Abstract
Persistence of effector cytotoxic T lymphocytes (CTLs) during an immunological response is critical for successfully controlling a viral infection or tumor growth. Various cytokines are known to play an important part in regulating the immune response. The IL-2 family of cytokines that includes IL-2 and IL-15 are known to function as growth and survival factors for antigen-experienced T cells. IL-2 and IL-15 possess similar properties, including the ability to induce T cell proliferation. Whereas long term IL-2 exposure has been shown to promote apoptosis and limit CD8+ memory T cell survival and proliferation, it is widely believed that IL-15 can inhibit apoptosis and helps maintain a memory CD8+ T-cell population. However, mechanisms for superior outcomes for IL-15 as compared to IL-2 are still under investigation. Our data shows that human T cells cultured in the presence of IL-15 exhibit increased expression of anti-oxidant molecules Glutathione reductase (GSR), Thioredoxin reductase 1 (TXNDR1), Peroxiredoxin (PRDX), Superoxide dismutase (SOD). An increased expression of cell-surface thiols, intracellular glutathione, and thioredoxins was also noted in IL-15 cultured T cells. Additionally, IL-15 cultured T cells also showed an increase in cytolytic effector molecules. Apart from increased level of Granzyme A and Granzyme B, IL-15 cultured T cells exhibit increased accumulation of reactive oxygen (ROS) and reactive nitrogen (RNS) species as compared to IL-2 cultured T cells. Overall, this study suggests that T cells cultured in IL-15 show increase persistence not only due to increased anti-apoptotic proteins but also due to increased anti-oxidant levels, which is further complimented by increased cytolytic effector functions.
Keywords: Cytokine, thiols, apoptosis
1. Introduction
Expansion of the CTL population and maintenance of its long-term functional activity is central to the success of T cell immunotherapy [1]. Various strategies are being developed to increase T-cell persistence to control tumor growth or viral infections [2-5]. To this end, the role of cytokines in maintaining T cells growth is well known and has been widely exploited in T-cell immunotherapy [6-7]. In fact, immunotherapy protocols employing IL-2 as a T-cell growth/activation factor have been used extensively for treating patients with metastatic melanoma and renal cell carcinoma [6, 8]. IL-15, a member of the same cytokine family as IL-2, is considered to be more efficacious than IL-2 in immunotherapy regimens [7, 9-12], but the mechanisms for this increased efficacy are still under investigation [11, 13-14].
An important aspect of increased efficacy of IL-15 as compared to IL-2 is attributed to its anti-apoptotic activity, enhancing the survival of effector and memory CD8+ CTLs [15-18]. Earlier studies suggest that despite the differences in anti-apoptotic protein bcl-xl expression IL-15 equally promotes the survival of CD8+ T cells with both naïve and activated phenotype as determined by expression of CD44 – an adhesion molecule that is expressed at low levels in naïve and at high levels in activated T cells [19]. Thus, the mechanism how IL-15-mediated rescue from T-cell apoptosis occurs is not completely understood. Here, we show that IL-15 pretreated T cells have higher level of free sulfhydryl groups (-SH; also referred as thiol) on cell surface and intracellular glutathione (iGSH) compared to IL-2 treated T cells. Increase in these key anti-oxidants are accompanied by elevated thioredoxins (Trx) in IL-15 treated cells irrespective of the phenotype. Interestingly, an evaluation of antioxidant and oxidative stress related genes on T cells cultured in the presence of IL-15 vs. IL-2 revealed that IL-15 induced up-regulation of 11 genes whereas 8 genes were down-regulated. Further our data also suggests that when T cells cultured in presence of IL-15 were compared to the ones cultured in IL-2 an increase in expression of cytolytic proteins Granzyme A, Granzyme B as well as increased accumution of reactive oxygen (ROS) and nitrogen species (RNS) upon restimulation was observed. Thus, while increased antioxidant level in T cells cultured with IL-15 could protect against oxidative stress-induced cell death, a concomitant increase in cytolytic molecules support the increased effector function.
2. Materials and Methods
2.1. Cells, culture medium, and reagents
Peripheral blood mononuclear cells (PBMC) from healthy donors were obtained after approval by institutional review board from a commercial vendor Research Blood Components, LLC (Brighton, MA, USA). Culture medium was Iscove’s Modified Dulbecco’s Medium (GIBCO BRL, Grand Island, NY) supplemented with 10 % fetal bovine serum (Gemini Bioproducts Inc., Calabasas, CA). Ficoll-Paque was obtained from Amersham Bioscience (Piscataway, NJ). Recombinant cytokines were purchased from R & D Systems (Minneapolis, MN).
2.2. Hydrogen peroxide mediated cell death
Hydrogen peroxide (H2O2) was used to mimic conditions of oxidative stress as described previously [20]. T cells obtained from PBL and expanded in the presence of either IL-2 (100 IU/ml) or IL-15 (10 ng/ml) were cultured with different concentrations (5–200 μM) of H2O2 for 24–48 hrs. Using a flow cytometry-based method, cell death measurements were performed by staining for Annexin V according to the manufacturer’s protocol (BioVision, Mountain View, CA) and acquired on a FACSCalibur (Becton Dickinson, Mountain View, CA) or an Accuri C6 (Accuri Cytometers, Ann Arbor, MI) flow cytometer. Data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
2.3. Western blot
Protein extractions were performed in RIPA buffer and samples were separated on 15% SDS polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes (Millipore, Burlington, MA) as described earlier [21]. The primary antibodies (anti-Trx, anti-GAPDH) and secondary antibodies were obtained from Cell Signaling Technology (Danvers, MA) or Santa Cruz Biotechnology, (Santa Cruz, CA).
2.4. Staining for intracellular GSH and surface thiols
Intracellular GSH was evaluated using Monochlorobimane (MCB) staining with slight modification as described earlier [22-23]. Monochloromobimane is essentially non-fluorescent until conjugated, readily reacts with low molecular weight thiols. Briefly, 1-2 × 106 cells were stained with 40 μM MCB (Molecular Probes, Eugene, OR) in ice for precisely 15 min that forms the blue fluorescent adduct with iGSH. Cells were chilled by addition of ice-cold FBS at 0°C to stop the enzyme dependent staining reaction. Cells were then washed and maintained at 4°C during the remaining treatments. 2.5 mM Probenecid was added to the staining medium to prevent outflow of the fluorescent MCB conjugate (GSB) during the MCB staining step and thereafter. Cells were excited with a UV 405 nm laser, and emission was acquired with a 440/40 filter.
To stain cell surface thiols, Alexa Fluor® 633 C5 maleimide dye (ALM) (Invitrogen, Carlsbad, CA) was used. Alexa dyes coupled to maleimide (ALMs) do not interfere with the efficieny of the maleimide-thiol reaction. They are charged molecules, do not enter cells and can be detected by FACS and reveal the overall thiol levels present on the cell surface. Briefly, 1-2 × 106 cells were washed with 1X PBS, and 1 μM ALM was added in cell suspension and incubated for 10 min on ice. Cells were then extensively washed and kept at 4°C.
2.5. Measurement of mitochondrial membrane potential (Δψ)
Δψ was estimated by staining with 20 nmol/L 3,3′-dihexyloxacarbocyanine iodide (DiOC6) (Molecular Probes, Eugene, OR), a cationic lipophilic dye, for 10 min at 37°C in the dark before analysis by flow cytometry. Fluorescence of DiOC6 is oxidation independent and correlates with Δψ [24].
2.6. Determination of intracellular reactive oxygen species (ROS)
Dihydroethydium (DHE) (Sigma Aldrich, USA), 5-(and -6) carboxy -2 ′ , 7 ′-dichlorodihydrofluorescein diacetate (Carboxy-H2DCFDA) (Molecular Probes, Eugene, OR), an ROS sensitive dyes, were used to evaluate the intracellular ROS level. T cells were stained with HE (1 μM) or CMH2-DCFDA (10 μm) for at 37°C for 10 min. The intracellular ROS were measured by flow cytometry. At least 10,000 cells were analyzed in each group using FACScalibur (BD Instruments, Mountain View, CA).
2.7. Intracellular nitric oxide (NO) determination
T cells were loaded with Diacetate (4-amino -5 methylamino- 2′, 7′- difluorescein diacetate) (DAF-FM) (1 μm; Molecular Probes, Eugene, OR) and incubated (10 min, 37°C) in the dark. Care was taken to prevent exposure to light throughout the rest of the experimentation, as the probe is light sensitive. After incubation with DAF-FM, the cells were analysed by flow cytometry. At least 10,000 cells were analyzed.
2.8. Total RNA Extraction and Reverse Transcription
T cells cultured and maintained in the presence of IL-2 and IL-15 from three different donors for 10 days. 5×106 cells from each treatment were pellet down and total RNA was isolated by TRIzol (Invitrogen, Carlsbad, CA) Reagent method. Reverse transcript (the cDNA) was synthesized from 2.5μg total RNA using iScript cDNA Synthesis Kit (SABiosciences Corp, Frederick, MD). Reverse transcript PCR quantification was performed. Briefly, 50μl reaction mix contained: cDNA, 10X PCR buffer, dNTPs, Taq DNA polymerase and primers at final concentration of 20 μM. The sequences of the primers used for RT PCR are bcl-2 (forward, 5′-GGAGGATTGTGGCCTTCT-3′; reverse, 5′- GTTATCCTGGATCCAGGTGT-3′), bcl-xl (forward, 5 ′-CTTGGATGGCCACTTACCTGA-3′; reverse 5 ′-TCTTCTGGTCATTTCCGACTGAAG), iNOS (forward, 5′-GTTCTCAAGGCACAGGTCTC-3 ′ ; reverse 5 ′-GCAGGTCACTTATGTCACTTATC-3 ′ ) T-bet (forward, 5 ′-CCCCCAAGGAATTGACAGTTG-3 ′ ; reverse 5 ′-GGGAAACTAAAGCTCACAAAC-3′), GAPDH (forward, 5 ′-GAAGATGGTGATGGGATTTC-3 ′ ; reverse 5′-GAAGGTGAAGGTCGGAGTC -3′). PCR products were separated by 1% agarose gel.
2.9. Real Time PCR-Based Array Analysis
The relative expression of genes involved in oxidative stress was determined in each of the four RNA samples from two different donors treated with IL-2 or IL-15 alone for 7 days using an RT2 Profiler™ Human Oxidative Stress and Antioxidant Defense PCR Array (Cat. No. PAHS-065, SABiosciences Corp, Frederick, MD) that profiles the expression of 84 genes related to oxidative stress. Equal amounts of total RNA from each sample were reverse transcribed to form cDNA. Samples were diluted in RT2 SYBR® Green qPCR master mix according to the supplier’s directions and pipetted into 96-well PCR array plates to evaluate the expression of 84 oxidative stress and antioxidant defense related genes. Real-time PCR was performed in technical duplicates using a Bio Rad iCycler Detection System. Raw data from the real-time PCR was uploaded using the RT2 Profiler PCR Array Data Analysis Template available at SABiosciences web site. Quality controls included within the array plates confirmed the lack of DNA contamination and successfully tested for RNA quality and PCR performance. The integrated web-based software package for the PCR array system automatically performed all comparative threshold cycle based fold-change calculations from the up-loaded data. For these calculations, the average expression of three housekeeping genes (hypoxanthine, beta-actin and ribosomal protein L13a) was used for the normalization of the data. After normalization, the relative change in the expression of each gene ≥ 2-fold in IL-15 treated cultures to that of IL-2 from two different donors was considered to be significant.
3. Results
3.1. Effect of IL-2 and IL-15 on T-cell apoptosis
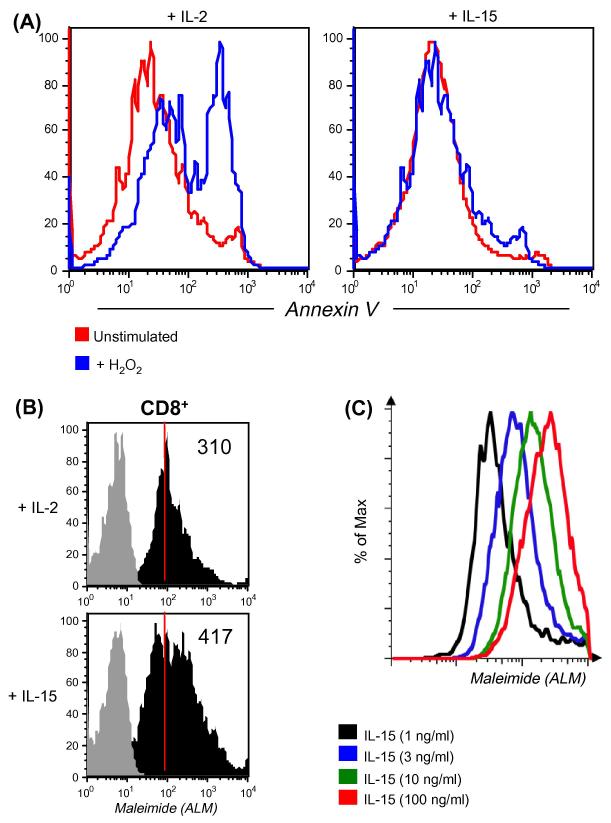
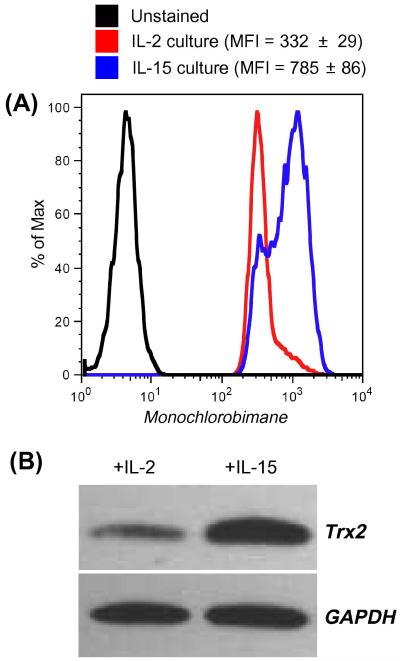
Human T cells were cultured with IL-2 and IL-15 for 7 days to evaluate the effect of IL-2 and IL-15. Our data indicate that oxidative stress-mediated apoptosis induced by H2O2 was more prominent in T cells cultured with IL-2 than in those cultured with IL-15 (Figure 1A). These results are similar to those shown by earlier studies highlighting anti-apoptotic characteristic of IL-15 [25-27]. However, we reasoned that for an oxidative agent such as H2O2 to induce cell death differentially, differences must exist in reduced or oxidized surface molecules/proteins which could correlate to the cytokines used for pretreatment and hence the difference in susceptibility to H2O2-mediated apoptosis. Because recent studies have implicated reduced thiols (cysteine –SH) in the function of individual cell surface proteins [28-29], we evaluated the level of cell surface thiols (cs-SH) on T cells cultured in IL-2 and IL-15 using fluorochrome conjugated melamide dye as described earlier [29]. Our data show that CD8+ T cells cultured in IL-15 had more cs-SH expression compared to those cultured in IL-2 (Figure 1B). Importantly, a dose-response analysis performed after culturing CD3+ T cells in the presence of increasing concentrations of IL-15 revealed a linear increase in cs-SH (Figure 1C), thereby favoring a direct role of IL-15 in regulating T cell thiols. It has also been documented that overall cs-SH content on cell surface molecules could be correlated to the level of intracellular glutathione (iGSH) [30]. Measuring iGSH on CD8+ T cells using the monochlorobimane staining [28] revealed higher expression of this key anti-oxidant molecule in cells cultured in the presence of IL-15, as opposed to cells cultured in the presence of IL-2 (Figure 2A). A further evaluation of Thioredoxins (Trx), proteins that acts as antioxidant by facilitating the reduction of other protein by cysteine thiol-disulfide exchange [31], revealed increased in mitochondria specific Trx-2 in T cells cultured with IL-15 (Figure 2B). These data suggest that increased level of reduced –SH groups and iGSH after IL-15 treatment could be responsible for the increased ability of T cells to persist in a tumor-induced oxidative stress microenvironment. Next we investigated whether these protective effects of IL-15 could be due to the differential expression level of genes involved in oxidative stress and ROS metabolism.
Figure 1. Effect of IL-15 on T cells.
(A). CD8+ T cells cultured in the presence of IL-2 and IL-15 for 7 days were exposed to 25 μM of H2O2. Cells were stained for CD8 and Annexin V. Histogram represents level of Annexin V on cells with or without H2O2 stimulation after 16 h.
(B). CD8+ T cells cultured in the presence of IL-2 and IL-15 for 7 days were evaluated for expression of cell surface thiols using fluorochrome conjugated maleimide (ALM). Grey histogram represents unstained, whereas black histogram represents fluorescence from stained samples. Numerical values on right corner represent Mean Fluorescence Intensity (MFI).
(C). Level of surface thiols on the T cells cultured for 7 days in presence of increasing dose of IL-15 was evaluated using fluorochrome conjugated maleimide (ALM). Each histogram represents fluorescence on cells obtained after co-culture. Data shown in A, B and C are from one of three experiments with similar results.
Figure 2. Expression of intracellular glutathione and thioredoixn-2 in IL-2 and IL-15 cultured T cells.
(A). CD8+ T cells cultured in the presence of IL-2 and IL-15 for seven days were tested for expression of intracellular glutathione using monochlorobimane. Histogram represents fluorescence from stained samples.
(B). Cell lysates were prepared using CD8+ T cells cultured in the presence of IL-2 and IL-15 for 7 days and probed for the expression of thioredoxin-2. Blots were probed with GAPDH for loading control. Data shown in A and B are from one of three experiments with similar results.
3.2. Expression of Oxidative stress and antioxidative defense related genes
A Real time PCR-based array was used to compare the relative expression of 84 oxidative stress and antioxidant defense related genes in IL-2 and IL-15 expanded T cell cultures from two different HLA-A2-positive healthy donors. Data from the IL-15 culture was then compared with that from the IL-2 culture. A relative change in expression of genes ≥ 2 fold was considered to be significant. As detailed in Table 1 of the 84 genes tested, 19 were uniquely differentially expressed (11 over-expressed and 8 under-expressed). Genes that were significantly overexpressed included FOXM1 (Forkhead box M1), GSR (Glutathione reductase), MLT5 [Metallothionein-like 5, testis-specific (tesmin)], NUDT1 Nudix (nucleoside diphosphate linked moiety X)-type motif 1, IPCEF1 (Interaction protein for cytohesin exchange factors 1), PRDX1 (Peroxiredoxin 1), PRDX2 (Peroxiredoxin 2), SIRT2 [Sirtuin (silent mating type information regulation 2 homolog)2 (S. cerevisiae)], SOD1 (Superoxide dismutase 1, soluble), SOD2 (Superoxide dismutase 2, soluble) and TXNRD1 (Thioredoxin reductase 1). Genes that were significantly under-expressed in IL-15 cultures included CYGB (Cytoglobin), DUSP1 (Dual specificity phosphatase 1), MPO (Myeloperoxidase), NOX5 (NADPH oxidase, EF-hand calcium binding domain 5), PRG3 (Proteoglycan 3), PTGS2 [Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase)], PXDNL [Peroxidasin homolog (Drosophila)-like], TPO (Thyroid peroxidase), TXNDC2 [Thioredoxin domain containing 2 (spermatozoa)]. After individually confirming the Real-Time PCR results of key anti-oxidant molecules that were up-regulated in presence of IL-15 we then investigated its ability to modulate anti-apoptotic and cytolytic molecules in T cells.
Table 1.
Oxidative stress and antioxidant defense related genes analyzed by real time PCR array.
| UniGene ID |
Accession No. |
Symbol | Description | Fold change |
|---|---|---|---|---|
| Genes Up- regulated in presence of IL-15 as compared to IL-2 | ||||
| Hs.239 | NM_021953 | FOXM1 | Forkhead box M1 | 14.25 |
| Hs.271510 | NM_000637 | GSR | Glutathione reductase | 2.89 |
| Hs.145932 | NM_004923 | MLT5 | Metallothionein-like 5, testis-specific (tesmin) |
2.35 |
| Hs.534331 | NM_002452 | NUDT1 | Nudix (nucleoside diphosphate linked moiety X)-type motif 1 |
5.40 |
| Hs.146100 | NM_015553 | IPCEF1 | Interaction protein for cytohesin exchange factors 1 |
3.32 |
| Hs.180909 | NM_002574 | PRDX1 | Peroxiredoxin 1 | 2.83 |
| Hs.432121 | NM_005809 | PRDX2 | Peroxiredoxin 2 | 3.25 |
| Hs.466693 | NM_012237 | SIRT2 | Sirtuin (silent mating type information regulation 2 homolog) 2 (S. cerevisiae) |
3.82 |
| Hs.443914 | NM_000454 | SOD1 | Superoxide dismutase 1, soluble |
12.41 |
| Hs.487046 | NM_000636 | SOD2 | Superoxide dismutase 2, mitochondrial |
9.40 |
| Hs.708065 | NM_003330 | TXNRD1 | Thioredoxin reductase 1 | 5.04 |
| Genes Down- regulated in presence of IL-15 as compared to IL-2 | ||||
| Hs.95120 | NM_134268 | CYGB | Cytoglobin | −19.25 |
| Hs.171695 | NM_004417 | DUSP1 | Dual specificity phosphatase 1 |
−11.06 |
| Hs.458272 | NM_000250 | MPO | Myeloperoxidase | −16.76 |
| Hs.657932 | NM_024505 | NOX5 | NADPH oxidase, EF-hand calcium binding domain 5 |
−4.19 |
| Hs.251386 | NM_006093 | PRG3 | Proteoglycan 3 | −5.53 |
| Hs.196384 | NM_000963 | PTGS2 | Prostaglandin- endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
−3.40 |
| Hs.444882 | NM_144651 | PXDNL | Peroxidasin homolog (Drosophila)-like |
−3.17 |
| Hs.467554 | NM_000547 | TPO | Thyroid peroxidase | −3.17 |
| Hs.98712 | NM_032243 | TXNDC2 | Thioredoxin domain containing 2 (spermatozoa) |
−2.96 |
3.3. Induction of anti-apoptotic and cytolytic protein expression following IL-15 stimulation
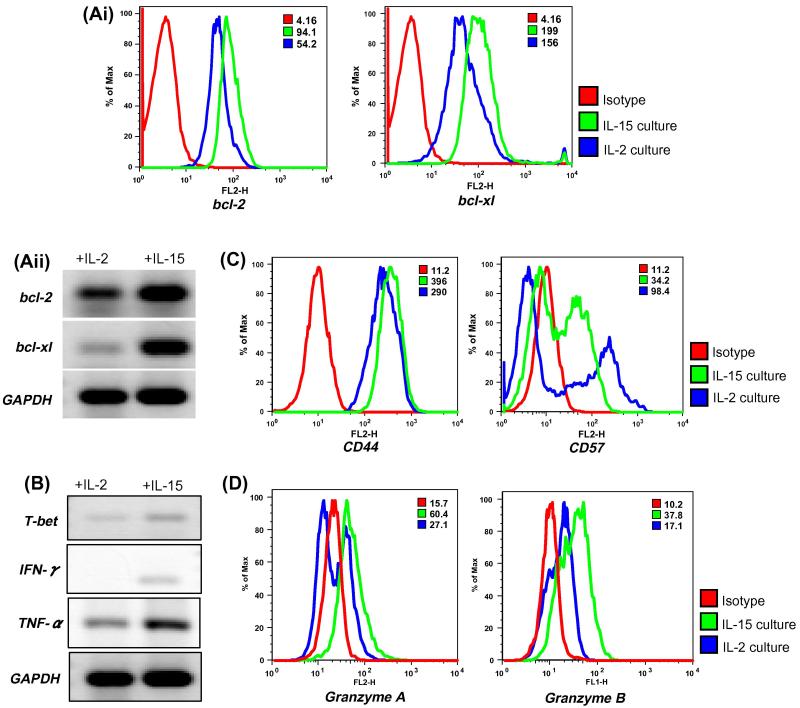
While we uncovered the novel role of IL-15 in up-regulating the antioxidant capacity of a T cell we wanted to confirm the role of IL-15 in up-regulating anti-apoptotic protein as has been shown earlier [32]. Thus, T cells were expanded from PBMC of healthy donors in the presence of IL-2 or IL-15 for 7 days. Total RNA was isolated and mRNA expression of key anti-apoptotic molecules bcl-2, bcl-xl and Th1 transcription factor T-bet along with cytokines IFN-γ and TNF-α was assessed. Our data showed that cells cultured in the presence of IL-15 exhibit elevated levels of bcl-2 and bcl-xl as evaluated both by flow cytometery and RT-PCR (Figure 3Ai & Figure 3Aii). Similarly, increased transcripts for T-bet, a Th1-specific T box transcription factor that controls the expression of Th1 cytokine [33] was noted in T cells cultured in presence of IL-15 as compared to IL-2. Additionally, IFN-γ and TNF-α transcripts were also found to be elevated in IL-15 cultures (Figure 3B). Increased cytokine secretion from T cell cultured in IL-15 was also accompanied by increased cell surface expression of CD44 – a widely expressed adhesion receptor that is an indicator of a previous immune response and elevated on the surface of memory T cells [34]. In addition, a comparison of T cells cultured in IL-15 vs. IL-2 showed reduced expression of CD57 - a marker for replicative senescence and immune deficiency on IL-15 cultured derived T cells (Figure 3C) [35]. The expression levels of CD44 and CD57 on CD8+ T cells obtained after in vitro expansion with IL-15 supports the fact that IL-15 results in increased persistence and higher cytokine accumulation upon re-stimulation [36].
Figure 3. Comparison of IL-15 and IL-2 treatment on expression of anti-apoptotic proteins and cytokine secretion.
(Ai). IL-15 and IL-2 cultured CD8+ T cells were used to determine the expression level of anti-apoptotic proteins bcl-2 and bcl-xl. Histograms represent fluorescence intensity of proteins after intracellular staining. Red histogram represents isotype, whereas blue and green histogram represents fluorescence from stained samples. Numerical values on right corner represent Mean Fluorescence Intensity (MFI).
(Aii). T cells cultured in the presence of IL-2 and IL-15 for 7 days were used to extract total RNA and RT PCR was performed as described in Material and Methods. Expression of bcl-2, bcl-xl, and GAPDH was assessed.
(B). T cells were expanded in the presence of IL-2 or IL-15 for 7 days. Total RNA was isolated and mRNA expression levels of T-bet, IFN-γ and TNF-α were evaluated by RT-PCR.
(C). Cell surface marker expression on CD8+ T cells gated population obtained from culture maintained in IL-2 or IL-15 was determined by using fluorochrome conjugated antibodies for CD44and CD57. Histogram represents fluorescence from stained samples. Numerical values on right corner represent Mean Fluorescence Intensity (MFI).
(D). CD8+ T cells expanded in either in IL-2 or IL-15 was evaluated for the expression of Granzyme A and Granzyme B. Histogram represents fluorescence from stained samples. Data shown in A - D are from one of three experiments with similar results.
To further compare the effect of IL-15 vs. IL-2 on cytolytic capacity of T cells we evaluated the expression levels of cytolytic molecules using flow cytometry. We observed that CD8+ T cells when cultured and maintained in IL-15 exhibits higher expression of Granzyme A and Granzyme B as compared to IL-2 (Figure 3D). The expression of Granzyme A and Granzyme B was not detected in fresh isolated CD8+ T cells (data not shown). The above data confirms that IL-15 promotes antigen independent in vitro expansion, promotes level of cytolytic molecules and long-term survival of cytotoxic T lymphocytes [37-39]. Since, IL-15 has been recently shown to possess innate anti-tumor activity independent of NK and CD8+ T cells [40] we further examined the potential of IL-15 cultured T cells in contributing to ROS accumulation and innate immunity [41].
3.4. Effect of IL-15 on Reactive Nitrogen Species (RNS) and Reactive Oxygen Species (ROS)
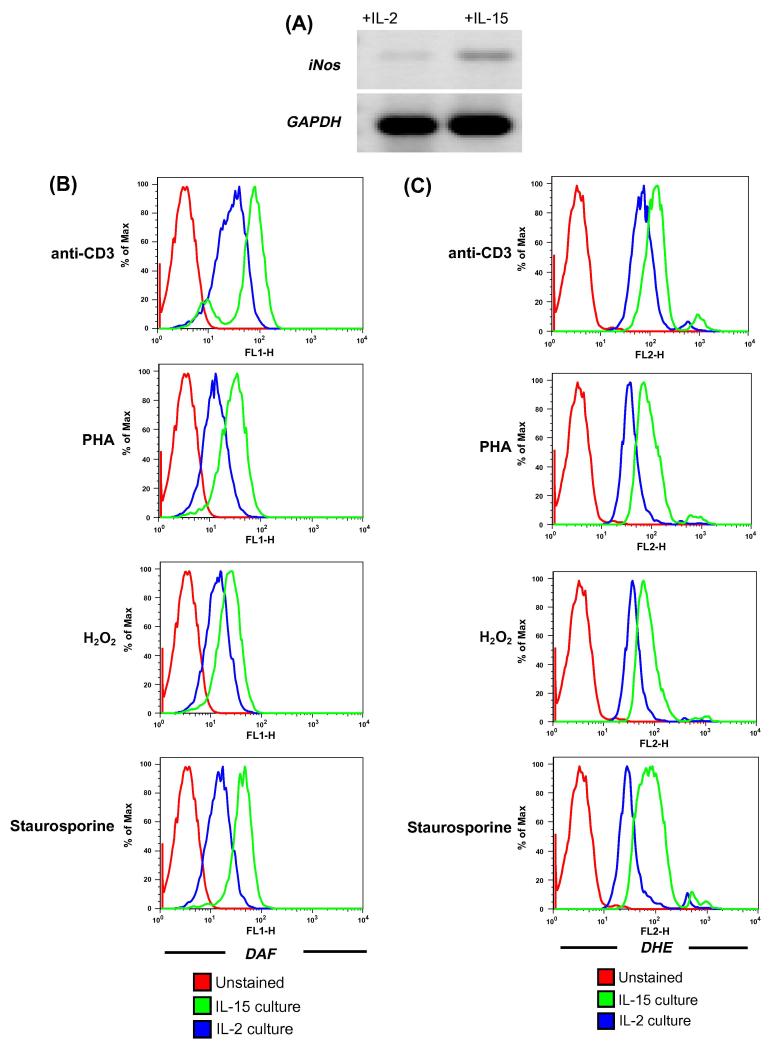
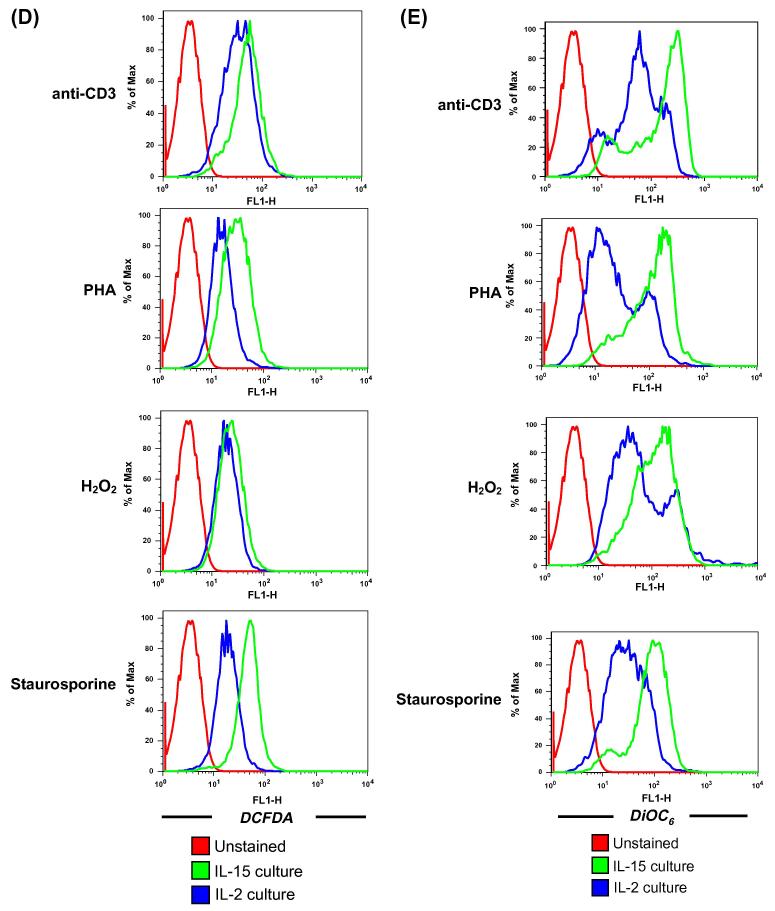
Reverse transcription PCR-based analysis for inducible nitric oxide synthase (iNOS) showed elevated levels of iNOS (Figure 4A) in cells obtained from culture maintained in IL-15 than in IL-2. We further tested if increased iNOS expression in IL-15 cultured T cells results in increased generation of NO and other free radical moieties in CD8+ T cells using flow cytometry. T cells expanded and maintained in IL-2 and IL-15 cytokine were activated with different stimuli: anti-CD3 for TCR engagement, H2O2 for oxidative stress, Phytohaemagglutinin – a lectin, and Staurosporine - a general kinase inhibitor. Our data shows that IL-15 cultured T cells showed increase generation of NO as measured by Diacetate (4-amino -5 methylamino- 2′, 7′-difluorescein diacetate) (DAF-FM) using flow cytometry (Figure 4B). Since, free radical are cytolytic and could result for increased overall cytolytic ability of a T cell we further compared the level of other reactive oxygen species between IL-15 vs. IL-2 cultures. Our data shows that T cell cultured in presence of IL-15 showed increase generation of superoxide and hydrogen peroxide as measured by Dihydroethidium (DHE) (Figure 4C) and 5-(and -6) carboxy -2′, 7′-dichlorodihydrofluorescein diacetate (DCFDA) staining respectively (Figure 4D). Further, evaluation of mitochondrial membrane potential – a hallmark of apoptosis – shows that under different conditions of stimulation tested the T cell cultured in presence of IL-15 maintained membrane integrity better than IL-2 cultured T cells (where an increased loss of membrane potential was noticeable) (Figure 4E). These results indicate that IL-15 play an important role in inducing cytotoxic activity through free radical induction while avoiding apoptosis.
Figure 4. Comparison of IL-15 vs. IL-2 on free radical accumulation and mitochondrial membrane potential.
(A). T cells cultured and maintained in the presence of IL-15 or IL-2 for 7 days were evaluated for iNOS expression by RT-PCR.
(B-E). CD8+ T cells expanded and maintained in IL-2 and IL-15 were restimulated with anti-CD3 (5μg/ml), PHA (1 μg/ml), H2O2 (100 μM) for overnight and Staurosporin for 2 hours and followed by staining with: (B) DAF to track NO; (C) DHE to track superoxide; (D) DCFDA to track hydrogen peroxide; and (E) DiOC6 to track mitochondrial membrane potential. Histograms represent fluorescence intensity of DAF, DHE, DCFDA and DiOC6 on the CD8+ T cells. Data shown in Figure A - D are from one of three experiments with similar results.
4. Discussion
The immune system is primarily dedicated to the maintenance of innate and adaptive immunity via the involvement of various immune mediators (as cytokines, chemokines etc.) or through executioner cells (as T, B, NK cells etc). Some degree of redundancy in the function of various cytokines or the lymphocyte population exists; however, the mechanisms for such observations are unclear. For the IL-2 family of cytokines, which includes IL-2 and IL-15, this redundancy can be explained by the sharing of the common receptor subunits. However, distinct actions of IL-2 and IL-15 in AICD and in the survival of memory phenotype CD8+ T cells have been highlighted [11]. Although IL-2 is an important growth and survival factor, it also plays a pivotal role in AICD [18, 42]. IL-15, in contrast, extends the survival of lymphocytes by acting as a growth factor and by inhibiting IL-2-mediated CD4+ T cell AICD [16]. The mechanism underlying AICD inhibition by IL-15 has not been completely defined. Our data show that, in addition to increased bcl-2 and bcl-xl, IL-15 confers protection against apoptosis by increasing the anti-oxidant capacity of T cells as indicated by increased cell surface thiol, iGSH in an antigen independent manner in T cells. This data is in line with earlier reports of an equivalent effect of IL-15 on both naïve and memory T cells [19]. The IL-15-mediated increase in thiols that protect cells from reactive oxygen species (ROS) and imparts an antioxidant capacity is also supported by over-expression of several antioxidant defense related genes as mentioned in Table 1.
Importantly, the increased anti-oxidant capacity in the presence of IL-15 attributed to the increased expression of thiols is novel. The thiol levels can be manipulated in vitro by altering iGSH [30]. iGSH, a cysteine-containing tripeptide (γ-glutamylcysteinylglycine), and its oxidized dimer GSSG, have key roles in regulating the intracellular redox balance and the status of -SH groups on proteins and other molecules [43]. Results of the real-time PCR based array provide the evidence that levels of gluthathione reductase (GSR), peroxiredoxin 1 (PRDX1) and peroxiredoxin 2 (PRDX2) were increased in the presence of IL-15 as compared to IL-2. Interestingly, superoxide dismutase-1 (SOD1), superoxide dismutase-2 (SOD2) and sirtuin 2 (SIRT2) levels were also elevated in IL-15 cultures. The enzyme GSR is essential for the functioning of reduced glutathione (GSH). GSR catalyzes the reaction that converts oxidized glutathione (GSSG) to GSH using NAD(P)H as an electron donor [44]. GSR plays a key role in response to oxidative stress by maintaining the intracellular pool of GSH. Upregulated GSR levels protect cell mitochondria from thiol oxidative-stress induced injury, thereby preventing dysfunction or cell death [45]. Similarly, the expression of SIRT2 - a member of a highly conserved gene family (sirtuins) encoding nicotinamide adenine dinucleotide (NAD)(+)-dependent deacetylases - that function as a deacetylase for numerous protein targets involved in various cellular pathways, including stress responses, apoptosis was found to be upregulated in presence of IL-15 than IL-2. We also observed an up-regulation of Prdx-1 and Prdx-2 in IL-15 cultures. Prdxs are antioxidant enzymes that have peroxidase functions and are found in both the cytoplasm and nucleus and work with thioredoxin-1 to effectively detoxify hydrogen peroxide (H2O2). In addition, elevated Thioredoxin (Trx) and Thioredoxin reductase1 (TXNRD1) expression supports our observation that IL-15 increases anti-oxidant capacity of T cells. Trx are a vital component of the thiol-reducing system and regulate various cellular functions (redox regulation) [46-47]. Members of the Trx system regulate apoptosis through a wide variety of mechanisms. Previous studies have shown that mitochondrial Trx-2 is essential for cell viability and plays a crucial role in the scavenging ROS in mitochondria and in regulating the mitochondrial apoptosis signaling pathway [48]. TXNRD1 are a family of selenium-containing pyridine nucleotide-disulphide oxidoreductases with mechanistic and sequence identity to glutathione reductases. TrxRs catalyse the NADPH-dependent reduction of the redox protein thioredoxin (Trx), as well as of other endogenous and exogenous compounds [49]. TXNRD1, either alone or in combination with Trx, has also been shown to interact with apoptosis signal-regulating kinase 1 (ASK-1). Thus, this system is also a sensor of oxidative stress [50] and involved in various cellular processes, including proliferation, apoptosis and differentiation. In fact, recent progress in the understanding of the cellular function of the TXNRD1 and Trx systems has focused on the regulatory roles of these enzymes in ROS-mediated signalling [51]. The expression levels of Nudix (nucleoside diphosphate linked moiety X) type motif 1 (NUDT1) were higher in the presence of IL-15. This is an antimutagenic gene, encodes for an enzyme that can hydrolyze 8-oxo-dGTP to 8-oxo-dGMP NUDT1 [52], thereby preventing the mis-incorporation of 8-oxo-dGTP produced by reactive oxygen species normally formed during cellular metabolic processes. The expression of Forkhead box M1 (FOXM1) was also found to be elevated in IL-15 cultures. FOXM1 is known to accelerate cell entry into S phase and mitosis and earlier expression of genes involved in cell cycle progression and proliferation.
On the other hand, Real-time PCR based array data also showed that NADPH oxidase, EF-hand calcium binding domain 5 (NOX5) expression was decreased in the presence of IL-15 by 4.19-fold in comparison with IL-2. NOX enzymes reduce molecular oxygen to superoxide that can be further converted to various secondary ROS. Since several factors other than Nox family of enzymes control the generation ROS [53] - efforts are underway to identify Nox independent molecules that control ROS generation in IL-15 cultured T cells. Further, we noticed that expression of, PTGS2, also known as cyclooyygenase-2 (COX-2); a well-characterized enzyme that contributes to the inflammatory process was 3.40-fold downregulated in IL-15 culture. The other most significantly down regulated genes identified by the PCR-based array were Cytoglobin (−19.25), Dual specificity phosphatase 1 (−11.06), Myeloperoxidase (−16.76), Proteoglycan 3 (−5.53) and Thyroid peroxidase (−3.17). Dual specificity protein phosphatase 1 (DUSP-1), also known as MKP-1 can dephosphorylate MAP kinases, terminate MAP kinase-regulated gene transcription [54]. MKP-1/DUSP1 also acts as negative regulator of cellular proliferation. Our data shows that IL-15 could inhibit DUSP-1/MKP-1 and effect cell viability and function. The other molecule found to be down regulated was myeloperoxidase (MPO). MPO is an enzyme that is primarily found in neutrophils and monocytes/macrophages. Intracellularly, it plays a major role in microbial killing, but extracellularly, it may cause host tissue damage [55]. An earlier study suggested that MPO could serve as a sensitive predictor for myocardial infarction in patients presenting with chest pain and elevated MPO levels predict more than doubled the risk for cardiovascular mortality [56]. A recent study has also suggested the differential expression of MPO on T cells and showed that MPO gene expression was greater in CD62L− vs. CD62L+ memory CD4+ T cells [57]. Since IL-15 is also known to preserve CD62L expression and promotes central memory phenotype [58] our data highlights another important attribute of IL-15, i.e. modulate antioxidant molecules to increase persistence. Thyroid peroxidase (TPO) is a type of enzyme produced in the thyroid involved in iodination that occurs via reactive iodine species released from TPO. Higher levels of TPO are common in those with Graves’ disease and Hashimoto’s disease. Earlier studies have isolated the TPO Specific T Cell Clones from patients with Hashimoto’s thyroiditis (HT) and implicated in its pathogenesis [59]. Since increased circulating pro-inflammatory cytokines and Th17 lymphocytes have been implicated in Hashimoto’s thyroiditis [60], a reduced expression of TPO and other inflammatory genes as COX-2 points to a possible role for IL-15 in controlling autoimmunity. Another important molecule that was down regulated in presence of IL-15 was PRG3 - a p53-inducible apoptogenic gene - that encodes a homologue of the apoptosis-inducing factor (AIF), a well-characterized mitochondrially released factor [61]. Redox regulation represents a fine balance between generation and scavenging of ROS by pro- and antioxidant pathway. Our observation that IL-15 results in significant up-regulation of antioxidant proteins such as Prdx, Trx, SOD, Sirt2, GSR, Nudix, while down-regulating the expression of pro-oxidant molecules like Nox, Cox, TPO, MPO implicates IL-15 as an important redox regulator and tilts the balance in favor of its role as cytokine promoting antioxidant levels. Thus, the PCR based array data supports the anti-apoptotic role of IL-15 and identifies novel anti-oxidant genes that are also modulated by IL-15.
While we report a novel role of IL-15 in controlling the anti-oxidant capacity of T cell we also confirmed that IL-15 affects bcl-2 family proteins. Several studies have invoked a role for bcl-2 in protection from ROS-induced apoptosis; the mechanism(s) by which bcl-2 prevents ROS-induced apoptosis is unknown. Anti-apoptotic bcl-2-like proteins do not themselves possess antioxidant enzymatic activity [62-63]. However, several studies have suggested that bcl-2 over expression can enhance the levels or redistribution of glutathione within cells [64-67] and modulate the release of apoptogenic factors, such as cytochrome c and apoptosis-inducing factor, from mitochondria. Further, anti-apoptotic effect of bcl-xl has also been attributed to the regulation of GSH homeostasis by preventing GSH loss [68]. In addition, long-term bcl-2 over expression induces the up regulation of superoxide dismutase (SOD)[65]. Thus, overexpression of bcl-2 may allow cells to cope better with the effects of ROS, possibly by allowing increases in endogenous antioxidant enzymes. We also examined that the cells cultured in the presence of IL-15 showed elevated levels of bcl-2, bcl-xl and SOD - the first line of defense against superoxide radicals to form H2O2 and oxygen. These results are in agreement with previous studies which have shown that IL-15 inhibits the apoptosis of HIV-specific CD8+ T cells [69-70] and induces both bcl-2 and bcl-xl levels in these cells, suggesting the up-regulation of these antiapoptotic molecules might be corealted to the upregulation of intracellular glutathione - a potential mechanism by which IL-15 acts to inhibit apoptosis of these cells.
Our data also show that the IL-15 treated CD8+ T cells with more thiols had increased activity of T-bet. Up regulated Th1 transcription factor T-bet signifies active transcription of the cytokine genes [71] and correlates to increased cytokine levels in T cells. Additionally, IL-15 also induced the expression of iNOS and lead to higher production of NO upon exposure to different stress stimuli. The results are in agreement with a recent report where IL-15 was shown to enhance the production of NO by overexpression of iNOS to mediate antitumor activity [40]. Further, we found that CD8+ T cells cultured and maintained in the presence of IL-15 attained increased levels of intracellular Granzyme A and Granzyme B expression to maintain cytolytic activity in comparison to IL-2 treated cultures. This finding extends the previous observations where IL-15 was found to induce Granzyme B and lytic capacity of CD8+ T cells in the absence of antigen and TCR stimulation [39]. Further, our data shows that CD8+ T cells cultured in the presence of IL-15 maintained their mitochondrial membrane integrity (as shown by DiOC6 staining) as compared with IL-2 cultures upon activation. Mitochondrial membrane permeability changes are accompanied by release of various proteins from the inter-membrane space that initiate and maintain a caspase cascade, chromatin condensation, and DNA fragmentation. Release of these proteins results in permeabilization of the inner mitochondrial membrane and dissipation of Δψ [72]. The elevated levels of anti-apoptotic bcl-2 and bcl-xl proteins in presence of IL-15 could be implicated in conserving the mitochondrial membrane potential in T cells, since these molecules have been shown to target the permeability transition pore complex of the mitochondrial membrane. In addition, our data showed an increased accumulation of ROS and RNS as tracked using intracellular stains and flow cytometry [24, 73-74]. These results indicate that IL-15 play an important role in inducing cytotoxic activity through free radical induction and maintenance of anti-oxidative properties by over-expression of key anti-oxidant genes as Trx, Sirt2, SOD1 and SOD2 in T cells when compared with IL-2. Thus, it is likely that the reduced extent of death and increased cytolytic effect of T cells in IL-15 cultures found in our experiments could be attributed to the homeostatic balance between oxidative stress and anti-oxidant defense related machinery.
While our results can be potentially important in adoptive transfer protocols where IL-15 instead of IL-2 could be used to enhance tumor specific cytotoxicity there still exists an in vivo scenario where T cells will likely encounter a mixture of cytokines that can either synergize to augment T cell function [75] or antagonize to inhibit T cell function [76]. Therefore, further studies to address the role of cytokines as redox regulators will be important. However, we conclude that ’redox remodeling’ [77] could be an important immunoregulatory strategy and identification of factors (cytokines or pharmacological compounds) [5, 78-84] responsible for maintenance or increasing anti-oxidant capacity of T cells could be employed to increase T-cell activity and function and effectively increase the potential of T cells in immunotherapy.
Acknowledgement
We are thankful to Drs. Michael Nishimura and Pravin Kesarwani in the Department of Surgery and Dr. Jennifer Schnellmann in the Office of Scientific Editing & Publications at Medical University of South Carolina for their help in preparation of this manuscript.
The work was supported by grants from the Charlotte-Geyer Foundation, NIH R21CA137725 and NIH R01CA138930 to SM.
Abbreviations Used
- IL
Interleukin
- AICD
Activation Induced Cell Death
- CTLs
Cytotoxic T lymphocytes
- GSB
Glutathione S-bimane
- Trx
Thioredoxin
References
- [1].Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–370. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med. 2009;1:11ps12. doi: 10.1126/scitranslmed.3000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vaglio A, Alberici F, Maggiore U, Buti S, Potenzoni D, Passalacqua R, et al. Chronically administered immunotherapy with low-dose IL-2 and IFN-alpha in metastatic renal cell carcinoma: a feasible option for patients with a good prognostic profile. Oncology. 2009;76:69–76. doi: 10.1159/000178810. [DOI] [PubMed] [Google Scholar]
- [7].Waldmann TA. The IL-2/IL-15 receptor systems: targets for immunotherapy. J Clin Immunol. 2002;22:51–56. doi: 10.1023/a:1014416616687. [DOI] [PubMed] [Google Scholar]
- [8].Arienti F, Belli F, Rivoltini L, Passerini C. Gambacorti, Furlan L, Mascheroni L, et al. Adoptive immunotherapy of advanced melanoma patients with interleukin-2 (IL-2) and tumor-infiltrating lymphocytes selected in vitro with low doses of IL-2. Cancer Immunol Immunother. 1993;36:315–322. doi: 10.1007/BF01741170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- [11].Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- [14].Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4 Suppl 3:S161–167. doi: 10.1186/ar584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Konczalik J. Marks, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tang C, Yamada H, Shibata K, Yoshida S, Wajjwalku W, Yoshikai Y. IL-15 protects antigen-specific CD8+ T cell contraction after Mycobacterium bovis bacillus Calmette-Guerin infection. J Leukoc Biol. 2009;86:187–194. doi: 10.1189/jlb.0608363. [DOI] [PubMed] [Google Scholar]
- [18].Shrikant P, Mescher MF. Opposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J Immunol. 2002;169:1753–1759. doi: 10.4049/jimmunol.169.4.1753. [DOI] [PubMed] [Google Scholar]
- [19].Berard M, Brandt K, Paus S. Bulfone, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- [20].Takahashi A, Hanson MG, Norell HR, Havelka AM, Kono K, Malmberg KJ, et al. Preferential cell death of CD8+ effector memory (CCR7-CD45RA-) T cells by hydrogen peroxide-induced oxidative stress. J Immunol. 2005;174:6080–6087. doi: 10.4049/jimmunol.174.10.6080. [DOI] [PubMed] [Google Scholar]
- [21].Gilbertson S. White, Mullen T, Senkal C, Lu P, Ogretmen B, Obeid L, et al. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene. 2009;28:1132–1141. doi: 10.1038/onc.2008.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sahaf B, Heydari K, Herzenberg LA. Lymphocyte surface thiol levels. Proc Natl Acad Sci U S A. 2003;100:4001–4005. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sahaf B, Heydari K, Herzenberg LA. The extracellular microenvironment plays a key role in regulating the redox status of cell surface proteins in HIV-infected subjects. Arch Biochem Biophys. 2005;434:26–32. doi: 10.1016/j.abb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- [24].Banki K, Hutter E, Gonchoroff NJ, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- [25].Rappl G, Abken H, Hasselmann DO, Tilgen W, Ugurel S, Reinhold U. The CD7(−) subset of CD4(+) memory T cells is prone to accelerated apoptosis that is prevented by interleukin-15 (IL-15) Cell Death Differ. 2001;8:395–402. doi: 10.1038/sj.cdd.4400825. [DOI] [PubMed] [Google Scholar]
- [26].Mueller YM, Makar V, Bojczuk PM, Witek J, Katsikis PD. IL-15 enhances the function and inhibits CD95/Fas-induced apoptosis of human CD4+ and CD8+ effector-memory T cells. Int Immunol. 2003;15:49–58. doi: 10.1093/intimm/dxg013. [DOI] [PubMed] [Google Scholar]
- [27].Demirci G, Li XC. IL-2 and IL-15 exhibit opposing effects on Fas mediated apoptosis. Cell Mol Immunol. 2004;1:123–128. [PubMed] [Google Scholar]
- [28].Gelderman KA, Hultqvist M, Holmberg J, Olofsson P, Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci U S A. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sahaf B, Heydari K, Herzenberg LA, Herzenberg LA. Lymphocyte surface thiol levels. Proc Natl Acad Sci U S A. 2003;100:4001–4005. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sahaf B, Heydari K, Herzenberg LA, Herzenberg LA. The extracellular microenvironment plays a key role in regulating the redox status of cell surface proteins in HIV-infected subjects. Arch Biochem Biophys. 2005;434:26–32. doi: 10.1016/j.abb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- [31].Franco R, Panayiotidis MI, Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem. 2007;282:30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010;120:2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- [34].Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010;32:104–115. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- [36].Liebenberg LJ, Adedeji AL, Martin DP, Gumbi PP, Denny L, Passmore JA. CD57 expression by T cells in the female genital tract of HIV-zx1 infected women. Clin Immunol. 2010;135:137–145. doi: 10.1016/j.clim.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu J, Giuntoli RL, 2nd, Omiya R, Kobayashi H, Kennedy R, Celis E. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8:3877–3884. [PubMed] [Google Scholar]
- [39].Tamang DL, Redelman D, Alves BN, Vollger L, Bethley C, Hudig D. Induction of granzyme B and T cell cytotoxic capacity by IL-2 or IL-15 without antigens: multiclonal responses that are extremely lytic if triggered and short-lived after cytokine withdrawal. Cytokine. 2006;36:148–159. doi: 10.1016/j.cyto.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Davies E, Reid S, Medina MF, Lichty B, Ashkar AA. IL-15 has innate antitumor activity independent of NK and CD8 T cells. J Leukoc Biol. 2010;88:529–536. doi: 10.1189/jlb.0909648. [DOI] [PubMed] [Google Scholar]
- [41].Kohchi C, Inagawa H, Nishizawa T, Soma G. ROS and innate immunity. Anticancer Res. 2009;29:817–821. [PubMed] [Google Scholar]
- [42].Thoren FB, Romero AI, Hermodsson S, Hellstrand K. The CD16-/CD56bright subset of NK cells is resistant to oxidant-induced cell death. J Immunol. 2007;179:781–785. doi: 10.4049/jimmunol.179.2.781. [DOI] [PubMed] [Google Scholar]
- [43].Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- [44].Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- [45].Qiao M, Kisgati M, Cholewa JM, Zhu W, Smart EJ, Sulistio MS, et al. Increased expression of glutathione reductase in macrophages decreases atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1375–1382. doi: 10.1161/ATVBAHA.107.142109. [DOI] [PubMed] [Google Scholar]
- [46].Masutani H, Ueda S, Yodoi J. The thioredoxin system in retroviral infection and apoptosis. Cell Death Differ. 2005;12 Suppl 1:991–998. doi: 10.1038/sj.cdd.4401625. [DOI] [PubMed] [Google Scholar]
- [47].Bouzar AB, Boxus M, Florins A, Francois C, Reichert M, Willems L. Reduced levels of reactive oxygen species correlate with inhibition of apoptosis, rise in thioredoxin expression and increased bovine leukemia virus proviral loads. Retrovirology. 2009;6:102. doi: 10.1186/1742-4690-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tanaka T, Hosoi F, Yamaguchi-Iwai Y, Nakamura H, Masutani H, Ueda S, et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346 Pt 1:1–8. [PMC free article] [PubMed] [Google Scholar]
- [50].Bishopric NH, Webster KA. Preventing apoptosis with thioredoxin: ASK me how. Circ Res. 2002;90:1237–1239. doi: 10.1161/01.res.0000025101.04065.83. [DOI] [PubMed] [Google Scholar]
- [51].Fujino G, Noguchi T, Takeda K, Ichijo H. Thioredoxin and protein kinases in redox signaling. Semin Cancer Biol. 2006;16:427–435. doi: 10.1016/j.semcancer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [52].Sakumi K, Furuichi M, Tsuzuki T, Kakuma T, Kawabata S, Maki H, et al. Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J Biol Chem. 1993;268:23524–23530. [PubMed] [Google Scholar]
- [53].Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110:243–253. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- [54].Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005;2:20–27. [PubMed] [Google Scholar]
- [55].Odobasic D, Kitching AR, Semple TJ, Holdsworth SR. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:760–770. doi: 10.1681/ASN.2006040375. [DOI] [PubMed] [Google Scholar]
- [56].Heslop CL, Frohlich JJ, Hill JS. Myeloperoxidase and C-reactive protein have combined utility for long-term prediction of cardiovascular mortality after coronary angiography. J Am Coll Cardiol. 2010;55:1102–1109. doi: 10.1016/j.jacc.2009.11.050. [DOI] [PubMed] [Google Scholar]
- [57].Hengel RL, Thaker V, Lempicki RA, Lane HC. Effector CD4 T-cell Myeloperoxidase expression: evidence of innate-type immune molecules in T-cell pools. Antivir Ther. 2003;8 abstract no-382. [Google Scholar]
- [58].Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fisfalen ME, Palmer EM, Van Seventer GA, Soltani K, Sawai Y, Kaplan E, et al. Thyrotropin-receptor and thyroid peroxidase-specific T cell clones and their cytokine profile in autoimmune thyroid disease. J Clin Endocrinol Metab. 1997;82:3655–3663. doi: 10.1210/jcem.82.11.4336. [DOI] [PubMed] [Google Scholar]
- [60].Figueroa-Vega N, Alfonso-Perez M, Benedicto I, Sanchez-Madrid F, Gonzalez-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;95:953–962. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- [61].Ohiro Y, Garkavtsev I, Kobayashi S, Sreekumar KR, Nantz R, Higashikubo BT, et al. A novel p53-inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF) FEBS Lett. 2002;524:163–171. doi: 10.1016/s0014-5793(02)03049-1. [DOI] [PubMed] [Google Scholar]
- [62].Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- [63].Aritomi M, Kunishima N, Inohara N, Ishibashi Y, Ohta S, Morikawa K. Crystal structure of rat Bcl-xL. Implications for the function of the Bcl-2 protein family. J Biol Chem. 1997;272:27886–27892. doi: 10.1074/jbc.272.44.27886. [DOI] [PubMed] [Google Scholar]
- [64].Lee M, Hyun DH, Marshall KA, Ellerby LM, Bredesen DE, Jenner P, et al. Effect of overexpression of BCL-2 on cellular oxidative damage, nitric oxide production, antioxidant defenses, and the proteasome. Free Radic Biol Med. 2001;31:1550–1559. doi: 10.1016/s0891-5849(01)00633-5. [DOI] [PubMed] [Google Scholar]
- [65].Voehringer DW, Meyn RE. Redox aspects of Bcl-2 function. Antioxid Redox Signal. 2000;2:537–550. doi: 10.1089/15230860050192314. [DOI] [PubMed] [Google Scholar]
- [66].Voehringer DW. BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic Biol Med. 1999;27:945–950. doi: 10.1016/s0891-5849(99)00174-4. [DOI] [PubMed] [Google Scholar]
- [67].Voehringer DW, McConkey DJ, McDonnell TJ, Brisbay S, Meyn RE. Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc Natl Acad Sci U S A. 1998;95:2956–2960. doi: 10.1073/pnas.95.6.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bojes HK, Datta K, Xu J, Chin A, Simonian P, Nunez G, et al. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem J. 1997;325(Pt 2):315–319. doi: 10.1042/bj3250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, Altman JD, et al. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- [70].Petrovas C, Mueller YM, Dimitriou ID, Bojczuk PM, Mounzer KC, Witek J, et al. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J Immunol. 2004;172:4444–4453. doi: 10.4049/jimmunol.172.7.4444. [DOI] [PubMed] [Google Scholar]
- [71].Dong C, Flavell RA. Control of T helper cell differentiation--in search of master genes. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.49.pe1. [DOI] [PubMed] [Google Scholar]
- [72].Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- [73].Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- [74].Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J Leukoc Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- [75].Pouw N, Treffers-Westerlaken E, Kraan J, Wittink F, ten Hagen T, Verweij J, et al. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol Immunother. 2010;59:921–931. doi: 10.1007/s00262-010-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yang L. TGFbeta, a potent regulator of tumor microenvironment and host immune response, implication for therapy. Curr Mol Med. 2010;10:374–380. doi: 10.2174/156652410791317039. [DOI] [PubMed] [Google Scholar]
- [77].Yan Z, Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry. 2010;49:1059–1066. doi: 10.1021/bi902022n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Checker R, Sharma D, Sandur SK, Subrahmanyam G, Krishnan S, Poduval TB, et al. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J Cell Biochem. 2010;110:1082–1093. doi: 10.1002/jcb.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010;140:1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Haddad JJ, Fahlman CS. Redox- and oxidant-mediated regulation of interleukin-10: an anti-inflammatory, antioxidant cytokine? Biochem Biophys Res Commun. 2002;297:163–176. doi: 10.1016/s0006-291x(02)02094-6. [DOI] [PubMed] [Google Scholar]
- [81].Hellstrand K, Brune M, Dahlgren C, Hansson M, Hermodsson S, Lindner P, et al. Alleviating oxidative stress in cancer immunotherapy: a role for histamine? Med Oncol. 2000;17:258–269. doi: 10.1007/BF02782190. [DOI] [PubMed] [Google Scholar]
- [82].Li-Weber M, Weigand MA, Giaisi M, Suss D, Treiber MK, Baumann S, et al. Vitamin E inhibits CD95 ligand expression and protects T cells from activation-induced cell death. J Clin Invest. 2002;110:681–690. doi: 10.1172/JCI15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jariwalla RJ, Lalezari J, Cenko D, Mansour SE, Kumar A, Gangapurkar B, et al. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection. J Altern Complement Med. 2008;14:139–146. doi: 10.1089/acm.2006.6397. [DOI] [PubMed] [Google Scholar]
- [84].Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]