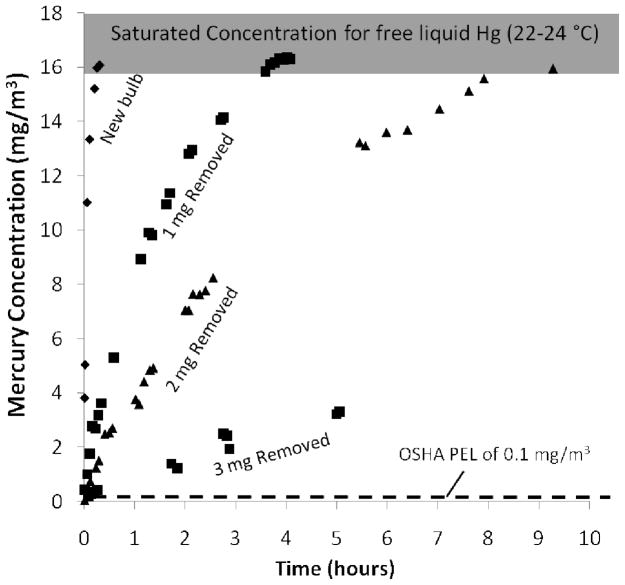
A significant unknown in the estimation of actual vapor concentrations in small confined spaces is the equilibrium vapor pressure of Hg above lamp debris. Most Hg in lamps is not free liquid, but associated with the phosphor, glass, endcaps, or dosing alloys [1] and thus has unknown phase behavior. Figure 2 shows results of an experiment specially designed to determine the Hg vapor pressure above CFL debris to define the thermodynamic equilibrium state. Fig. 2 shows that the mercury vapor pressure above CFL debris is approximately the same as that of pure liquid mercury at ambient temperature (16,000 – 18,000 μg/m3), which is over 150 times the OSHA-PEL. As mercury is removed from the CFL debris by gas purging, the saturated vapor pressure remains at these high values although the rate of approach is reduced. However, the mercury release rate is non linear with respect to the Hg remaining on the CFL. Rather, it appears that Hg on the CFL is bound to various surfaces at different energies. In addition to defining the thermodynamic equilibrium state, this data has the practical implication that very high Hg values can occur in confined spaces such as boxes or drawers, where the volume is small enough for the concentrations not to be limited by available Hg mass. The data in Figure 2 data will be used later in the modeling and design of reactive barriers.
Figure 2.
Equilibrium mercury vapor concentrations above CFL debris, and rate of approach to the equilibrium values in a 0.5 L flask. Although most Hg is associated with the phosphor, glass, or endcaps, its equilibrium vapor pressure is similar to that of free liquid Hg and over 150 times the OSHA-PEL. Partial removal of Hg (1, 2, and 3mg) by heating and gas purging reduces the release rates, but does not affect the equilibrium vapor concentrations. After 3 mg removal, the equilibrium Hg concentration returns to >15,000, but over 30 hours (data not shown). This equilibrium data is relevant to Hg-vapor concentrations expected in small confined spaces.
Reactive Barrier Concept
To reduce human exposure to mercury vapor that slowly volatilizes from condensed-phase lamp debris, we hypothesized the need for two elements: (i) a diffusive/convective barrier to Hg vapor transport, and (ii) a sorbent capable of reacting with and stabilizing mercury on the time scale over which the barrier is effective. The barrier alone would allow Hg vapor to reach saturation (Figure 2) and either slowly escape or pose a later hazard when the barrier was removed (e.g. opening of a bag or box). The sorbent alone, even if quite active, could not prevent loss of some Hg to the indoor space through vapor diffusion or convection away from the break site occurring in parallel with reaction. Thus a device that combines barrier and reactive elements is highly desirable for this application.
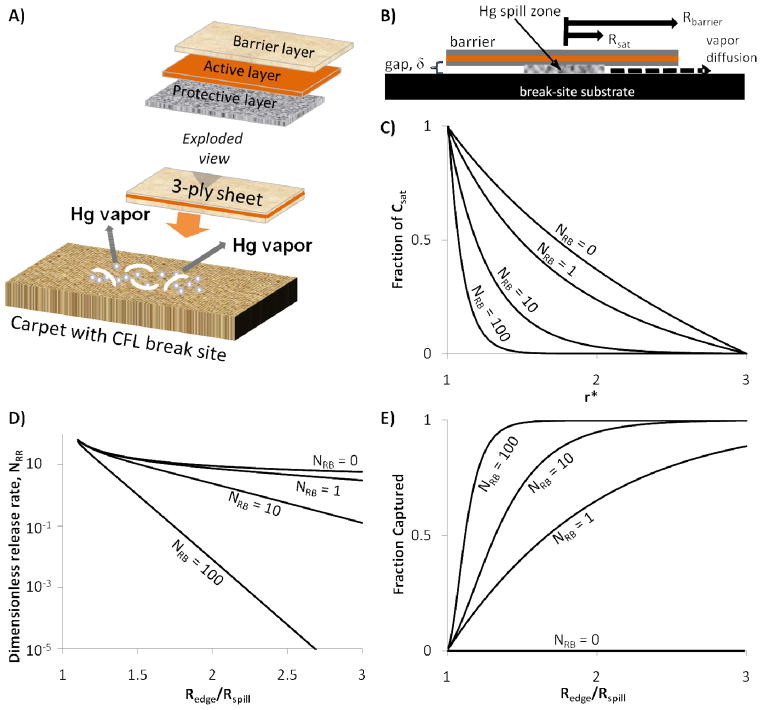
Our concept for a reactive mercury barrier is illustrated by the multi-layer structure in Figure 3-A. This reactive barrier concept can be generalized to boxes or bags, where the container itself may serve as a barrier layer, and the sorbent may be added as a separate layer, or may be coated or impregnated on the barrier layer itself. In all cases, however, capture in these quasi-open systems (indoor spaces) requires some means to both contain and react with mercury vapor. The multi-layer porous barrier structure is particularly suited to nanoparticle sorbents that can be synthesized by colloidal methods and impregnated by spontaneous capillary infiltration and drying to obtain uniform reactive films.
Figure 3.
Formulation and prediction of a coupled reaction and transport model of the reactive barrier technology applied to fluorescent lamp break sites. A) Exploded view of reactive barrier concept, B) Model formulation, C) Example Hg concentration profiles under a barrier three times the size of the spill, D) Release rates as a function of the dimensionless barrier number, NRB, and ratio of reactive barrier size Redge to spill size Rspill, E) Predictions of the fraction captioned of the mercury leaving the saturated zone.
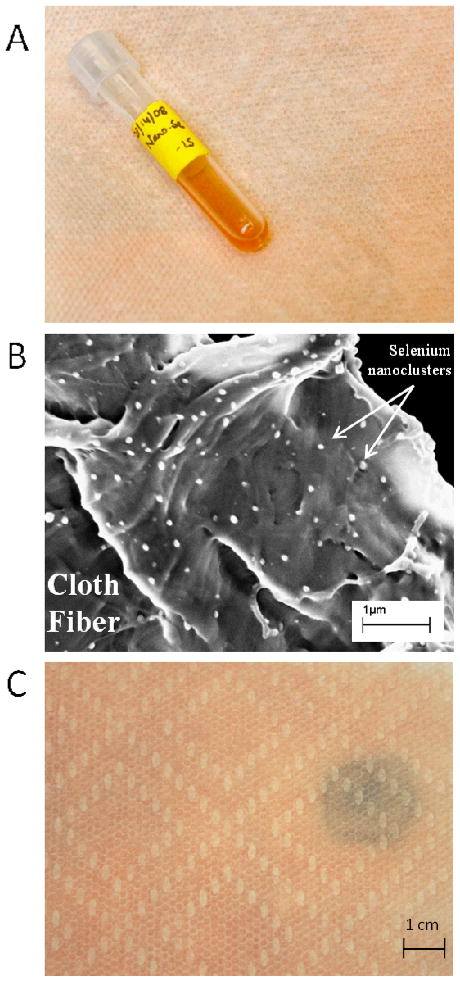
Figure 4 shows an example active layer fabricated by spontaneous capillary infiltration of uncoated nSe into a porous cellulose-based cloth. The nSe particle sizes in fresh colloidal solution were reported by Johnson et al. as 12 – 615 nm by dynamic light scattering [4]. The primary colloidal synthesis yields 0.2 mg-Se/ml, which impregnated into the cellulose cloth (0.03 ml/cm2 liquid at saturation), gives 6 μg-Se/cm2 upon drying. Over 780 cm2 of cloth area, the total nSe loading is approximately 5 mg, which is sufficient to capture at about 1 mg Hg based on the published capacity of 0.2 g-Se/g-Hg [4]. Upon reaction, this nanomaterial-doped thin film structure shows spatially resolved color change (Fig. 4), which may be useful for sorbent self-sensing, and/or to identify Hg “hot-spots” in some contamination scenarios.
Figure 4.
Nano-selenium formulations. A) nSe colloidal suspension (test-tube) and impregnated cloth fabricated by spontaneous capillary infiltration and drying. Nominal 5 mg nSe in 780 cm2 cloth. B) SEM image of impregnated cloth showing cellulose fibers decorated with ~20–200 nm selenium nanoclusters. C) nSe-impregnated cloth after placed over carpet containing a single small Hg droplet, showing the color change associated with the Hg/Se reaction demonstrating the indicator of self-sensing function.
Reactive barrier model
A one dimensional mass balance model of coupled reaction and transport was developed to help design the reactive barriers (Fig. 3).
| (1) |
The model considers diffusion of Hg vapor from the periphery of a circular spill zone of radius Rsat (assumed to contain saturated Hg vapor by the results in Figure 2), along a narrow gap, δ, between the substrate and an impermeable reactive barrier of radius, Rbarrier. C is the Hg vapor concentration, D is the diffusivity of mercury in air, is the sorbent concentration in the barrier, and k is the first-order reaction rate constant of the adsorption of Hg vapor onto the barrier. The size and activity of the barrier determine the Hg release rate at pseudo-steady-state. Diffusion of Hg vapor through the stagnant gap is determined by coupled reaction and diffusion. Recasting in dimensionless form gives:
| (2) |
with the dimensionless variables:
Where NRB is a “reactive barrier number”. At pseudo-steady-state, and with negligible external resistance to the room air, the boundary conditions are:
| (3) |
The Hg concentration is at saturation near the spill site and 0 at the periphery. The mercury release rate is determined from the concentration gradient at the periphery:
| (4) |
The solution of the second order ordinary differential equation (2) is presented in Figure 3-C, which can be used to design and size reactive barriers for CFL clean-up as described below.
Application to CFL Break Site Remediation
The results of Fig. 3 can be used to rationally design reactive barriers for effective suppression of Hg vapor release. To calculate the reactive barrier number, we use the nSe/Hg reaction rate reported by Johnson et al. [4] of 30 μg-Hg/g-Semin at 60 μg/m3 Hg vapor concentration, which corresponds to a pseudo-first-order rate constant of 0.5 m3/g-min. Using typical values for the remaining parameters: Rsat = 5 cm, m = 5 mg Se, a = 800 cm2, δ = 1 mm, and DHg = 0.13 cm2/s (estimated using the method of Fuller [9]) gives NRB of approx. 100. For the case where the Hg concentration in the saturated zone is 16 mg/m3, an Rbarrier to Rsat ratio of 2 can be chosen to give a Hg release rate of 0.1 ng/min and 99.99% capture efficiency (from Fig 3C, D). Thus we predict that effective Hg suppression can be achieved with convenient barrier sizes (Rbarrier) and relatively low nSe doping densities (m/a).
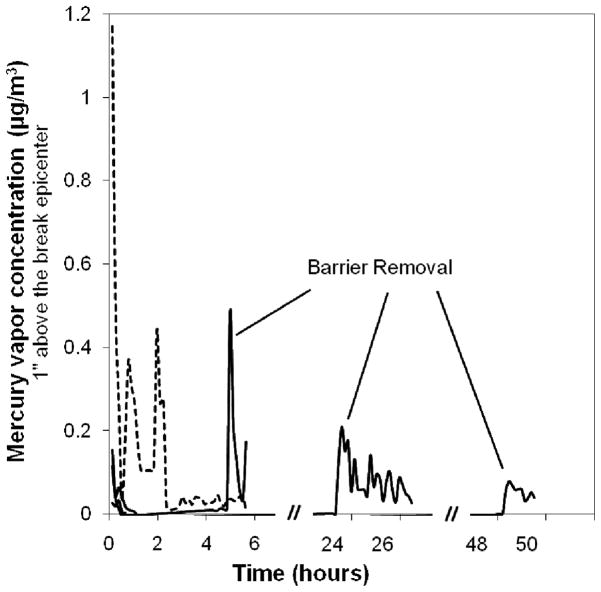
As validation, Figure 5 shows real-time Hg-vapor concentrations measured at 1 inch directly over the epicenter of a CFL break on the standard 18″ by 12″ carpet both with and without coverage by a 5 mg nSe three-layer reactive barrier of the structure shown in Figures 3-A, B. Without a barrier, the Hg-vapor concentration exceeds 1 μg/m3 in the first half hour and remains about the ASTDR limit for most of the first 2 hrs. Placing the 3-ply nSe barrier on the site reduces the early Hg-vapor concentrations to very low values indicating near-complete suppression. Removal of the barrier after 5 hrs leads to a measurable pulse of Hg that reflects as-yet unreacted Hg vapor trapped beneath the barrier. This Hg pulse decreases when the barrier is left in place for 24 hrs and drops to a very low value after 48 hrs (all measured in separate experiments). A cloth-based, 5-mg-nSe reactive barrier left for 48 hrs or more is capable of suppressing almost all Hg release to indoor spaces, after which the residual phosphor and glass can be removed by vacuuming.
Figure 5.
Mercury vapor concentrations 1 inch above the epicenter of CFL break sites in an open laboratory with (solid curves) and without (dashed curve) treatment by coverage with a 5 mg nSe reactive barrier. The curve segments at 5, 24, and 48 hrs show the effects of barrier removal. The use of the reactive barrier for at least 48 hrs suppresses almost all of the Hg vapor released to the indoor space.
As a control, the experiment in Figure 5 was repeated but without the nano-selenium impregnation. The three-ply “dummy” (non-reactive) barrier suppressed mercury release at early times relative to the open (untreated) case, but there was measurable vapor leakage (0.1–0.2 μg/m3 at 1 inch height), and removal after 5 hrs led to a large spike in Hg vapor concentration that exceeded the initial spike in the untreated case (peak value 1.7 vs. 1.2 μg/m3). Not surprisingly, the dummy barrier slowed Hg-vapor mass transfer to the room air, but only by trapping a high-concentration vapor film that is released after barrier removal.
Application as disposal/recycle bag liners
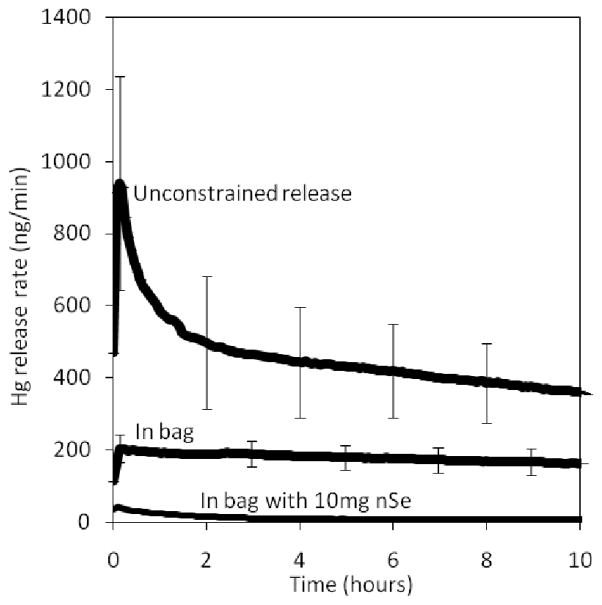
Figure 6 gives total Hg release rates measured after controlled CFL break experiments in polyethylene bags with and without nSe reactive barriers. The polymer bag alone does not prevent Hg-vapor release (though the release is slowed by a factor of 2–4), whereas the bag/nSe combination reduces Hg vapor release to levels close to the detection limit.
Figure 6.
Effect of reactive barriers on Hg-vapor release from 1 liter sealed polyethylene bags used for single-lamp handling and disposal. Experiments carried out in 1 L/min flow gas inside a 2 liter PTFE bag that is flexible enough to allow a single internal CFL to be fractured from the outside. A range of values are given for the polyethylene bag and unconstrained release (no bag) cases reflecting lamp-to-lamp variability seen in replicates. The nSe was introduced as single-layer doped porous cloth internal linings with 10 mg nSe.
Application as shipping/recycle box liners
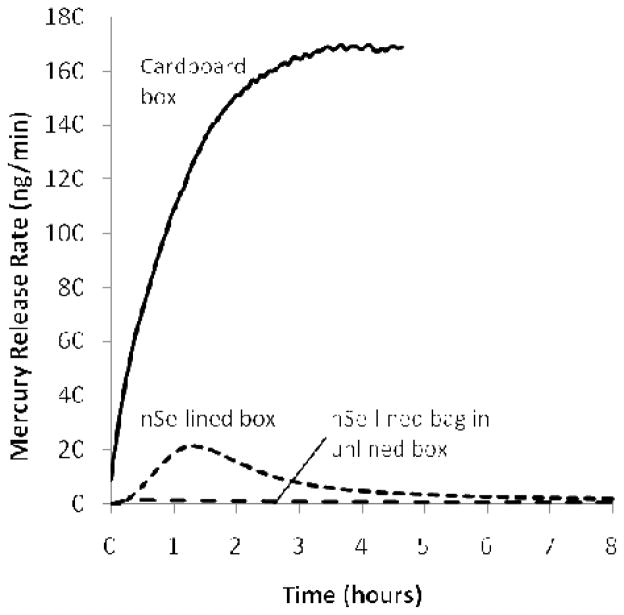
Figure 7 shows the effect of including nSe active layers on the inner surfaces of boxes containing a broken CFL. The nSe liner dramatically decreases the mercury release rate, though the leakage is still measurable. We hypothesized that the finite Hg levels reflect the poor barrier properties of the box, which is a folded cardboard structure with visible air gaps. Figure 7 shows that the nSe-lined bag within an unlined box essentially eliminates the Hg-vapor leakage, suggesting the usefulness of the bag/box composite structure to support nSe-based Hg exposure prevention.
Figure 7.
Effect of nSe reactive barriers on Hg-vapor release from a cardboard box used for single-lamp handling and disposal. Experiments carried out in 0.21 L/min flow gas inside a 5 gallon polyethylene pail. 5 mg nSe sorbent is introduced as a single-layer doped porous cloth internal lining to the box or bag.
TCLP testing and material safety considerations
Table 1 shows TCLP results for both Hg and Se for the various barriers and barrier/bulb combinations. The leachable Hg is higher in spent bulbs then new bulbs, consistent with previous studies that show the gradual transformation of Hg metal to soluble forms through oxidative reaction with the phosphor [10–12]. The Se leachate concentrations were below the detection limit in almost every case, which reflects the known low water solubility of elemental selenium and the small amounts of Se needed due to its ultra-high Hg-capture activity. The case with measurable Se was a fresh active layer (nSe-doped cloth) with no associated CFL, so its mass was very small (1 – 2 g), leading to very low TCLP water addition and the highest possible solute concentrations. The disposal of a doped cloth with no other mass (no barrier backing, cardboard, or lamp) represents a worst case for Se leachate concentration.
Table 1.
TCLP test results on CFLs and reactive barriers
| Test Sample* | Hg in leachate (mg/L)** | Se in leachate (mg/L)** |
|---|---|---|
| Before treatment | ||
| nSe-doped cloth, no lamp | <0.001 | 0.6 ± 0.3 |
| Spent lamp, no sorbent | 0.098 ± 0.11* | <0.1 |
| New lamp, no sorbent | 0.011 ± 0.009 | <0.1 |
| After treatment (combined lamp/sorbent disposal) | ||
| Spent lamp with nSe | 0.101 ± 0.036 | <0.1 |
| New lamp with nSe | 0.005 ± 0.001 | <0.1 |
S-AC cases: nominal 2 g sample, nSe cases: 5 mg
TCLP Limit is 0.2 mg/L for Hg and 1 mg/L for Se.
standard deviations, n=3
In summary, a reactive barrier approach appears promising for reducing Hg vapor exposure from CFLs, and could be the basis for a suite of products tailored for various stages of the CFL life-cycle. Uncoated nano-selenium is particularly well suited for the active component in these barriers due to its ultra-high activity, ease of uniform impregnation, and indicator function through local reaction color change. Other sorbents may be viable alternatives for the active layer, but will require higher amounts and may lack the indicator feature and ease of impregnation of nSe. More work is needed on the long-term aging, kinetics, coating and imbedding chemistry, and material safety issues associated with nSe reactive barriers.
Acknowledgments
Financial support was provided by the NIEHS Superfund Basic Research Program Grant at Brown University: P42 ES013660. While this work was supported financially by the NIEHS, the article does not necessarily reflect the views of the agency. The technical contributions of Indrek Kulaots, Aihui Yan, Shin Bowers, and Professor Steven Hamburg from Brown University are gratefully acknowledged.
References
- 1.Corazza A, Boffito C. Mercury dosing solutions for fluorescent lamps. Journal of Physics D: Applied Physics. 2008;41:144007. [Google Scholar]
- 2.Eckelman MJ, Anastas PT, Zimmerman JB. Spatial Assessment of Net Mercury Emissions from the Use of Fluorescent Bulbs. Environmental Science & Technology. 2008 Oct;42:8564–8570. doi: 10.1021/es800117h. [DOI] [PubMed] [Google Scholar]
- 3.Hildenbrand VD, Denissen CJM, van der Pol A, Hendriks AHC, van der Marel C, Snijders JHM, Tamminga Y, Brongersma HH, Viitanen MM. Reduction of Mercury Loss in Fluorescent Lamps Coated with Thin Metal-Oxide Films. Journal of The Electrochemical Society. 2003;150:H147–H155. [Google Scholar]
- 4.Johnson NC, Manchester S, Sarin L, Gao Y, Kulaots I, Hurt RH. Mercury Vapor Release from Broken Compact Fluorescent Lamps and In Situ Capture by New Nanomaterial Sorbents. Environmental Science & Technology. 2008;42:5772–5778. doi: 10.1021/es8004392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ralston N. Nanomaterials: Nano-selenium captures mercury. Nature Nanotechnology. 2008;3:527–528. [Google Scholar]
- 6.Engelhaupt E. Do compact fluorescent bulbs reduce mercury pollution? Environmental Science & Technology. 2008;42:8176–8176. [Google Scholar]
- 7.Stahler D, Ladner S, Jackson H. Maine Compact Fluorescent Lamp Study. Maine Department of Environmental Protection; 2008. [Google Scholar]
- 8.Agency for Toxic Substances and Disease Registry. Suggested Action Levels for Indoor Mercury Vapors in Homes or Businesses with Indoor Gas Regulators. 2000. [Google Scholar]
- 9.Fuller E, Schettler P, Giddings J. A new method for prediction of binary gas-phase diffusion coefficients. Industrial and Engineering Chemistry. 1966;58:19–27. [Google Scholar]
- 10.Dang TA, Frisk TA, Grossman MW, Peters CH. Identification of Mercury Reaction Sites in Fluorescent Lamps. Journal of The Electrochemical Society. 1999 Oct;146:3896–3902. [Google Scholar]
- 11.Raposo C, Windmoller CC, Durao Junior WA. Mercury speciation in fluorescent lamps by thermal release analysis. Waste Management. 2003;23:879–886. doi: 10.1016/s0956-053x(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 12.Thaler EG, Wilson RH, Doughty DA, Beersb WW. Measurement of Mercury Bound in the Glass Envelope during Operation of Fluorescent Lamps. Journal of The Electrochemical Society. 1995 Jun;142:1968–1970. [Google Scholar]