Abstract
XPC protein is a critical DNA damage recognition factor in nucleotide excision repair (NER) for which genetic deficiency confers a predisposition to cancer. In this study we demonstrate that XPC has a function that is independent of its canonical function in DNA repair, potentially altering the interpretation of how XPC deficiency leads to heightened cancer susceptibility. XPC enhances apoptosis induced by DNA damage in a p53 nullizygous background, acting downstream of mitochondrial permeabilization and upstream of caspase-9 activation in the DNA damage-induced apoptosis cascade. We found that deficiency in XPC upregulated production of the short isoform of caspase-2 (casp-2S). This upregulation occurred at both protein and mRNA levels through repression of the caspase-2 promoter by XPC protein. Targeted RNAi-mediated downregulation of casp-2S enhanced UV-induced apoptosis as well as activation of caspase-9 and caspase-6 in XPC-deficient cells, but not in XPC-proficient cells. In addition, XPC overexpression in various p53-deficient cancer cells resistant to cisplatin improved their sensitivity to cisplatin-induced apoptosis. Given that casp-2S functions as an anti-apoptotic protein, our findings suggest that XPC enhances DNA damage-induced apoptosis through inhibition of casp-2S transcription. Together, these findings offer a mechanistic foundation to overcome the resistance of highly prevalent p53-deficient tumors to cell death induced by DNA-damaging therapeutic agents, by targeting strategies that inhibit the expression or function of casp-2S.
Keywords: XPC, caspase-2S, apoptosis, DNA damage
Introduction
DNA lesions induced by UV light and the chemotherapeutic agent cisplatin are mainly removed by the nucleotide excision repair (NER) consisting of two sub-pathways termed transcription-coupled repair (TCR) and global genomic repair (GGR) (1). TCR is required for the preferential removal of lesions from the transcribed strand of active genes, whereas GGR is responsible for the removal of DNA lesions from the bulk of the genome (1). Cell lines derived from individuals suffering from the disorders like Xeroderma pigmentosum (XP) and Cockayne syndrome (CS) have defects in one or both of these NER sub-pathways. Specifically, XP-C cells have a defect in GGR but not TCR. In contrast, CS-A and CS-B cells are defective in TCR but not GGR (2), whereas XP-A cells have defects in both TCR and GGR (1).
TCR-deficient fibroblasts (e.g., CS-A and XP-A) are extremely sensitive to UV and cisplatin-induced apoptosis (3–5), whereas XP-C cells with selective defect in GGR but with proficient TCR have normal resistance to cisplatin (6;7). In addition, murine Xpc−/− keratinocytes are characterized by a complete absence of apoptosis after UV exposure, in contrast to Xpa−/− and Csb−/− cells (8). These reports suggest that unrepaired DNA damage in the transcribed strand is an indispensable trigger for UV/cisplatin-induced apoptosis. However, increasing evidence has suggested that unrepaired DNA lesions in the non-transcribed strands or transcriptionally inactive genes can cause replication blockage, which also acts as an apoptosis-inducing signal (9). It has been reported that Xpa−/− keratinocytes (TCR and GGR deficiency) showed greater apoptosis than Csb−/− cells (TCR only deficiency) following UV irradiation (8). Given that both cells have deficient TCR, the additional GGR deficiency in Xpa−/− cells must be a contributing factor in UV-induced apoptosis. Although transcription blockage is absent in XP-C cells due to proficient TCR, the replication blockage induced by deficient GGR is sufficient to trigger apoptosis in these cells. Therefore, the resistance of XP-C cells to UV/cisplatin induced apoptosis could not be simply attributed to the proficient TCR. This prompts us to hypothesize that an important apoptosis element must be lost in XP-C cells.
XPC is a 940-amino acid protein, and harbors domains that can bind to damaged DNA and repair factors (10–12). The major function of XPC is to recognize helix-distorting lesions located in a transcriptionally inactive genome or a non-transcribed strand of actively transcribed genes (13). Recent studies have suggested that beyond its role in DNA repair, the XPC protein is also involved in transcriptional process including both transcription activation and repression (14;15). However, the relationship between XPC and apoptosis-related gene transcription is yet to be established.
Caspases are a family of cysteine aspartate-specific proteases involved in the initiation or execution of apoptosis. Caspase-2 (casp-2) is the most conserved caspase across species, and is one of the initiator caspases activated by various stimuli (reviewed in (16)). The casp-2 gene produces several alternative splicing isoforms. The inclusion of exon 9 leads to the inclusion of an in-frame stop codon in casp-2 short isoform (casp-2S) mRNA, thus producing a truncated protein that inhibits cell death. Whereas the exclusion of exon 9 results in casp-2 long isoform (casp-2L) mRNA, whose protein product induces cell death (17;18).
In this study, we have uncovered a novel function of XPC as a potent enhancer of apoptosis in the absence of any influence by p53. From a mechanistic standpoint, XPC protein down-regulates anti-apoptotic casp-2S through inhibition of its promoter activity, and thus promotes DNA damage-induced activation of casp-9 and casp-6, which ultimately enhances cellular death.
Materials and Methods
Cell Culture and Treatment
SV40-transformed XP-C (GM15983) and fully corrected XP-C (GM16248) cells were purchased from NIGMS Human Genetic Cell Repository (Coriell Institute for Medical Research, Camden, NJ). HCT116(p53−/−) and HCT116(p53+/+) colorectal carcinoma cells were kindly provided by Dr. B. Vogelstein (Johns Hopkins University). 041-TR cells were provided by Dr. G. Stark (Cleveland Clinic Foundation). A2780-CP70 ovarian cancer cell line was provided by Dr. P. Modrich (Duke University), SKOV3 ovarian cancer cell line was provided by Dr. T. C. Hamilton (Fox Chase Cancer Center), H1299 and A549 cell lines were kindly provided by Dr. W. Duan (The Ohio State University). Except for the regular testing for mycoplasma contamination using a PCR based assay, the cell lines were not specifically authenticated. The cultures were maintained as described in Supplementary Data and the cells were treated with cisplatin or UV as described before (19).
Plasmids and Transfection
pXPC3 plasmid-containing XPC cDNA and its corresponding empty vector pEBS7 were kindly provided by Dr. R. Legerski (The University of Texas MD Anderson Cancer Center) (20). XPC shRNA-containing plasmids (pLKO.1-puro) were purchased from Sigma (St. Louis, MO). p3XFLAG-CMV-14-casp-2S construct was generated in our lab (Supplementary Data). Casp-2S promoter-luciferase construct Del4 has been described (21;22). The plasmids were transfected into cells using either FuGENE6 (Roche Applied Science, Indianapolis, IN), or Lipofectamine LTX reagents (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions.
Western Blotting Analysis
Whole cell lysates were prepared by boiling cell pellets for 10 min in lysis buffer (2% SDS, 10% Glycerol, 62 mM Tris-HCl, pH 6.8 and a complete mini-protease inhibitor cocktail [Roche Applied Science]). For the detection of cytochrome c release (cyto c), cells were harvested by trypsinization and the cytosol was separated as described (23). Equal amounts of proteins were loaded, separated on a polyacrylamide gel, and transferred to a nitrocellulose membrane. Protein bands were immuno-detected with appropriate antibodies (Supplementary Data).
Apoptosis Analysis by Annexin V Staining
Phosphatidylserine exposure on the outer leaflet of the plasma membrane was detected using the Annexin V-GFP apoptosis detection kit II (BD Pharmingen, San Diego, CA) according to the manufacturer’s instructions. Briefly, 1 × 106 cells were pelleted following treatment and washed in PBS. Cells were then resuspended in 500 µl of binding buffer, mixed with Annexin V-GFP and propidium iodide (PI) and incubated at room temperature (22°C) for 5–10 min in the dark. The Annexin V-positive cells were analyzed by flow cytometry.
Mitochondria Transmembrane Potential Detection
XP-C and XP-C+XPC cells growing on the coverslips were either treated with UV or untreated. The cells were further cultured for 24 h, and stained with MitoCapture reagents (BioVision, Mountain View, CA) according to the manufacture’s instruction. Cells were observed immediately under a Nikon Fluorescence Microscope E80i (Nikon, Tokyo, Japan) fitted with appropriate filters for FITC and Texas Red. The digital images were then captured with a cooled CCD camera and processed with the help of its SPOT software (Diagnostic Instruments, Sterling Heights, MI, USA).
Transfection with siRNAs
siRNA directed against casp-2S (5’- GAAUACUACUGGUAAACUAUU -3’) (5’UTR), and a scramble non-targeting siRNA (5’- UUCUCCGAACGUGUCACGUdTdT -3’), were synthesized by Dharmacon Inc. (Lafayette, CO). siGENOME SMARTpool XPC siRNA was purchased from Dharmacon. 100 nM siRNA was transfected into cells using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacture’s instruction.
Real-time PCR
Real-time PCR was conducted as described previously (19). Special primers were designed to only amplify casp-2S or casp-2L, e.g., the forward sequence of casp-2S primer covers the exon 9, while the forward sequence of casp-2L primer covers the junction of exon 8 and 10. The sequence of the primers are as follows: casp-2L, forward: 5 ’- GCCTGCCGTGGAGATGAGACTGAT -3 ’; casp-2S, forward: 5 ’- CACCGCCTCTCTTGCTCTAT -3 ’; casp-2L and -2S reverse: 5 ’- TTACCGGCATCACTCTCCTC -3’; GAPDH, forward, 5’- GAAGGTGAAGGTCGGAGT -3’; reverse, 5’- GAAGATGGTGATGGGATTTC -3’.
Promoter Activity Assay
Cells were transiently transfected with casp-2S promoter-luciferase constructs (Del4). As an internal control, the pGL4.73 plasmid (Promega, Madison, WI), which carries a renilla luciferase gene, was also co-transfected into the cells to normalize for transfection efficiency. Luciferase assays were performed using Dual-Luciferase® Reporter Assay System (Promega) according to manufacturer’s instruction.
Chromatin Immunoprecipitation (ChIP) Assay
HCT116(p53−/−) cells were fixed with 1% formaldehyde for 10 min at room temperature. The ChIP assay was performed as described previously (24) using 2 µg of either normal rabbit IgG or rabbit anti-XPC antibodies. The casp-2S promoter-specific PCR reactions were performed using four different pairs of primers (Supplementary Data).
RESULTS
XPC Facilitates DNA Damage-Induced Apoptosis
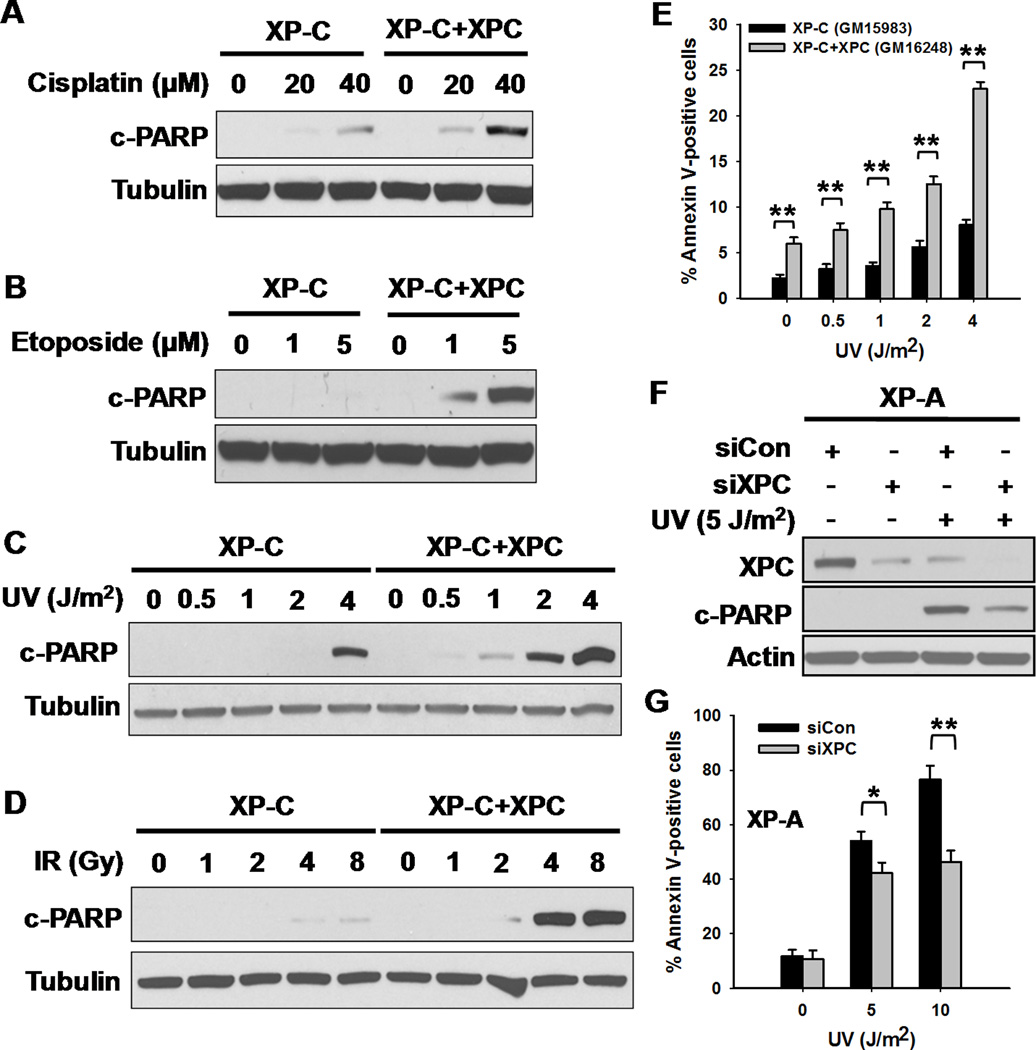
Since our results (Supplementary Data S1A–B & S2A–D) and others (6;7) have shown that XPC-deficient cells have lower UV and cisplatin sensitivity than other NER-deficient cells, we wanted to determine whether XPC-deficient cells are also resistant to DNA damage-induced apoptosis. A SV40-transformed human XP-C cell line and its stably XPC-restored counterpart were used to investigate the role of XPC protein in various DNA damaging agent-induced apoptosis. As shown in Fig. 1A–D, following treatment with diverse DNA damaging-agents, XPC-restored cells exhibited enhanced apoptosis than parental XPC-deficient cells, as reflected by the characteristic increase of poly(ADP-ribose) polymerase (PARP) cleavage in these cells. Enhanced UV-induced apoptosis in XPC-restored cells was also confirmed by Annexin V-staining (Fig. 1E) and sub-G1 cell analysis (Supplementary Data S3). Furthermore, we transiently transfected XPC cDNA-containing plasmids into XP-C cells and again found the increased apoptosis upon UV irradiation compared to the empty vector transfected XP-C cells (Supplementary Data S4A–B). To further confirm the independent role of XPC in apoptosis, without the potential influence via its canonical function in NER, we knocked down the expression of XPC in NER-deficient XP-A cells and analyzed their apoptosis upon UV irradiation. Our findings indicated that XPC-deficiency compromised UV-induced apoptosis in XP-A cells which are completely deficient in both GGR and TCR (Fig. 1F,G). These observations demonstrated that XPC protein can facilitate DNA damage-induced apoptosis independent of its known function in DNA repair.
Figure 1.
XPC Facilitates DNA-damaging Agent-induced Apoptosis. A–D, XP-C and XP-C+ XPC cells were treated with cisplatin (A), etoposide (B), UV radiation (C) or ionizing radiation (D), and further cultured for 24 h (cisplatin: treated for 1 h, further cultured in drug-free medium for 24 h. Etoposide: treated for 24 h). Whole cell lysates were prepared and the same amounts of proteins were loaded for Western blotting analysis of cleaved PARP. Tubulin was detected as loading control. E, XP-C and XP-C+XPC cells were irradiated with UV and further cultured for 24 h. Apoptotic cells were detected with Annexin V staining. The percentage of Annexin V-positive cells is an average of three independent repeats. Statistical significance was determined by two-sample Student’s t-test. Bars represent standard deviation (SD), *: p< 0.05, **: p< 0.01, compared with XP-C cells. F,G, XP-A cells were transfected with either control or XPC siRNA for 48 h, UV irradiated and further cultured for 24 h. the expression of XPC and the amount of cleaved PARP were detected with Western blotting (F). Apoptotic cells were detected with Annexin V staining (G). N = 3, bars: SD, *: p< 0.05, **: p< 0.01, compared with control siRNA transfected cells.
XPC-Mediated Apoptosis Occurs Without an Influence by p53
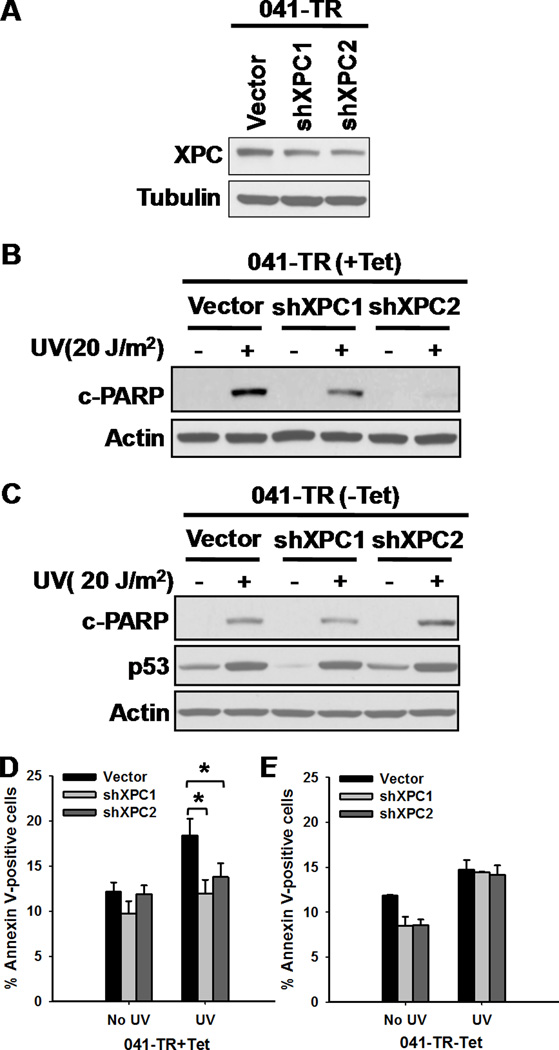
The above-mentioned data were obtained from SV40-transformed XP-C and XP-A cells, in which the transcriptional properties of p53 are suppressed. To further investigate the role of an involvement of p53 in the XPC-mediated apoptosis, we analyzed the effect of XPC-deficiency on UV-induced cellular apoptosis in the presence or absence of p53. XPC shRNA was stably transfected into 041-TR cells carrying tetracycline (Tet)-regulated p53 and two new cell lines with downregulated XPC were established (Fig. 2A). These cell lines were irradiated with UV either in the presence or absence of Tet, and the apoptosis was analyzed by PARP cleavage and Annexin-V staining. As shown in Fig. 2B–E, XPC-knockdown compromised UV-induced apoptosis when p53 is eliminated by Tet, while had no significant influence on apoptosis when p53 was present and responded normally to induction by DNA damage. This finding was also confirmed in HCT116 cells with differing status of p53 (Supplementary Data S5A–D). These data indicated that XPC-mediated apoptosis is independent of functional p53, and this novel effect can be obscured by the regular p53-mediated apoptosis.
Figure 2.
XPC-mediated Apoptosis is Independent of p53. A, 041-TR cells were stably transfected with empty vector or shXPC, the expression of XPC was detected using Western blotting in the presence of Tet (p53−/−). B, 041-TR cells stably transfected with empty vector or shXPC were cultured in the presence of Tet (p53−/−), UV irradiated and cultured for 24 h. Cleaved PARP was detected using Western blotting. C, Tet was withdrawn from the culture of 041-TR cells 16 h prior to UV irradiation (p53+/+), and further cultured in the absence of Tet for 24 h. Cleaved PARP was detected. D,E, Apoptotic cells in (B, C) were detected with Annexin V staining. N = 3, Bars: SD, *: p< 0.05 compared with empty vector transfected cells.
XPC Functions Downstream of Mitochondrial Events in Facilitating Apoptosis
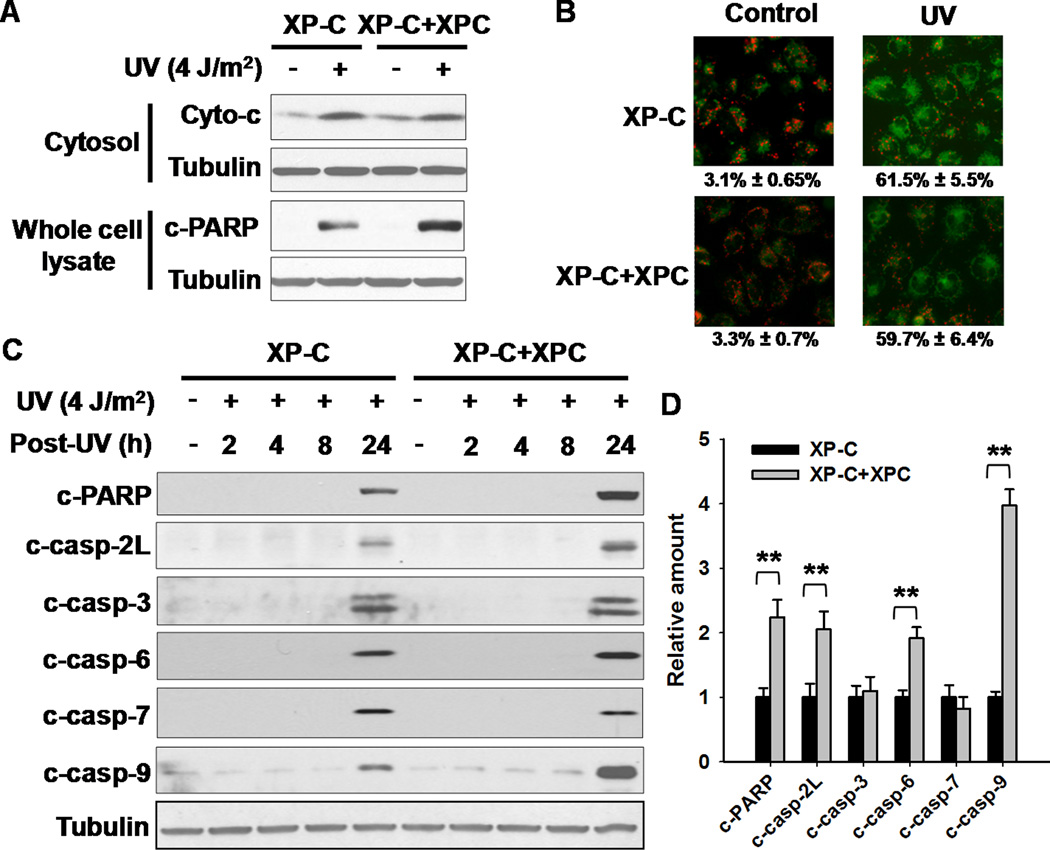
For insights into the mechanism by which XPC protein enhances apoptosis, we focused on the contribution of XPC to the intrinsic apoptotic pathway. We first analyzed cytochrome c (cytoc) release from mitochondria following UV irradiation of XPC-deficient and -proficient cells. As shown in Fig. 3A, despite the higher level of apoptosis in XPC-proficient cells as reflected by increased PARP cleavage, UV irradiation caused a comparable release of cyto c into cytosol fraction from both cell lines. Furthermore, we analyzed the disruption of the mitochondrial trans-membrane potential, and found that both XPC-deficient and -proficient cells displayed the similar diffused green fluorescence upon UV irradiation (Fig. 3B), further supporting that XPC protein does not influence DNA damage-induced mitochondrial permeabilization, and indicated that XPC could be playing its role at steps downstream of cyto c release during apoptotic cascade. We then determined the activation of various caspases functioning in the downstream of cyto c release in both XP-C and XP-C+XPC cells following UV irradiation. As shown in Fig. 3C,D, casp-2L, casp-6 and casp-9 activation prominently coincided with the enhanced PARP cleavage of XPC-proficient cells, while casp-3 and casp-7 activation was comparable in XP-C and XP-C+XPC cells. This suggested that XPC-mediated apoptosis might be occurring through the enhanced activation of casp-2L, casp-9 and casp-6. Furthermore, we also found that pan-caspase inhibitor z-VAD blocked, while casp-3 inhibitor z-DEVD had no influence on UV-induced apoptosis in both XP-C and XP-C+XPC cells, although casp-3 activity was higher in XP-C+XPC cells than XP-C cells upon UV irradiation (Supplementary Data S6A–D). This indicates that the functional role of casp-3 in UV-induced apoptosis in XP-C cells is apparently being substituted by other executor caspases.
Figure 3.
XPC Functions Downstream of Mitochondrial Events in Facilitating Apoptosis. A, Cytosol was isolated from both XP-C and XP-C+XPC cells at 24 h after UV irradiation. The presence of cyto c in cytosol and the cleaved PARP in whole cell lysates were detected using Western blotting. B, XP-C and XP-C+XPC cells growing on the coverslips were UV irradiated at 4 J/m2, further cultured for 24 h. Mitochondrial trans-membrane potential was detected using MitoCapture probe under fluorescence microscope. The percentages of cells with diffused green fluorescence (apoptotic cells) were calculated. C, XP-C and XP-C+XPC cells were UV irradiated at 4 J/m2, and further cultured for 24 h. Whole cell lysates were prepared and subjected to Western blotting for the detection of cleaved PARP, and various caspases cleavage. D, Quantification of cleaved PARP and various caspases at 24 h after UV irradiation. N = 3, bars: SD, **: p< 0.01, compared with XP-C cells.
XPC Inhibits the Expression of Casp-2S through Repressing Casp-2S Promoter Activity
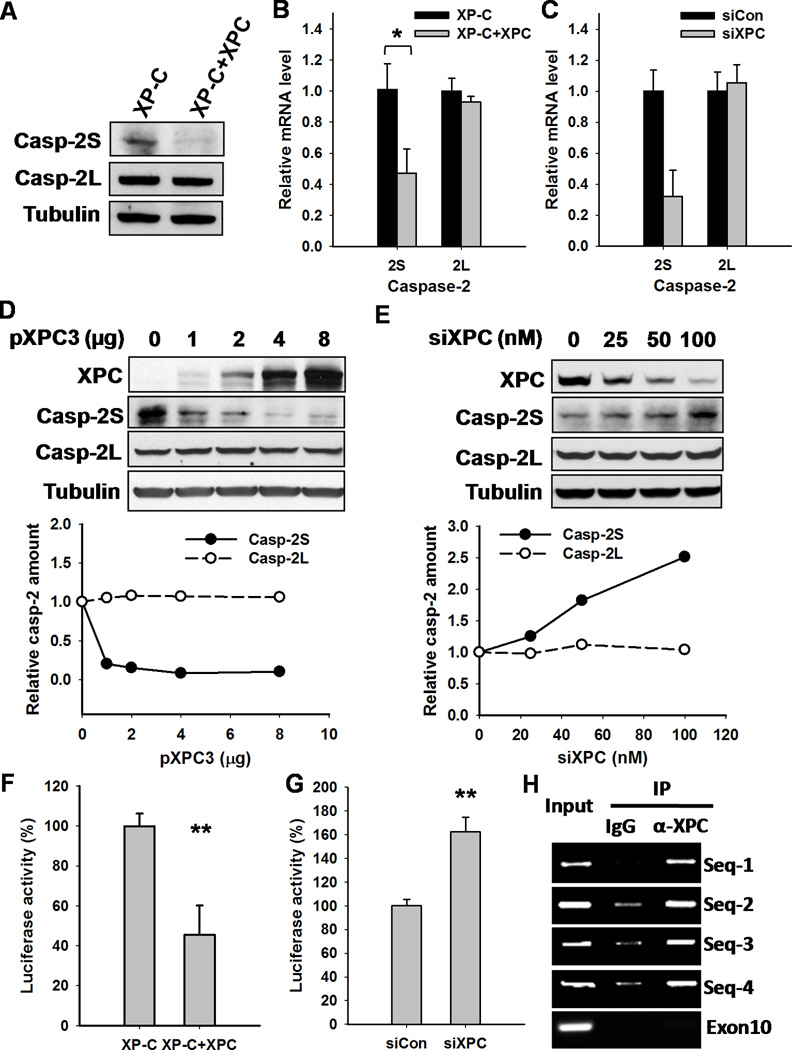
Casp-2 exists as two distinct isoforms, pro-apoptotic casp-2L and anti-apoptotic casp-2S (17;18). So, we determined the effects of XPC on the expression of both casp-2L and casp-2S in cultured cells. As shown in Fig. 4A, in contrast to casp-2L, casp-2S protein was up-regulated in XPC-deficient cells, indicating that XPC protein inhibits the expression of casp-2S. Real-time quantitative RT-PCR analysis further indicated that XPC repressed the transcription of casp-2S without an influence on casp-2L transcription (Fig. 4B, C). To further confirm the inhibitory effect of XPC on the expression of casp-2S, we transiently transfected various amounts of XPC cDNA-containing constructs into XP-C cells, or down-regulated XPC expression by transfecting XPC siRNA into HCT116(p53−/−) cells, and determined the protein levels of casp-2S and casp-2L. As expected, with the increase of XPC expression, casp-2S demonstrated decreased level while casp-2L level did not change. Similarly, with the decrease of XPC expression, casp-2S exhibited increased level and again, casp-2L level kept constant (Fig. 4D, E).
Figure 4.
XPC Inhibits the Expression of Casp-2S at Both Protein and mRNA Levels. A, The expression of casp-2S and casp-2L in XP-C and XP-C+XPC cells were detected using Western blotting. B,C, The total RNA was isolated from XP-C and XP-C+XPC cells (B) or HCT116(p53−/−) cells transfected with either control or XPC siRNA (C), the transcript levels of casp-2S and casp-2L were determined using quantitative RT-PCR. N = 3, bars: SD, *: p< 0.05, compared with XP-C cells (B), **: p< 0.01 compared with control siRNA transfected cells (C). D, XP-C cells were transfected with different amounts of XPC containing pXPC3 constructs for 48 h. Whole cell lysates were prepared and the expression of XPC, casp-2S, casp-2L and Tubulin were analyzed using Western blotting. The intensity of each band was scanned; the relative amounts of casp-2 normalized to Tubulin were plotted. E, HCT116(p53−/−) cells were transfected with different amounts of XPC siRNA for 48 h. Casp-2S and -2L were detected as described in (D). F, Casp-2S promoter-luciferase construct (Del4) was transfected together with pGL4.73 plasmid into XP-C and XP-C+XPC cells for 48 h. The cultures were lysed and subjected to luciferase assays. N = 3, bars: SD, **: p< 0.01, compared with XP-C cells. G, HCT116(p53−/−) cells were transfected with control or XPC siRNA for 24 h. Casp-2S promoter activity was detected as (F). N = 3, bars: SD, **: p< 0.01, compared with cells transfected with control siRNA. H, ChIP analysis was performed with anti-XPC antibody and normal rabbit IgG in HCT116(p53−/−) cells. Four promoter sequences on the casp-2S promoter were analyzed by PCR.
It has been reported that casp-2S can be transcribed from an alternate promoter within intron 1 of the casp-2 gene (22). Thus, we utilized the casp-2S promoter-luciferase reporter construct, described earlier (22), to investigate the repressive effect of XPC on the casp-2S promoter activity. Our data demonstrated that XPC expression led to a substantial decrease in the reporter activity from the construct containing casp-2S promoter (Del4) (Fig.4F), indicating that XPC may reduce the synthesis of casp-2S pre-mRNA. We further detected the influence of down-regulation of XPC on the activity of casp-2S promoter in HCT116 cells. As shown in Fig. 4G, knockdown of XPC increased the casp-2S promoter activity in HCT116 cells. To gain insight into the mechanism through which XPC regulates casp-2S promoter activity, we performed ChIP assay to investigate whether XPC protein directly binds to the putative casp-2S promoter (-3006--1952) site (21). As shown in Fig. 4H, the strong casp-2S promoter (Seq 1–4), but not casp-2 exon-10 PCR product, in anti-XPC antibody-recruited DNA fragment indicated the enrichment of XPC to casp-2S promoter. Taken together, these data demonstrated that XPC protein specifically regulates the expression of anti-apoptotic casp-2S by directly binding to casp-2S promoter region and inhibiting its activity.
Enhanced Casp-2S is Responsible for the Resistance of XPC-deficient Cells to Apoptosis
Earlier it has been reported that casp-2 is required for stress-induced apoptosis (25). However, that study could not have separated the roles of casp-2L and casp-2S, since the siRNA used in their experiments actually targeted both casp-2L and casp-2S (25). By using the siRNA targeting of casp-2L and casp-2S (Supplementary Data S7A–B) and caspase-2 inhibition (Supplementary Data S7C), we failed to find any influence of casp-2 down-regulation on UV-induced apoptosis in both XP-C and XP-C+XPC cells observed in this study.
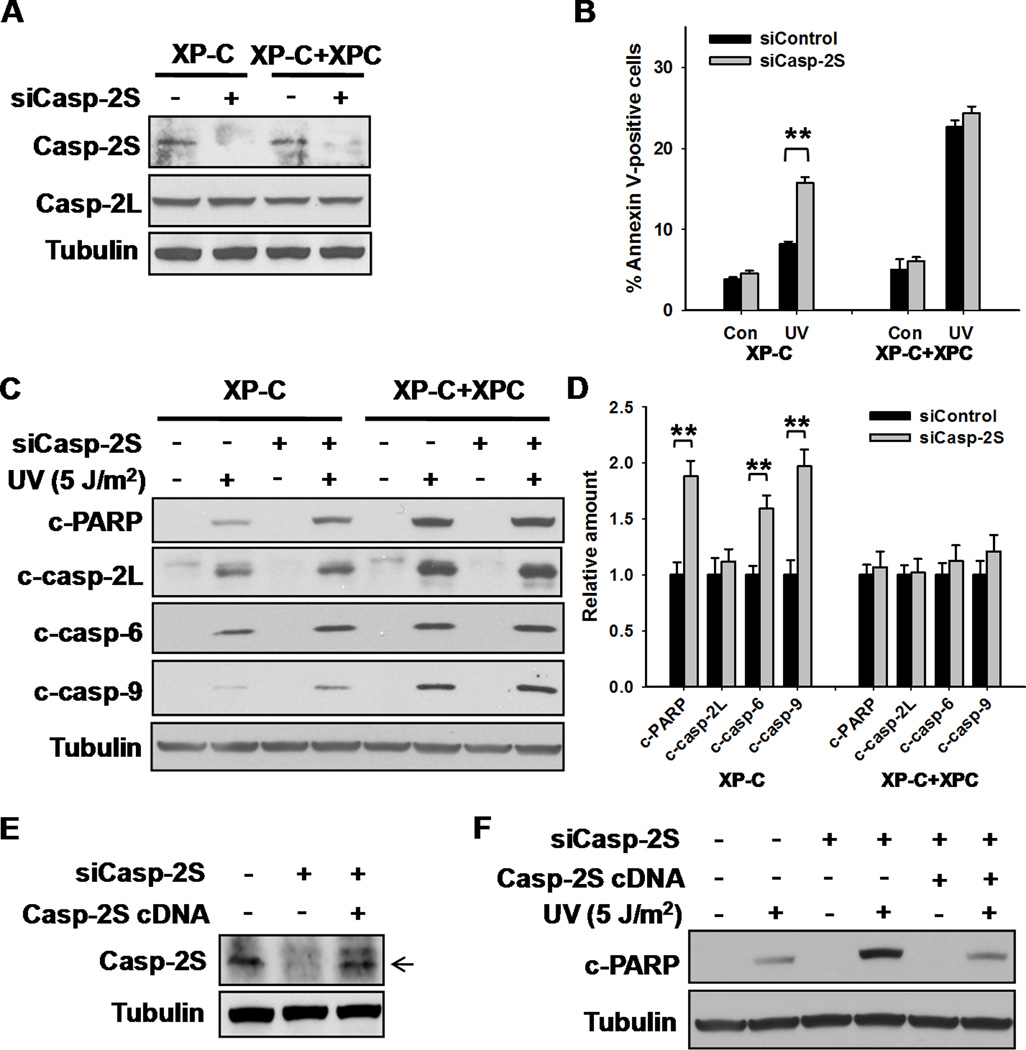
To specifically investigate the role of casp-2S in XPC-mediated apoptosis, we designed a siRNA that only targeted casp-2S (Fig. 5A), and analyzed the UV-induced apoptosis in both XPC-deficient and -proficient cells before and after casp-2S siRNA knockdown. The results indicated that down-regulation of casp-2S significantly enhanced the apoptosis in the deficient XP-C cells but not the restored XP-C+XPC cells following UV irradiation (Fig. 5B–D). Over-expression of casp-2S in casp-2S siRNA transfected XP-C cells reduced UV-induced apoptosis to the original level (Fig. 5E,F). Furthermore, down-regulation of casp-2S simultaneously enhanced the activation of casp-9 and casp-6 in XPC-deficient cells, but not in XPC-proficient cells (Fig. 5C,D). Notably, we were unable to observe any influence of casp-2S knockdown on the casp-2L activation. The above results demonstrated that XPC enhances apoptosis through down-regulation of anti-apoptotic casp-2 short isoform, which is an inhibitor of casp-9 and casp-6 activation.
Figure 5.
Casp-2S is Responsible for the Resistance to Apoptosis in XP-C cells. A, XP-C and XP-C+XPC cells were transfected with either control or casp-2S siRNA for 48 h, the expression of casp-2S and casp-2L were detected using Western blotting. B, XP-C and XP-C+XPC cells were transfected with either control or casp-2S siRNA for 48 h, UV irradiated at 5 J/m2 and further cultured for 24 h. Apoptotic cells were detected with Annexin V staining by using Flow cytometry. N = 3, bars: SD, **: p< 0.01, compared with siControl transfected cells. C, Whole cell lysates from cells in (B) were prepared and the cleavages of PARP and various caspases were detected using Western blotting. D, Quantification of cleaved PARP and cleaved caspases after UV irradiation in (C). N = 3, bars: SD, **: p< 0.01, compared with siControl transfected cells. E, XP-C cells were transfected with either casp-2S siRNA, or casp-2S siRNA together with casp-2S cDNA for 48 h, the expression of casp-2S was detected using Western blotting with anti-casp-2S antibody. The Arrow indicates the casp-2S band, the upper band in the third lane is a variant of casp-2S(21). F, Cells in (E) were UV irradiated and further cultured for 24 h. The amount of cleaved PARP and Tubulin was detected using Western blotting.
XPC Over-expression Enhances Cisplatin-induced Apoptosis in Various Cancer Cell Lines
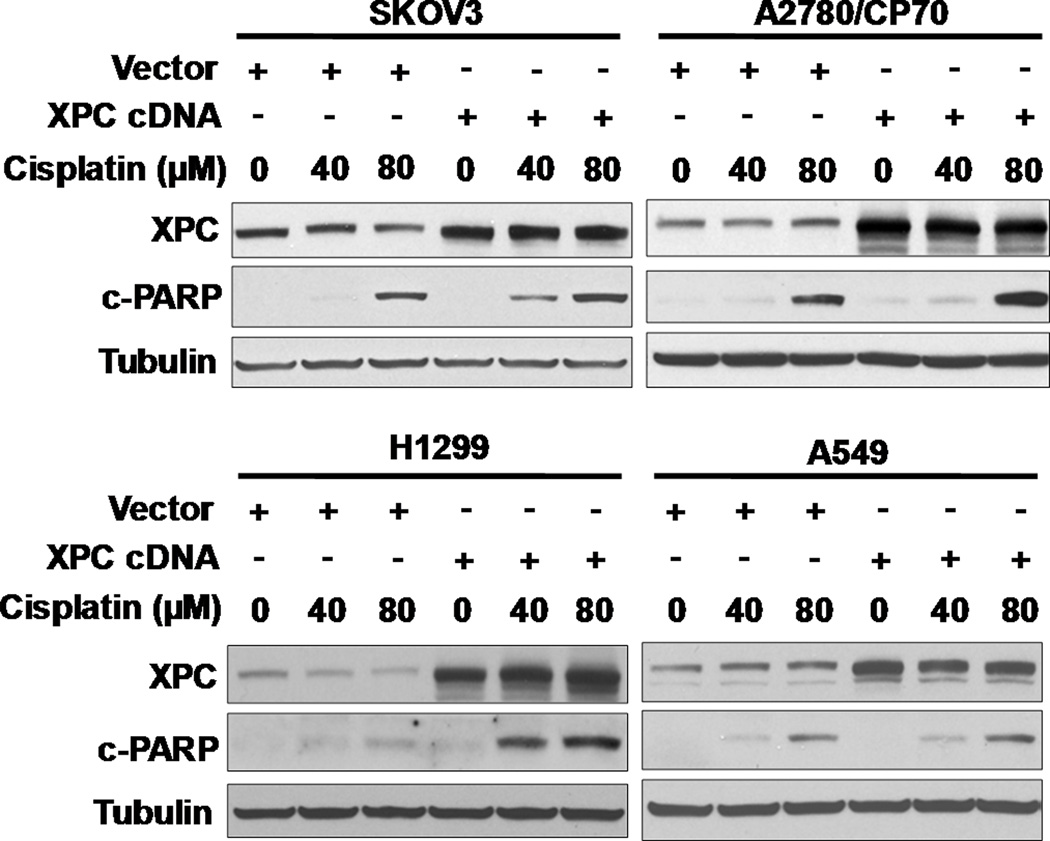
To determine whether the level of XPC affects chemotherapeutic agent-induced apoptosis in human cancer cells, we over-expressed XPC in various human cancer cell lines of different p53 status, and analyzed cisplatin-induced apoptosis. As shown in Fig. 6, XPC over-expression enhanced the cisplatin-induced apoptosis in p53-deficient human ovarian carcinoma cells SKOV3 and human non-small cell lung carcinoma cells H1299, as well as p53-heterozygous ovarian carcinoma cells A2780/CP70. However, XPC over-expression did not exhibit augmented apoptosis in p53-proficient A549 cells upon cisplatin treatment. These data indicated that elevation of XPC level in p53-deficient cancer cells can overcome their resistance to cisplatin.
Figure 6.
XPC Over-expression Enhances Cisplatin-induced Apoptosis in Various Cancer Cell Lines. SKOV3, A2780/CP70, H1299 and A549 were transfected with either empty vector (pEBS7) or XPC cDNA-containing vector (pXPC3) for 24 h, treated with cisplatin for 1 h, and further cultured in drug-free medium for another 48 h. Whole cell lysates were prepared and subjected to Western blot analysis for the detection of XPC and cleaved PARP.
Discussion
XPC deficiency has been associated with cancer predisposition in both human and animal models (26–30). Deficient NER is established as the main contributor to the increasing cancer rates. Here, we have provided evidence showing that XPC protein can also enhance DNA damage-induced apoptosis. Thus, the loss of XPC-mediated apoptotic pathway could also be a contributory factor in the cancer susceptibility of XPC-deficient individuals.
The resistance of XPC-deficient cells to apoptosis was initially attributed to the proficient TCR in these cells (6;7). The intact TCR pathway removes UV or cisplatin-induced DNA lesions from the transcribed strand of the active genes, thus eliminates the apoptosis triggers. However, our data demonstrated that XPC-deficient cells are not only resistant to apoptosis induced by UV and cisplatin treatment, which induce DNA lesions repaired by NER, but also resistant to apoptosis induced by IR and etoposide, which induce DSBs. Given the putative role of XPC in the repair of DSBs (31), XPC-deficient cells should have more DNA lesions, or in other words apoptosis triggers, than XPC–proficient cell. Therefore, the compromised apoptosis, in response to IR and etoposide in XPC-deficient cells, could not be attributed to diminished apoptosis signals. This indicates that XPC protein may participate in apoptosis process through a novel function beyond its canonical role in DNA repair. Furthermore, our finding that down-regulation of XPC in XP-A cells compromised UV-induced apoptosis further reinforced this notion. Since XP-A cell line is deficient in both GGR and TCR, the knockdown of XPC expression does not further reduce its NER efficiency, and the DNA lesions in the entire genome including transcribed and non-transcribed strands are comparable between XPC-knockdown and XPC-proficient XP-A cells. Thus, the reduced apoptosis in XP-A cells transfected with XPC siRNA can only be attributed to the loss of XPC protein, and indicates that XPC participates in DNA damage-induced apoptosis independent of NER.
It was previously reported that XPC protein plays an essential role in cisplatin-induced apoptosis, perhaps through the activation of p53 and p73-dependent apoptotic pathways (32). Here, we have identified a new pathway in which XPC is shown to enhance DNA damage-induced apoptosis in the absence of functional p53. More interestingly, XPC seems to enhance apoptosis only when p53 function is deficient, indicating that XPC is redundant in cisplatin or UV-induced apoptosis in the presence of functional p53, probably because the dominant p53-driven apoptosis obscures the underlying contribution of the XPC protein. Therefore, the role of this XPC-casp-2S pathway, similar to the role of p73 pathway (33), is not critical to apoptosis in the cells with wild-type p53. However, considering the high p53 mutation prevalence among human tumors, induction of XPC-mediated apoptosis pathway offers a unique strategy for sensitizing p53-deficient tumors to cancer therapy.
Although we were unable to alter UV-induced apoptosis by using casp-2 siRNA that targets both casp-2L and casp-2S, we clearly showed that specific knockdown of casp-2S overcomes the resistance of XPC-deficient cells to apoptosis. As indicated above, casp-2 exists as two distinct isoforms with opposing functions: casp-2L induces while casp-2S inhibits cell death upon over-expression (17;18). Since both isoforms were knocked out or down-regulated simultaneously in casp-2 knockout mice studies and the RNA interference cell culture studies, the biological function of these two casp-2 isoforms, especially the anti-apoptotic function of casp-2S was not clarified (34). In addition, the results regarding the anti-apoptotic function of casp-2S obtained by over-expression experiments are also controversial (18;21;35). By using a specific casp-2S siRNA designed in this study, we have unambiguously revealed that down-regulation of casp-2S can enhance UV-induced apoptosis in XPC-deficient cells, but not XPC-proficient cells. This indicates that the cellular endogenous XPC level plays a critical role in investigations of the function of casp-2S by manipulation of cellular casp-2S levels.
The expression of casp-2S can be regulated by alternative promoter activation (21). It has been reported that p73 is able to specifically transactivate the expression of casp-2S through directly binding to a Sp-1-binding site-containing region in the promoter of casp-2S. In contrast, our data clearly demonstrated that the binding of XPC protein to casp-2S promoter inhibits casp-2S expression. Given XPC is able to regulate gene transcription in two opposite ways with different mechanisms (14;15), it is possible that XPC can function as a direct transcriptional repressor of casp-2S.
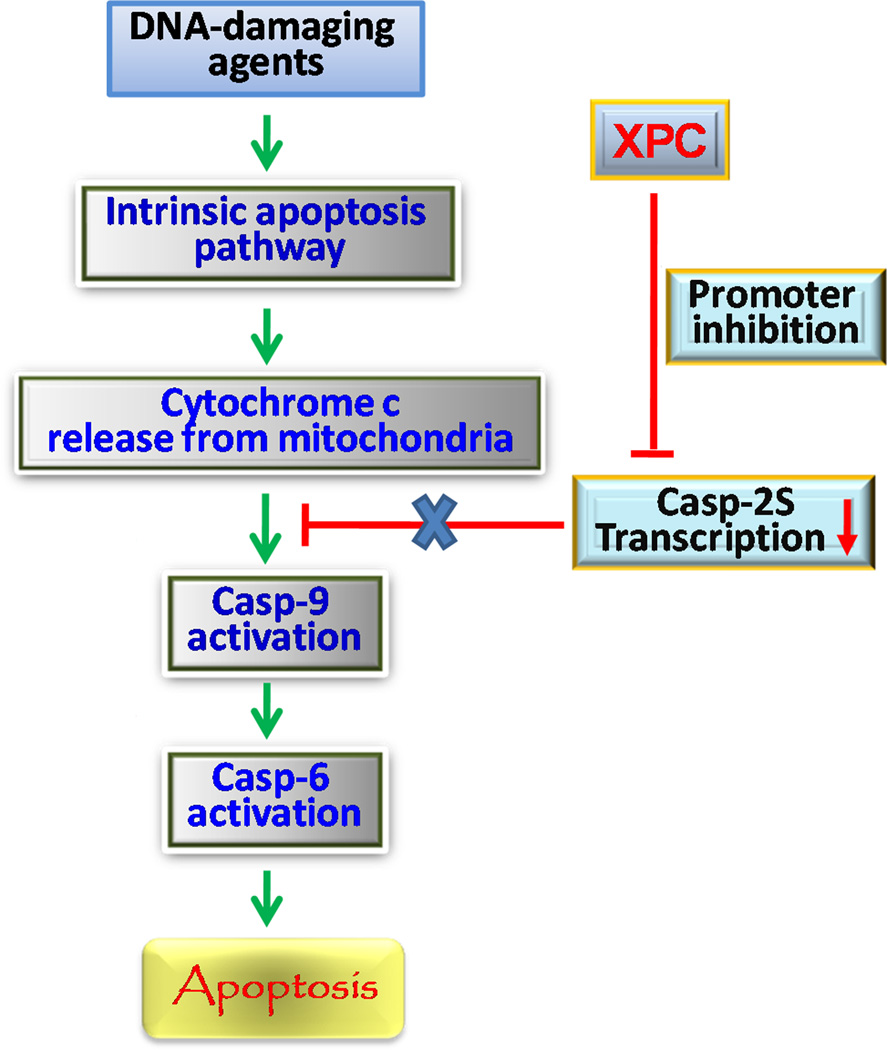
In summary, XPC protein is proposed to enhance DNA damage-induced apoptosis through intrinsic apoptotic pathway (Fig. 7). XPC protein down-regulates the expression of anti-apoptotic casp-2S through inhibiting its promoter activity. The down-regulation of casp-2S would attenuate its inhibitory effect on the activation of casp-9 and casp-6, resulting in augmented apoptosis.
Figure 7.
A Schematic Model for XPC Function in the Enhancement of DNA Damage-induced Apoptosis Cascade. XPC protein represses the transcription of anti-apoptotic casp-2S through inhibiting casp-2 promoter activity. The down-regulation of casp-2S attenuates its anti-apoptosis effect, thus enhances DNA damage-triggered casp-9 and casp-6 activation, and ultimately augments apoptosis.
Supplementary Material
Acknowledgments
We greatly acknowledge Drs. George Stark for 041-TR cell line; Bert Vogelstein for HCT116 cell line; Paul Modrich for A2780/CP70 cell line; Thomas Hamilton for SKOV3 cell line; and Wenrui Duan for H1299 and A549 cell lines. We also thank Dr. Randy Legerski for providing pXPC3 plasmids, and Yi-Wen Huang for assisting Real-time PCR analysis.
Grant Support
This study was supported by NIH grants ES2388, ES12991& CA93413 to AAW.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Friedberg EC. DNA repair and Mutagenesis. Washington: AVM Press; 1995. [Google Scholar]
- 2.Van Hoffen A, Natarajan AT, Mayne LV, Van Zeeland AA, Mullenders LHF, Venema J. Deficient repair of the transcribed strand of active genes in Cockayne's syndrome cells. Nucleic Acids Res. 1993;21:5890–5895. doi: 10.1093/nar/21.25.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andera L, Wasylyk B. Transcription abnormalities potentiate apoptosis of normal human fibroblasts. Mol Med. 1997;3:852–863. [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungman M, Zhang F. Blockage of RNA polymerase as a possible trigger for u.v. light-induced apoptosis. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- 5.McKay BC, Ljungman M, Rainbow AJ. Persistent DNA damage induced by ultraviolet light inhibits p21waf1 and bax expression: implications for DNA repair, UV sensitivity and the induction of apoptosis. Oncogene. 1998;17:545–555. doi: 10.1038/sj.onc.1201963. [DOI] [PubMed] [Google Scholar]
- 6.Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002;62:4899–4902. [PubMed] [Google Scholar]
- 7.McKay BC, Becerril C, Ljungman M. P53 plays a protective role against UV- and cisplatin-induced apoptosis in transcription-coupled repair proficient fibroblasts. Oncogene. 2001;20:6805–6808. doi: 10.1038/sj.onc.1204901. [DOI] [PubMed] [Google Scholar]
- 8.Stout GJ, Oosten M, Acherrat FZ, Wit J, Vermeij WP, Mullenders LH, et al. Selective DNA damage responses in murine Xpa−/−, Xpc−/− and Csb−/− keratinocyte cultures. DNA Repair (Amst) 2005;4:1337–1344. doi: 10.1016/j.dnarep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Batista LF, Kaina B, Meneghini R, Menck CF. How DNA lesions are turned into powerful killing structures: insights from UV-induced apoptosis. Mutat Res. 2009;681:197–208. doi: 10.1016/j.mrrev.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Uchida A, Sugasawa K, Masutani C, Dohmae N, Araki M, Yokoi M, et al. The carboxy-terminal domain of the XPC protein plays a crucial role in nucleotide excision repair through interactions with transcription factor IIH. DNA Repair (Amst) 2002;1:449–461. doi: 10.1016/s1568-7864(02)00031-9. [DOI] [PubMed] [Google Scholar]
- 11.Araki M, Masutani C, Takemura M, Uchida A, Sugasawa K, Kondoh J, et al. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J Biol Chem. 2001;276:18665–18672. doi: 10.1074/jbc.M100855200. [DOI] [PubMed] [Google Scholar]
- 12.Nishi R, Okuda Y, Watanabe E, Mori T, Iwai S, Masutani C, et al. Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol Cell Biol. 2005;25:5664–5674. doi: 10.1128/MCB.25.13.5664-5674.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugasawa K, Ng JMY, Masutani C, Iwai S, Van der Spek P, Eker A, et al. Xeroderma pigmentosum group C complex is the initiator of global genome nucleotide excision repair. Mol Cell. 1998;2:223–232. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 14.Le MN, Mota-Fernandes D, Velez-Cruz R, Iltis I, Biard D, Egly JM. NER factors are recruited to active promoters and facilitate chromatin modification for transcription in the absence of exogenous genotoxic attack. Mol Cell. 2010;38:54–66. doi: 10.1016/j.molcel.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Frechet M, Warrick E, Vioux C, Chevallier O, Spatz A, Benhamou S, et al. Overexpression of matrix metalloproteinase 1 in dermal fibroblasts from DNA repair-deficient/cancer-prone xeroderma pigmentosum group C patients. Oncogene. 2008;27:5223–5232. doi: 10.1038/onc.2008.153. [DOI] [PubMed] [Google Scholar]
- 16.Vakifahmetoglu-Norberg H, Zhivotovsky B. The unpredictable caspase-2: what can it do? Trends Cell Biol. 2010;20:150–159. doi: 10.1016/j.tcb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1β- converting enzyme. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang QE, Milum K, Han C, Huang YW, Wani G, Thomale J, et al. Differential contributory roles of nucleotide excision and homologous recombination repair for enhancing cisplatin sensitivity in human ovarian cancer cells. Mol Cancer. 2011;10:24. doi: 10.1186/1476-4598-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legerski R, Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992;359:70–73. doi: 10.1038/359070a0. [DOI] [PubMed] [Google Scholar]
- 21.Logette E, Wotawa A, Solier S, Desoche L, Solary E, Corcos L. The human caspase-2 gene: alternative promoters, pre-mRNA splicing and AUG usage direct isoform-specific expression. Oncogene. 2003;22:935–946. doi: 10.1038/sj.onc.1206172. [DOI] [PubMed] [Google Scholar]
- 22.Toh WH, Logette E, Corcos L, Sabapathy K. TAp73beta and DNp73beta activate the expression of the pro-survival caspase-2S. Nucleic Acids Res. 2008;36:4498–4509. doi: 10.1093/nar/gkn414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baptiste-Okoh N, Barsotti AM, Prives C. A role for caspase 2 and PIDD in the process of p53-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105:1937–1942. doi: 10.1073/pnas.0711800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Q, Wani G, Yao J, Patnaik S, Wang QE, El-Mahdy MA, et al. The ubiquitin-proteasome system regulates p53-mediated transcription at p21waf1 promoter. Oncogene. 2007;26:4199–4208. doi: 10.1038/sj.onc.1210191. [DOI] [PubMed] [Google Scholar]
- 25.Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297:1352–1354. doi: 10.1126/science.1074721. [DOI] [PubMed] [Google Scholar]
- 26.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 27.Thielmann HW, Popanda O, Edler L, Jung EG. Clinical symptoms and DNA repair characteristics of xeroderma pigmentosum patients from Germany. Cancer Res. 1991;51:3456–3470. [PubMed] [Google Scholar]
- 28.Sands AT, Abuin A, Sanchez A, Conti CJ, Bradley A. High susceptibility to ultraviolet-induced carcinogenesis in mice lacking XPC. Nature. 1995;377:162–165. doi: 10.1038/377162a0. [DOI] [PubMed] [Google Scholar]
- 29.Cheo DL, Burns DK, Meira LB, Houle JF, Friedberg EC. Mutational inactivation of the xeroderma pigmentosum group C gene confers predisposition to 2-acetylaminofluorene-induced liver and lung cancer and to spontaneous testicular cancer in Trp53−/− mice. Cancer Res. 1999;59:771–775. [PubMed] [Google Scholar]
- 30.Hollander MC, Philburn RT, Patterson AD, Velasco-Miguel S, Friedberg EC, Linnoila RI, et al. Deletion of XPC leads to lung tumors in mice and is associated with early events in human lung carcinogenesis. Proc Natl Acad Sci U S A. 2005;102:13200–13205. doi: 10.1073/pnas.0503133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despras E, Pfeiffer P, Salles B, Calsou P, Kuhfittig-Kulle S, Angulo JF, et al. Long-term XPC silencing reduces DNA double-strand break repair. Cancer Res. 2007;67:2526–2534. doi: 10.1158/0008-5472.CAN-06-3371. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Yang J, Wang G, Song B, Li J, Xu Z. Attenuated expression of xeroderma pigmentosum group C is associated with critical events in human bladder cancer carcinogenesis and progression. Cancer Res. 2007;67:4578–4585. doi: 10.1158/0008-5472.CAN-06-0877. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Sancar A. Circadian clock disruption improves the efficacy of chemotherapy through p73-mediated apoptosis. Proc Natl Acad Sci U S A. 2011;108:10668–10672. doi: 10.1073/pnas.1106284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitevska T, Spencer DM, Hawkins CJ. Caspase-2: controversial killer or checkpoint controller? Apoptosis. 2009;14:829–848. doi: 10.1007/s10495-009-0365-3. [DOI] [PubMed] [Google Scholar]
- 35.Droin N, Rebe C, Bichat F, Hammann A, Bertrand R, Solary E. Modulation of apoptosis by procaspase-2 short isoform: selective inhibition of chromatin condensation, apoptotic body formation and phosphatidylserine externalization. Oncogene. 2001;20:260–269. doi: 10.1038/sj.onc.1204066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.