Abstract
Background
Handheld spirometers have several advantages over desktop spirometers but worries persist regarding their reproducibility and validity. We undertook an independent examination of an ultrasonic flow-sensing handheld spirometer.
Methods
Laboratory methods included reproducibility and validity testing using a waveform generator with standard American Thoracic Society (ATS) waveforms, in-line testing, calibration adaptor testing, and compression of the mouthpiece. Clinical testing involved repeated testing of 24 spirometry-naive volunteers and comparison to a volume-sensing dry rolling seal spirometer.
Results
The EasyOne Diagnostic spirometer exceeded standard thresholds of acceptability for ATS waveforms. In-line testing yielded valid results with relative differences (mean ± SD) between the EasyOne and the reference spirometer for the forced vital capacity (FVC) of 0.03±0.23 L and the forced expiratory volume in one second (FEV1) of −0.06±0.09 L. The calibration adaptor showed no appreciable problems, but extreme compression of the mouthpiece reduced measures. In clinical testing, coefficients of variation and limits of agreement were, respectively: 3.3% and 0.24 L for the FVC; 2.6% and 0.18 L for the FEV1; and 1.9% and 0.05 for the FEV1/FVC ratio. The EasyOne yielded lower values than the reference spirometry (FVC: −0.12 L; FEV1: −0.17 L; FEV1/FVC ratio: −0.02). Limits of agreement were within criteria for FVC but not for the FEV1, possibly due to a training effect.
Conclusion
The EasyOne spirometer yielded generally reproducible results that were generally valid compared to laboratory-based spirometry. The use of this handheld spirometer in clinical, occupational and research settings seems justified.
Introduction
Potentially millions of Americans have symptomatic chronic obstructive pulmonary disease (COPD) but are undiagnosed [1], in part due to the lack of widespread acceptance of office spirometry by primary care providers [2]. One of the barriers to the more widespread use of office spirometers includes a perception that they are inaccurate [3]. Accuracy of portable spirometers is also important for screening and surveillance programs in occupational settings, epidemiological studies, and clinical trials.
Handheld, flow-sensing spirometers have several advantages over traditional volume-sensing, desktop spirometers for clinical and epidemiological purposes including portability, utility in the field or home settings, lesser risk of cross-contamination, battery power, and ease of cleaning [4]. However, because early models of flow-sensing spirometers were less accurate than volume-sensing spirometers, a perception persists that flow-sensing spirometers are less accurate, even for current fourth-generation models [5].
The EasyOne handheld spirometer (ndd Medical Technologies, Chelmsford, MA) has been used for tens of thousands of spirometry tests in epidemiological surveys of COPD [6, 7] and for the detection of the respiratory effects of occupational exposures [8]. Yet validation of this portable spirometer against a gold-standard, dry-rolling spirometer in adult subjects is limited. One study reported its suitability for clinical use; however, only 32% of the participants who underwent testing with the EasyOne had a follow-up laboratory spirometry test, and comparisons for the forced vital capacity (FVC) were not reported [9]. A prior report evaluating 10 portable spirometers suggested that the EasyOne showed inadequate reproducibility for the FVC and overestimated the forced expiratory volume in one second (FEV1) compared to laboratory spirometry [10], but this report has not been confirmed.
Given the widespread use of the EasyOne spirometer in clinical and research settings, we undertook an independent examination of the reproducibility of this handheld spirometer and its validity compared to rolling-barrel spirometers using laboratory-based diagnostics and clinical measurements.
Methods
Description of the Device
The EasyOne spirometer is comprised of a small hand-held unit that contains the flow sensor, electronics, a data-entry keyboard, and a digital display which shows menus, subject data, waveforms, quality control results, and test results (Figure 1). Instead of a conventional hose and mouthpiece, the unit employs a disposable spirette that is a 5-inch pliable plastic tube that is inserted through the body of the spirometer. The spirette has an oval-shaped mouthpiece at one end, an opaque screen in the middle that allows transmission of ultrasound waves for velocity measurement, and is open on the non-mouthpiece end. The EasyOne spirometer can be used by itself, or connected to a personal computer upon which software will show flow-volume graphs and the results of quality checks in real time [11]. At the time of publication, there were two models of EasyOne spirometer: the Diagnostic model and the Frontline model. These appear identical and use the same spirette mouthpieces. We evaluated the Diagnostic model with version 1.12 firmware without the use of a connected personal computer.
Figure 1.
The EasyOne Diagnostic spirometer and spirette.
The EasyOne spirometer uses an ultrasonic transit time analysis to measure airflow [12]. Ultrasound transducers located diagonally on either side of the spirette cavity emit and receive sound in alternating directions. The gas flow through the disposable spirette slows the transmission of sound waves traveling against the gas flow and speeds up the transmission of sound waves traveling in the direction of the gas flow. The rate of gas flow can therefore be calculated from the difference between the upstream and downstream transit times. This relationship is theoretically independent of gas composition, pressure, temperature, and humidity, and hence should eliminate errors due to these variables. The spirette has no sensor elements and therefore should not become inaccurate due to condensed moisture or exhaled phlegm (as with screen and capillary tube pneumotachometers).
Laboratory Testing
Standardized volume waveform testing was performed by an experienced technologist (KJS) at the National Institute for Occupational Safety and Health (NIOSH) laboratory following the 1995 American Thoracic Society (ATS) guideline [13], which were current at the time the study was performed. The EasyOne was attached to a pulmonary waveform generator (Pulmonary Waveform Generator System; MH Custom Design, Midvale, UT) which produced ambient temperature and humidity air (body temperature and pressure saturated, or BTPS, standarized). The 24 volume ATS waveforms and the 26 ATS flow waveforms were tested three times each.
In-line testing
Tests were performed using the EasyOne spirometer set up in-line with a reference spirometer, a volume-sensing, dry rolling seal spirometer (Ohio 827, SensorMedics, Yorba Linda, California), interfaced to an IBM-compatible notebook computer, with custom software written by NIOSH staff to provide automated calibration and operation, comparison of the subject's performance, and a real-time display of flow-volume curves. This HF5 spirometry system is very similar to the HF4 spirometry system used in the NHANES III survey, from which the ATS-recommended spirometry reference equations were derived [14].
For the in-line testing, the subject blew into the EasyOne's spirette mouthpiece. Since the tubular spirette goes through the body of the spirometer and the non-mouthpiece side of it protrudes out from the spirometer, a hose was attached to the latter side and connected to the HF5 spirometer, as shown in Figure 2. The EasyOne was clamped in place during testing. In-line spirometry was then performed by 12 volunteers in the laboratory, most of whom had extensive experience with spirometry testing.
Figure 2.
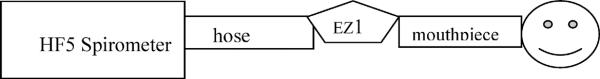
Setup for in-line EasyOne comparisions with the dry rolling seal HF5 spirometer
Testing using the calibration adaptor
A calibration adapter is required to connect the spirette and the round opening of a 3-liter calibration syringe or waveform generator. It is a 4 ½ inch long, grey plastic tube that has two screens within the body (newer models of the EasyOne now have a different calibration adaptor that no longer has the mesh screens). The screens help with noise reduction issues (acoustics) that are relevant when using the calibration syringe or the waveform generator but not when testing human subjects. A selection of five flow waveforms were each run twice using the calibration adaptor. The same flow waveforms were then also run (twice) by attaching the meter directly to the waveform generator. Then a selection of five volume waveforms were run twice using the calibration adaptor the first time and then connecting the meter directly to the waveform generator and repeating the process as mentioned above.
Testing the effects of mouthpiece compression
The spirette mouthpieces were made of a white plastic material, which was found to be somewhat pliable. When subjects were being tested they sometimes bit down on the disposable mouthpiece. Testing was performed to see if the biting affected accuracy. The EasyOne and HF5 were attached to the waveform generator and a set of two flow waveforms were run two times each, starting with the Spirette being pinched with a clamp to a ½ inch opening, then 3/8 inch opening, then ¼ inch, then 1/8 inch opening, repeating the two flow waveforms times two each time. The tests were done with the calibration adaptor in-line. Further testing compared results in which subjects `bit down' on the mouthpiece during expiration. Since this testing, the manufacturer has replaced this spirette with a less pliable one.
Clinical Testing
We recruited 24 subjects who were not familiar with spirometry via fliers. Those with poorly controlled lung disease were excluded. The volunteers received standardized instructions on the use of the EasyOne according to ATS guidelines. These volunteers underwent two sets of expiratory measurements using the EasyOne Diagnostic spirometer followed by a third, reference set using a standard rolling-barrel spirometer system 2130 Computerized Spirometer (SensorMedics Corporation, Yorba Linda, CA) in a university-based, certified pulmonary function laboratory following the same protocol.
All spirometry measures followed the 1995 ATS guidelines, which were current at the time the testing was performed [13]. Participants were asked to perform five to eight forced expiratory maneuvers in an effort to meet the ATS acceptability and reproducibility goals. The highest values from all acceptable maneuvers were used in analyses.
Ethics
Bench testing was originally performed for quality control purposes only at NIOSH and did not meet the definition of research as detailed in 45CFR46.102(d) according to the Human Subjects Review Board of NIOSH and the Institutional Review Board of Columbia University. The protocol for clinical testing at Columbia University was approved by the Institutional Review Board of Columbia University Medical Center and informed consent was obtained for all participants.
Statistical Analysis
Descriptive statistics included means and standard deviations and proportions. Comparisons were made with t-tests, or Chi-square tests, as appropriate. All participants were retained in analyses regardless of spirometry quality to maximise generalisability. Reproducibility was assessed as coefficient of variation (CVi = SDi / Meani) and 95% limits of agreement (1.96 * SDi) between the two measures made on the EasyOne. The validity was assessed with absolute differences between spirometers (e.g., FVCEasyOne − FVClab), with negative numbers implying lower values from the EasyOne. Limits of agreement were calculated as mean difference ± 1.96 * SD and plots were estimated following Bland-Altman methods allowing for proportionality of error and mean values [15]. This approach to the calculation of limits of agreement is conservative should proportionality be present. We did not specify acceptable limits of agreement a priori but followed ATS standards for reproducibility for the FVC and FEV1 of 0.20 L [13] and, for comparative purposes, previously described 95% limits of agreement for validity of 0.50 L for the FVC and 0.35 L for the FEV1 [10]. Statistical significance was defined as two-tailed P < 0.05. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
Results
Waveform generator
The EasyOne spirometer passed the test in all categories according to the ATS criteria [13].
In line testing
The mean results for FVC and FEV1 for both spirometers were very similar (Table 1). The mean ± SD FVC for the HF5 and EasyOne spirometers were 4.29 ±0.85 and 4.32 ± 1.04 L, respectively, with relative mean difference of 0.0% between the spirometers. The mean ± SD FEV1 for the HF5 and EasyOne spirometers were 3.43 ± 0.66 and 3.38 ± 0.7 L, respectively, with a −1.9% mean difference. Similar findings were observed for FEV1/FVC ratio. The mean difference for the peak expiratory flow (PEF) rate was greater (3.7%).
Table 1.
Spirometry values and differences between National Institute for Occupational Safety and Health HF5 spirometer and EasyOne spirometer from in-line testing.
| FVC* (L) | FEV1† (L) | FEV1/FVC | PEF‡ (L/sec) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference§ | Difference§ | Difference§ | Difference§ | ||||||||||||||
| Sex | Asthma | HF5 | EasyOne | Percent | Absolute | HF5 | EasyOne | Percent | Absolute | HF5 | EasyOne | Percent | Absolute | HF5 | EasyOne | Percent | Absolute |
| f | Maybe | 3.02 | 2.74 | −9.3 | −0.28 | 2.1 | 2.01 | −4.3 | −0.09 | 0.69 | 0.73 | 5.2 | 0.036 | 6.32 | 7.37 | 16.6 | 1.05 |
| f | n | 3.32 | 3.31 | −0.3 | −0.01 | 2.8 | 2.72 | −2.9 | −0.08 | 0.84 | 0.82 | −2.7 | −0.023 | 9.50 | 9.40 | −1.1 | −0.10 |
| f | y | 3.43 | 3.27 | −4.7 | −0.16 | 3.04 | 2.89 | −4.9 | −0.15 | 0.89 | 0.88 | −0.7 | −0.006 | 7.05 | 6.97 | −1.1 | −0.08 |
| f | n | 3.54 | 3.57 | 0.8 | 0.03 | 3.15 | 3.14 | −0.3 | −0.01 | 0.89 | 0.88 | −1.2 | −0.011 | 7.88 | 8.57 | 8.8 | 0.69 |
| f | n | 3.85 | 3.52 | −8.6 | −0.33 | 3.44 | 3.22 | −6.4 | −0.22 | 0.89 | 0.91 | 1.8 | 0.016 | 8.71 | 8.19 | −6.0 | −0.52 |
| f | n | 4.5 | 4.36 | −3.1 | −0.14 | 3.33 | 3.3 | −0.9 | −0.03 | 0.74 | 0.76 | −2.6 | 0.019 | 6.45 | 6.87 | 6.4 | 0.42 |
| f | n | 4.52 | 4.65 | 2.9 | 0.13 | 4.05 | 4.03 | −0.5 | −0.02 | 0.90 | 0.87 | −3.0 | −0.027 | 9.12 | 9.00 | −1.4 | −0.12 |
| f | n | 4.58 | 4.72 | 3.1 | 0.14 | 3.95 | 3.92 | −0.8 | −0.03 | 0.86 | 0.83 | −3.6 | −0.031 | 6.95 | 6.83 | −1.8 | −0.12 |
| f | y | 4.64 | 4.68 | 0.9 | 0.04 | 3.4 | 3.28 | −3.5 | −0.12 | 0.73 | 0.7 | −4.5 | −0.033 | 7.29 | 7.74 | 6.2 | 0.45 |
| m | n | 5.04 | 5.46 | 8.3 | 0.42 | 3.23 | 3.27 | 1.2 | 0.04 | 0.64 | 0.6 | −6.4 | −0.041 | 7.26 | 8.26 | 13.8 | 1.00 |
| m | n | 5.35 | 5.68 | 6.2 | 0.33 | 4.33 | 4.43 | 2.3 | 0.1 | 0.81 | 0.78 | −3.7 | −0.030 | 10.62 | 11.15 | 5.0 | 0.53 |
| m | n | 5.68 | 5.92 | 4.2 | 0.24 | 4.36 | 4.3 | −1.4 | −0.06 | 0.77 | 0.73 | −4.8 | −0.037 | 11.10 | 10.93 | −1.5 | −0.17 |
|
| |||||||||||||||||
| Average | 4.29 | 4.32 | 0.0 | 0.03 | 3.43 | 3.38 | −1.9 | −0.06 | 0.80 | 0.79 | −1.8 | −0.014 | 8.19 | 8.44 | 3.7 | 0.25 | |
| SD | 0.85 | 1.04 | 5.5 | 0.23 | 0.66 | 0.70 | 2.6 | 0.09 | 0.08 | 0.09 | 3.4 | 0.025 | 1.60 | 1.47 | 6.95 | 0.51 | |
FVC = forced vital capacity;
FEV1 = forced expiratory volume in one second;
PEF = peak expiratory flow rate;
difference calculated as EasyOne measurement – HF5 measurement
Although the absolute mean differences between the spirometers were small for the FVC, and FEV1 (Table 1), the differences for given individuals were larger. Nonetheless, the 95% limits of agreement for the FVC were −0.42 and 0.49 L, which were within the specified limits for validity. The 95% limits of agreement for the FEV1 were −0.23 and 0.12 L, which were also within the specified limits for validity.
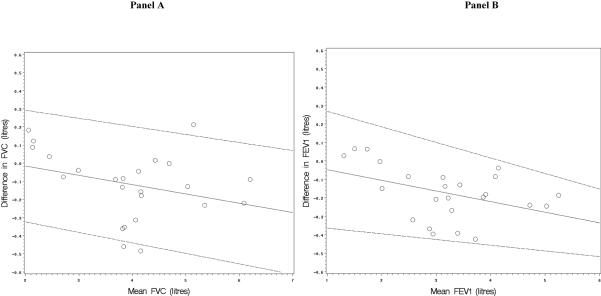
Figure 3 shows Bland-Altman plots of the difference between measures from the two spirometers compared to the mean value from two spirometers for FVC (Panel A) and FEV1 (Panel B). The plot for FVC suggested that the difference between measures from the spirometers was proportional to mean FVC, and linear regression confirmed that the slope of the line on the Bland-Altman plot was statistically significantly different from the null (β = 0.20; P=0.001). The plot for FEV1 showed less proportionality that was not statistically significant (β = 0.05, P=0.16). Figure 3 also shows 95% limits of agreement that allow for proportionality of mean differences vs. mean values and which are considerably narrower than those listed in the preceding paragraph. The 95% limits of agreement that ignore proportionality are conservative (i.e., wider), particularly for the FVC.
Figure 3.
Bland-Altman plots of the difference vs. mean value in measurements of the forced vital capacity (Panel A) and the forced expiratory volume in one second (Panel B) from the EasyOne and laboratory spirometry from in-line testing.
The solid lines of the Bland-Altman plots are regression lines of mean differences vs. mean values which allow for proportionality of mean differences vs. mean values (which is suggested by the non-zero slope of the line for FVC). The dashed lines are the 95% limits of agreement around the regression lines.
Calibration adaptor tests
The data collected using the calibration adapter was reproducible and within the targeted range. The data colleted without the adapter had a much wider range, but was also acceptable.
Mouthpiece compression
Compression of the spirette mouthpiece caused no appreciable change in values of the FEV1 and the PEF for compression up to ¼”, but a large reduction in values of the FEV1 and PEF with compression to 1/8” on the Easyone and the reference spirometer (Table 2). The readings from the two spirometers were similar except for the ⅛ inch opening measurements, where the difference in FEV1 was 0.39 L on waveform 1 and the differences in PEF were 1.40 and 1.66 L/sec on waveforms 1 and 19, respectively.
Table 2.
Differences in FEV1 and PEF values for the National Institute for Occupational Safety and Health HF5 and EasyOne spirometers during a pinch test of the spirette at ½, ⅜, ¼ and ⅛ inches of the spirette's original diameter using waveforms 1 & 19 from the 26 ATS standard flow-time waveforms.
| Diameter of mouthpiece (inches) | ATS Wave Form Number | FEV1 (L)* | PEF (L/sec)† | ||||
|---|---|---|---|---|---|---|---|
| HF5 | Easyone | Difference‡ | HF5 | Easyone | Difference‡ | ||
| .500 or ½” | 1 | 3.52 | 3.53 | 0.01 | 7.87 | 7.87 | 0.00 |
| 19 | 3.19 | 3.17 | 0.02 | 7.40 | 7.45 | 0.05 | |
|
| |||||||
| .375 or ⅜” | 1 | 3.61 | 3.59 | 0.02 | 8.13 | 8.00 | 0.13 |
| 19 | 3.21 | 3.05 | 0.16 | 7.43 | 7.02 | 0.41 | |
|
| |||||||
| .250 or ¼” | 1 | 3.56 | 3.6 | 0.04 | 7.77 | 7.75 | 0.02 |
| 19 | 3.24 | 3.25 | 0.01 | 7.47 | 7.50 | 0.03 | |
|
| |||||||
| .125 or ⅛” | 1 | 2.56 | 2.17 | 0.39 | 6.05 | 4.65 | 1.40 |
| 19 | 2.6 | 2.58 | 0.02 | 5.72 | 7.38 | 1.66 | |
FEV1 = forced expiratory volume in one second
PEF = peak expiratory flow rate
Difference calculated as EasyOne measurement – HF5 measurement
Similarly, `biting down' on the spirette to a ½ inch opening did not alter values appreciably. Further `biting down' to ⅛ inch did affect readings (data not shown) and caused air passing through the spirette and the EasyOne to sound obstructed.
Clinical reproducibility
Characteristics of the 24 spirometry-naïve volunteers are shown in Table 3. The reproducibility of the EasyOne spirometer differed by spirometry measure among these subjects. The CVs were lowest for the FEV1/FVC ratio, the FEV1 and the forced expiratory volume in six seconds (FEV6), and highest for the forced expiratory flow between 25% and 75% of vital capacity (FEF25–75) and PEF (Table 4). The 95% limits of agreement for reproducibility were within the specified limits for the FEV1 but not for the FVC.
Table 3.
Characteristics of participants in clinical reproducibility and validation study
| N (%) | 24 (100) |
|
| |
| Female gender (%) | 12 (50) |
|
| |
| Age (years), mean ± SD | 36.5 ± 13.1 |
|
| |
| Race/ethnicity | |
| White (%) | 3 (13) |
| African American (%) | 6 (26) |
| Hispanic (%) | 9 (39) |
| Asian/Other (%) | 6 (25) |
|
| |
| Smoking status | |
| Never (%) | 17 (71) |
| Former or current (%) | 7 (29) |
|
| |
| Height (cm), mean ± SD | 166.3 ± 9.5 |
|
| |
| Weight (kg), mean ± SD | 72.3 ± 13.0 |
|
| |
| FVC* (L), mean ± SD | 3.90 ± 1.12 |
|
| |
| FEV1† (L), mean ± SD | 3.11 ± 1.03 |
|
| |
| FEV1/FVC, mean ± SD | 0.79 ± 0.07 |
|
| |
| FEV6‡ (L), mean ± SD | 3.81 ± 1.13 |
|
| |
| FEF25–75§ (L), mean ± SD | 3.28 ± 1.45 |
|
| |
| PEF∥ (L/sec), mean ± SD | 8.30 ± 2.55 |
FVC = forced vital capacity
FEV1 = forced expiratory volume in one second
FEV6 = forced expiratory volume in six seconds
FEF25–75 = forced expiratory flow between 25% and 75% of vital capacity
PEF = peak expiratory flow rate
Table 4.
Reproducibility of the EasyOne spirometer in the clinical setting with spirometry naive participants
| Coefficient of variation (95% confidence intervals) | 95% limits of agreement (1.96* SDi) | |
|---|---|---|
| FVC* | 3.3% (2.1 – 4.5) | 0.24 L |
| FEV1† | 2.6% (1.8 – 3.3) | 0.18 L |
| FEV1/FVC | 1.9% (0.9 – 3.0) | 0.05 |
| FEV6‡ | 2.8% (1.7 – 3.8) | 0.23 L |
| FEF25–75§ | 6.8% (3.1 – 10.4) | 0.45 L |
| PEF∥ | 5.1% (3.1 – 7.1) | 1.05 L/sec |
FVC = forced vital capacity
FEV1 = forced expiratory volume in one second
FEV6 = forced expiratory volume in six seconds
FEF25–75 = forced expiratory flow between 25% and 75% of vital capacity
PEF = peak expiratory flow rate
Clinical validation
There were modest mean absolute differences between the two spirometers, with the EasyOne producing statistically significantly lower values for all measures except PEF (Table 5). The 95% limits of agreement were wider, particularly in the negative direction. The limits of agreement were within the specified boundaries for the FVC and the FEV6 but not for the FEV1. Mean differences for FVC and FEV1 were approximately proportional, such that the limits of agreement for the FEV1/FVC ratio were considerably narrower than for either measure alone.
Table 5.
Measures of validity of the EasyOne spirometer compared to laboratory spirometry in the clinical setting with spirometry-naive participants.
| Mean difference (95% confidence intervals) | 95% limits of agreement | Slope of line in Bland-Altman plots of absolute difference vs. mean value | ||
|---|---|---|---|---|
| β | P-value | |||
| FVC* (L) | −0.12 (−0.19, −0.04) | −0.48, 0.25 | −0.05 | 0.14 |
| FEV1† (L) | −0.17 (−0.23, −0.11) | −0.45, 0.11 | −0.06 | 0.04 |
| FEV1/FVC | −0.02 (−0.03, −0.01) | −0.05, 0.02 | 0.06 | 0.26 |
| FEV6‡ (L) | −0.14 (−0.21, −0.07) | −0.48, 0.19 | −0.05 | 0.14 |
| FEF25–75§ (L) | −0.27 (−0.40, −0.13) | −0.93, 0.40 | −0.04 | 0.37 |
| PEF∥ (L/sec) | 0.18 (−0.10, 0.45) | −1.18, 1.54 | 0.05 | 0.45 |
FVC = forced vital capacity
FEV1 = forced expiratory volume in one second
FEV6 = forced expiratory volume in six seconds
FEF25–75 = forced expiratory flow between 25% and 75% of vital capacity
PEF = peak expiratory flow rate
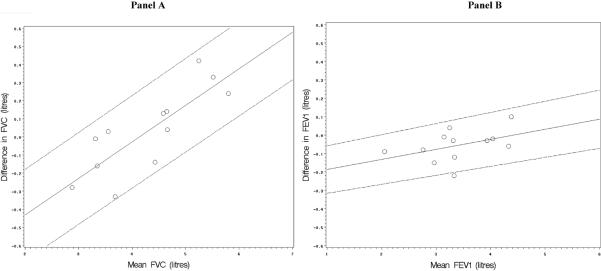
The slopes for FVC and FEV1 in Bland-Altman plots for clinical testing (Figure 4) were slightly negative – in contrast to the results for in-line testing. This proportionality of differences between measures to mean values was general small and not statistically significant with the exception of FEV1, which was just significant (Table 5).
Figure 4.
Bland-Altman plots of the difference vs. mean value in measurements of the forced vital capacity (Panel A) and the forced expiratory volume in one second (Panel B) from the EasyOne and laboratory spirometry in clinical testing among spirometry-naïve volunteers.
The solid lines of the Bland-Altman plots are regression lines of mean differences vs. mean values which allow for proportionality of mean differences vs. mean values. The dashed lines are the 95% limits of agreement around the regression lines.
Discussion
The results of independent laboratory and clinical testing of the EasyOne handheld spirometer are summarized here. Most of the laboratory based testing yielded satisfactory results, although some deviations were noted and are discussed below. In clinical testing, the EasyOne met criteria for reproducibility for the FEV1 but not for the FVC, and met criteria for validity for the FVC but not for the FEV1.
The EasyOne performed well in standardized waveform testing. The in-line testing showed little bias. The mean difference in the FVC was close to zero, and the slightly negative result for the FEV1 was probably attributable to a known error of 1–2% in the reference volume-sensing spirometer due to its the lack of real time BTPS correction [16] rather than to error in the Easyone. The limits of agreement for both the FVC and FEV1 were within the specified criteria for validity. The in-line results were similar to those from a study of children with asthma (mean age 9 years) that compared the EasyOne spirometer in-line to a volume-sensing spirometer [17]. That study found a similar lack of bias and similar limits of agreement – although the percent error on a relative scale in that study was 2–3 times greater given the smaller lung volumes of children.
Bland-Altman plots of in-line testing suggested that differences in FVC were proportional to mean FVC such that larger differences were present at larger values of mean FVC. Similar plots of clinical testing, however, revealed no increase in differences in FVC proportional to mean FVC. The in-line set-up may change the flow profile or increase downstream pressure for the EasyOne, and thereby produce erroneous results (according to discussions with ndd engineers). We were not entirely able to rule out such error via additional testing. However, in-line testing in the much larger sample of children revealed no similar proportionality in the FVC [17]. It is therefore possible that the proportionality for FVC in in-line testing in our study was a false positive finding.
An additional potential source of error in the EasyOne system was the pliable mouthpiece of the spirette. Compression of the spirette mouthpiece or biting down on it did affect measurements at more severe degrees of constriction. Since this testing was performed, however, the manufacturer has re-engineered the spirette to make it less pliable.
We further assessed the reproducibility and validity of the EasyOne compared to a dry rolling seal spirometer in a clinical setting among healthy, spirometry naive volunteers. The reproducibility of the FEV1 was adequate but the reproducibility of the FVC exceeded standard criteria for reproducibility of 200 ml. Both of these findings are consistent with a prior report [10]. Similar variability in the FVC was not evident in wave-form or in-line testing (data not shown), and hence may be related to the clinical use of the EasyOne rather than intra-device measurement error. The EasyOne's LCD display shows a flow-volume loop but not a volume-time curve and the unit relies on audible beeps to determine when plateau has been reached. The reproducibility of the FEV1 may therefore be easier for the technician to establish than the reproducibility of the FVC. Use of a connected personal computer to display both the flow-volume loop and the volume-time curve at the time of testing might improve reproducibility in the FVC; however, we did not test this strategy.
FVC values from the EasyOne were smaller when compared to the laboratory spirometer, which was at variance with the in-line results. However, this decrement was in the range of short-term, within-subject reproducibility of the FEV1 and FVC (about 5% CV or 0.20 L), and thus of limited clinical significance. The EasyOne produced slightly lower FEV1 values during both in-line and clinical testing. The limits of agreement met previously published criteria [10] for validity of the FVC but not of the FEV1.
These findings match and expand the results from a study of a convenience sample of people attending a community health fair. In that study, 32% of participants who underwent screening with the EasyOne returned for laboratory-based testing [9]. The results, although more variable than ours, also showed the mean FEV1 and mean FEV6 measured by the EasyOne to be lower – by 0.25 L and 0.29 L respectively – than laboratory based testing. That difference was probably due to a training effect, since participants performed spirometry first at the community health fair using the Easyone and again, on a separate occasion, using laboratory-based testing. On the other hand, the recent study that compared 10 portable spirometers to laboratory testing in a random fashion among patients with and without COPD found the EasyOne to yield slightly higher results for FEV1 and FVC – by 0.08 an 0.07L respectively – than laboratory based testing [10]. A study from Australia has also demonstrated the long-term reliability of EasyOne spirometers [18].
Our laboratory and in-line testing was not subject to training effects, but our clinical testing may have been. We performed two separate tests on the EasyOne before performing laboratory based spirometry and used the highest values from these tests in an effort to minimize training effect, however we did not randomize the order of testing. Some of the lower values obtained with the EasyOne therefore may have been due to training effect, which may have led to an overestimation of bias. Such a training effect may also have caused the limit of agreement for the FEV1 not to meet criteria for validity since width of the observed limits of agreement for FEV1 (0.56 L) were well within the targeted width (0.70 L) but the downward bias from a training effect resulted in the lower threshold of criteria for validity being crossed. We did not include patients with significant obstructive airways disease in the current study and therefore cannot make firm generalizations to that population. Finally, the EasyOne software (firmware) has been updated several times since our evaluation was completed. These updates may have an influence on the test accuracy and reproducibility of currently available models.
In conclusion, the EasyOne handheld spirometer yielded reproducible results for the FEV1 but not for the FVC in clinical testing. The latter result may be due to operating characteristics of the unit and might be improved by the use of a connected personal computer to allow simultaneous visualization of the flow-volume loop and volume-time curve. In-line testing suggested a lack of bias and valid results. Clinical testing in spirometry naïve volunteers showed validity for the FVC although not the FEV1, principally due to a training effect. The use of this spirometer in general clinic settings and for research and occupational purposes seems justified.
Acknowledgements
The EasyOne spirometers and spirettes were purchased from the U.S. distributor at standard government and institutional prices. The authors thank Paul Enright, MD, for their helpful comments on the manuscript.
Funding: This work was supported by grants HL075476 and HL077612 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Footnotes
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of any company or product does not constitute endorsement by NIOSH.
References
- 1.Mannino DM, et al. Chronic obstructive pulmonary disease surveillance -- United States, 1971–2000. Surveillance Summaries, August 2, 2002. MMWR. 2002;51(SS-6):1–8. [PubMed] [Google Scholar]
- 2.Ferguson GT, et al. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–61. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 3.Enright PL, Kaminsky DA. Strategies for screening for chronic obstructive pulmonary disease. Respir Care. 2003;48:1194–1201. [PubMed] [Google Scholar]
- 4.Hankinson JL. Beyond the peak flow meter: newer technologies for determining and documenting changes in lung function in the workplace. Occupational Medicine. 2000;15(2):411–20. [PubMed] [Google Scholar]
- 5.Enright P. Spirometers' technological evolution spurs increased confidence in results. Four generations of advances enable more useful diagnoses for asthma, emphysema or tuberculosis. Occup Health Saf. 1994;63:81–84. [PubMed] [Google Scholar]
- 6.Menezes AM, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 7.Buist AS, et al. The Burden of Obstructive Lung Disease initiative (BOLD): rationale and design. J COPD. 2005;2:277–283. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Physical health status of World Trade Center rescue and recovery workers and volunteers - New York City, July 2002-August 2004. MMWR Morb Mortal Wkly Rep. 2004;53:807–12. [PubMed] [Google Scholar]
- 9.Schoh RJ, et al. Performance of a new screening spirometer at a community health fair. Respir Care. 2002;47:1150–57. [PubMed] [Google Scholar]
- 10.Liistro G, et al. Technical and functional assessment of 10 office spirometers: a multicenter comparative study. Chest. 2006;130:657–65. doi: 10.1378/chest.130.3.657. [DOI] [PubMed] [Google Scholar]
- 11.EasyOne Model 2001 diagnostic spirometer website. 2006 www.ndd.ch/English/Products/EasyOne_fs.html.
- 12.EasyOne Model 2001 diagnostic spirometer website. 2006 www.ndd.ch/English/Technology/Technology_fs.html.
- 13.American Thoracic Society Standardization of spirometry. 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson J, Odencrantz J, Fedan K. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical method for assessing agreement between two methods of clinical measurement. The Lancet. 1986;i:307–10. [PubMed] [Google Scholar]
- 16.Johnson LR, et al. Volume spirometers need automated internal temperature sensors. Am J Respir Crit Care Med. 1994;150:1575–80. doi: 10.1164/ajrccm.150.6.7952617. [DOI] [PubMed] [Google Scholar]
- 17.Mortimer KM, et al. Evaluating the use of a portable spirometer in a study of pediatric asthma. Chest. 2003;123(6):1899–907. doi: 10.1378/chest.123.6.1899. [DOI] [PubMed] [Google Scholar]
- 18.Walters JAE, et al. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology. 2006;11:306–10. doi: 10.1111/j.1440-1843.2006.00842.x. [DOI] [PubMed] [Google Scholar]