Abstract
Purpose
To test AAV-mediated gene therapy in the rd10 mouse, a natural model of recessive RP caused by mutation of the β-subunit of rod photoreceptor cGMP phosphodiesterase.
Methods
One eye of a cohort of rd10 mice kept in a dark environment was subretinally injected at postnatal day (P) 14 with 1 μL AAV5-smCBA-PDEβ. The contralateral eye was not injected. The animals were then maintained for 2 weeks in the dark before they were moved to a normal 12-hour light/12-hour dark cycling light environment for visually guided behavioral training. Three weeks after injection, treated rd10 mice were examined by scotopic and photopic electroretinography and then killed for biochemical and morphologic examination.
Results
Substantial scotopic ERG signals were maintained in treated rd10 eyes, whereas untreated eyes in the same animals showed minimal signals. Treated eyes showed photopic ERG b-wave amplitudes similar to those of the normal eyes; in untreated partner eyes, only half the normal amplitudes remained. Strong PDEβ expression was observed in photoreceptor outer segments only in treated eyes. Light microscopy showed a substantial preservation of the outer nuclear layer in most parts of the treated retina only. Electron microscopy showed good outer segment preservation only in treated eyes. A visually guided water maze behavioral test under dim light showed significantly improved performance in one eye–treated rd10 mice compared with untreated mice.
Conclusions
These data demonstrate that P14 administration of AAV5-smCBA-PDEβ can prevent retinal degeneration in rd10 mice, as reflected by significant structural, biochemical, electrophysiological, and behavioral preservation/restoration. These results serve as a baseline for studying long-term retinal rescue in rd10 mice.
Retinal degeneration (RD) is a large family of inherited dystrophies characterized by photoreceptor dysfunction and eventual photoreceptor death. As many as 17 million persons worldwide have vision loss associated with RD, including patients with retinitis pigmentosa (RP), a disease for which no cure exists. A description of autosomal recessive mutations associated with retinal degeneration dates back to the discovery of the “rodless retina” mouse by Keeler in 1924, which later became known as the rd1 mouse.1–3 The rd1 mouse carries mutations in a gene (Pde6b) that encodes the β-subunit of rod photoreceptor cGMP phosphodiesterase (PDEβ).3,4 The biochemical result is a nonfunctional PDEβ and an accumulation of cGMP.5 Mutations in the human ortholog of Pde6b have been linked to autosomal recessive RP.6,7 In the rd1 mouse, photoreceptor degeneration begins at 1 week of age, when photoreceptor outer segments first begin to mature.8 Rods degenerate first, then cones; the culmination is complete ablation of photoreceptors by about 4 weeks of age.1,2,8–12 Until recently, the rd1 mouse was considered one of the best models of human autosomal recessive RP; however, because of the rapid rate of photoreceptor cell loss, providing effective, lasting gene replacement therapy has proven difficult. Subretinal injection of adenoviral, retroviral, or adenoassociated viral vectors encoding the PDEβ gene to neonatal rd1 mice resulted in partial preservation of photoreceptor structure but little, if any, ERG rescue.13–16 Because it takes at least 1 week for the viral vector–mediated gene to express in the retina and PDEβ is expressed in the retina by postnatal day (P)5 to P6, prenatal gene therapy in rd1 mice may be considered and has been achieved in mice with the RPE65 form of Leber congenital amaurosis.17 The course of retinal degeneration in humans, however, though often quicker than the course for other mutations, still takes decades. Thus, questions remain as to why the rd1 mouse has been relatively unresponsive to standard gene replacement therapy15 and, in particular, whether any element of recessive mutation in PDEβ makes the resultant retinal degeneration so rapid that the mice are refractory to gene replacement therapy in the mature photoreceptor.
A more recently identified mouse strain that exhibits autosomal recessive retinal degeneration, the rd10 mouse, has a point mutation in exon 13 of the Pde6b gene.18 Recent natural history studies of the rd10 mouse indicate that it better emulates the slow progression of typical human autosomal recessive RP than the previously described rd1 mouse.19,20 Loss of photoreceptors in the rd10 mouse begins after 2 weeks of age, with peak photoreceptor death occurring at P25.20 By 5 weeks most photoreceptor cells have been lost.19,20 This rate of photoreceptor loss is substantially slower than in the rd1 animal. Importantly, most photoreceptors in the rd10 retina are lost after the retina has terminally differentiated, whereas peak photoreceptor cell death in rd1 occurs before this developmental stage. In addition, it has been found that rearing rd10 mice in darkness further slows the rate of degeneration by as much as 4 weeks.19 Taken together, these findings suggest that retinal degeneration in the rd10 mouse is more analogous to the human condition and perhaps is better suited than the rd1 mouse for testing PDEβ gene replacement therapy. Recently, studies using stem cell, antiapoptotic, and antioxidant therapies have shown a measure of retinal rescue in rd10 mice,21–23 but the potential for gene therapy has not yet been reported.
Adenoassociated virus (AAV)-mediated gene replacement has already proven to be effective for restoring retinal function and for protecting photoreceptor structure in a number of mouse models of retinal disease.24–26 This approach has been optimized with the use of AAV serotypes that preferentially target photoreceptors in conjunction with ubiquitously expressed constitutive promoters that drive stronger transgene expression than obtained when using cell-specific promoters.26 The size of the Pde6b cDNA (2.6 kb) and the packaging limitation of AAV vectors (4.5 kb, excluding the required terminal DNA sequences) require the use of a relatively small promoter that directs strong and lasting expression. In this study, an AAV serotype 5 vector (AAV5) containing minimal chicken β-actin promoter/CMV enhancer (smCBA) was used to deliver the Pde6b gene to the rd10 mouse retina. The purpose of this gene replacement strategy was to determine whether retinal degeneration could be delayed in this new murine model of human autosomal recessive PDEβ-based RP, the rd10 mouse.
Materials and Methods
Animals
C57BL/6J mice and the congenic inbred strain of rd10 mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred at the University of Florida. Except where otherwise indicated, all mice were maintained in the University of Florida Health Science Center Animal Care Services Facilities under a 12-hour light/12-hour dark cycle with less than 15 ft-c environmental illumination. All experiments were approved by the local Institutional Animal Care and Use Committee and were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and with National Institutes of Health regulations.
Construction of AAV Vectors
AAV5 vectors exhibit higher transduction efficiency in photoreceptors and a faster onset of expression than other AAV serotypes when delivered to the subretinal space (Auricchio A, et al. IOVS 2001;42: ARVO Abstract 125)27 and were, therefore, used for packaging the current vector. Vector plasmids were constructed as previously described.28 Wild-type murine Pde6b cDNA was placed under the control of the ubiquitous, constitutive smCBA promoter28 to generate pTR-smCBA-PDEβ. Previously, we have shown that the smCBA promoter drives efficient and long-term transgene expression when targeted to photoreceptors through AAV5 (Boye SL, et al. IOVS 2006;47:ARVO E-Abstract 852). AAV vectors were packaged and purified according to previously reported methods.25
Subretinal Injections
Late-term pregnant rd10 females were kept in a continuously dark room, except for husbandry at 5 lux or less, and then pups were raised under these same conditions. When the pups were 14 days old, 1 μL AAV5-smCBA-PDEβ (1 × 1010 genome containing vector particles) was subretinally injected into one eye under dim light, and the animals were maintained for 2 more weeks in the same dark environment before they were moved to normal 12-hour light/12-hour dark vivarium cycling room light. The other eye remained uninjected. Subretinal injections were made under direct observation aided by a dissecting microscope under dim light. The injected retinal area was visualized by fluorescein-positive subretinal blebs demarcating the retinal detachment. Such detachments usually resolved within 1 to 2 days. Only animals with minimal surgical complications and initial retinal blebs occupying more than half the retina were retained for further evaluation.26 Approximately 20 rd10 mice met these criteria, which allowed at least three animals for each experiment. After all injections, 1% atropine eye drops and neomycin/polymyxin B/dexamethasone ophthalmic ointment were given.
Electroretinography
Three weeks after subretinal injection (P35; 1 week after the move to cyclic light environment), a semiautomated ERG recording instrument adapted for rodent analysis (Jaeger/Toennies) was used for ERG examination. All testing was performed in a climate-controlled and electrically isolated dark room with animals placed on a 37°C warming pad. After overnight dark adaptation, mice were anesthetized by ketamine (72 mg/kg)/xylazine (4 mg/kg) intraperitoneal injection in a dark room under dim red light illumination. Corneas were anesthetized with a drop of 0.5% proparacaine hydrochloride, and the pupils were dilated with 1% atropine and 2.5% phenylephrine hydrochloride. Small contact lenses scaled to mice with gold wire loop electrodes were placed on each cornea with a drop of 2.5% methylcellulose to maintain corneal hydration and to promote conductivity. A silver wire reference electrode was placed subcutaneously between the eyes, and a ground electrode was placed subcutaneously in a hind leg. For light-adapted electroretinography, the animals were put under a background light of 100 cd · s/m2 for 5 minutes before the recording. Two days after ERG examination, almost all injected rd10 mice were killed for morphologic and biochemical examination; the exception was one animal with almost 100% initial retinal detachment and minimal injection-related damage that was kept for another 2 weeks in a cyclic light environment for further ERG examination.
Immunocytochemistry for PDEβ Expression
Treated rd10 mice were killed 2 days after ERG examination for biochemical and morphologic examination. Eyes from treated and untreated rd10 mice, along with age-matched C57BL/6J mice, were enucleated, and the eyecups and frozen sections were processed as described previously.29 Retinal sections were permeabilized with 0.1% Triton X-100, rinsed in PBS, blocked in 20% normal goat serum (NGS), and incubated overnight at 4°C in a rabbit polyclonal anti–mouse PDEβ antibody (ABR-Affinity BioReagents, Golden, CO), diluted 1:400 in 20% NGS. This antibody reacts with human and mouse PDEβ protein. After three rinses with 0.1 M PBS, sections were incubated in goat anti–rabbit IgG conjugated with Texas Red (1:300; Molecular Probes, Eugene, OR) and DAPI (1:100; Molecular Probes, Eugene, OR) for 2 hours, followed by 3 rinses with 0.1 M PBS. Sections were then mounted with coverslips before fluorescence photography.
Western Blot Analysis
For PDEβ measurements, eyecups were carefully dissected from treated and untreated rd10 eyes and age-matched normal C57BL/6J eyes, pooled into separate groups (injected, uninjected, and normal, respectively), and homogenized by sonication in a buffer containing 0.23 M sucrose, 2 mM EDTA, 5 mM Tris-HCl (pH 7.5), and 0.1 mM phenylmethylsulfonyl fluoride. Samples were then centrifuged, and supernatants were collected. Protein concentrations were determined using a protein assay kit (Coomassie Plus; Pierce, Rockford, IL). After the addition of loading buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 200 mM dithiothreitol, 0.02% bromophenol blue), an equal amount (25 μg) of each sample was resolved by SDS-PAGE (10% Tris-glycine gel) and electrotransferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore, Bedford, MA). The membrane was blocked with 5% horse serum in PBS and incubated overnight with thesame PDEβ polyclonal antibody. The blot was then washed three times in PBS containing 0.05% Tween-20 (PBST) and was incubated with an anti–mouse IgG–conjugated alkaline phosphatase secondary antibody for 30 minutes at room temperature. After another wash in PBS, the blot was developed with a color assay using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. Treated and untreated rd10 and normal C57BL/6J samples were compared on the same blot with β-actin as an internal loading control.
Histology and Morphometry
Structural evaluation of the treated and untreated eyes has been described.26,29 Treated and untreated eyes from rd10 mutant mice were enucleated, and eyecups were prepared for light and electron microscopic examination.
Visually Guided Behavioral Test
The water maze visually guided behavioral test has been described previously by us.26 Briefly, 2 weeks after injection, one eye–treated rd10 mice reared in a dark environment, together with age-matched untreated rd10 and normal C57 mice, were initially trained in a plastic water tank with a platform positioned in a well-lit room. Training consisted of three blocks of four trials per day for 4 consecutive days. During each trial, the mouse was placed in the water from one of four equally spaced start locations. Behavioral data were acquired as the latency to escape to the platform during the training trials. After training in the well-lit room, the rod function of dark-adapted mice was measured using the same procedure but under very dim light (not detectable with the Datalogging Light Meter, model 401036; Extech Instruments, Waltham, MA).
Results
To maintain rd10 mice, we initially used lighting conditions that would allow a rate of retinal degeneration sufficiently slow for testing of a gene therapy approach. To that end, we maintained late-term pregnant rd10 females in a continuously dark room, except for husbandry at 5 lux or less, and then raised the pups under the same conditions. Light microscopic images of retinas from 4-week-old rd10 reared in a normal 12-hour light/12-hour dark cyclic light environment and from rd10 mice raised in dim light are compared in Figure 1. Approximately three layers of photoreceptors and minimal outer segments remained in mice reared in vivarium cyclic light, whereas the outer nuclear layer (ONL) was nearly normal in dark-reared rd10 mice. Thus, only dark-reared rd10 mice were used to test for therapy.
Figure 1.
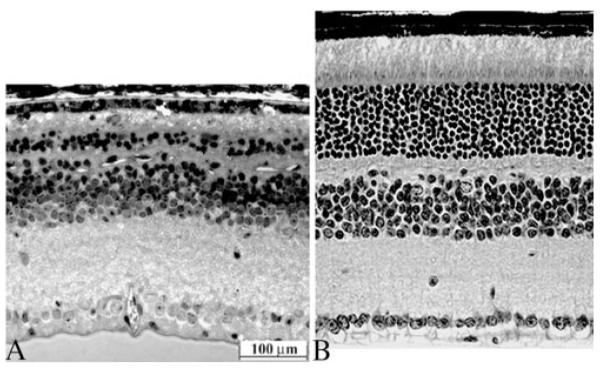
Light microscopic images of a 4-week-old rd10 mouse reared in normal cyclic light environment (left) compared with very dim light (right). Both images are from equivalent central regions of retina. Only two to three layers of ONL nuclei with residual outer segment material remain in the cyclic light–reared animal. In the dim light–reared animal, a nearly normal complement of 9 to 10 ONL nuclei are evident with clearly improved inner and outer segment morphology.
Electrophysiological Rescue
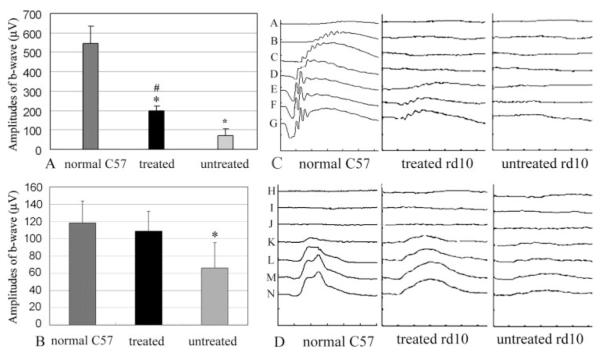
At P14, rd10 mice were subretinally injected with AAV5-smCBA-PDEβ. Three weeks after treatment (1 week after removal of mice to a cyclic light environment), rd10 mice were examined by dark-adapted and light-adapted electroretinography. Larger dark- and light-adapted ERG responses were evident in vector-treated eyes. When the stimulus intensity was 2.68 cd · s/m2, the average dark-adapted ERG b-wave amplitudes in PDEβ treated rd10 eyes were 200 ± 20 μV (Fig. 2A; n = 3), which was 37% of the isogenic wild-type mice (544 ± 89 μV; n = 3) and approximately threefold higher than in contralateral untreated eyes (70 ± 40 μV; n = 3). Paired t-test analysis showed significantly smaller dark-adapted b-wave amplitudes in untreated rd10 eyes compared with C57 eyes (P < 0.01). Although statistically not as good as those in normal C57 eyes (P < 0.05), dark-adapted b-wave amplitudes were significantly improved in treated rd10 eyes compared with those in untreated rd10 eyes (P < 0.05). Light-adapted ERG b-wave amplitudes elicited with a flash intensity of 12 cd · s/m2 were 118 ± 25 μV in normal, 109 ± 23 μV in treated rd10, and 66 ± 29 μV in untreated rd10 eyes (Fig. 2B). Statistical analysis showed similar light-adapted ERG b-wave amplitudes (P = 0.6) between normal C57 and treated rd10 eyes 3 weeks after injection (n = 6), whereas a significant difference was found between treated and untreated rd10 eyes (P < 0.05; n = 6). Figure 2C–D shows a representative rd10 mouse 5 weeks after one eye received subretinal vector at P14 (P49, 3 weeks after returning to cyclic light environment). In the untreated rd10 eye, dark-adapted ERG responses were minimal (Fig. 2C), whereas the light-adapted b-wave amplitudes (Fig. 2D) were approximately 25% of the wild-type controls elicited with flash intensity of 12 cd · s/m2. In the treated rd10 eye, approximately 22% of the normal dark-adapted b-wave (Fig. 2C) and 82% of the normal light-adapted b-wave amplitudes elicited with flash intensity of 2.68 cd · s/m2 (Fig. 2D) persisted. In time domain, the implicit times of the dark- and light-adapted ERG b-waves were approximately 75 ms (2.68 cd · s/m2) and 45 ms (12 cd · s/m2), respectively. In the treated rd10 mouse eye, the implicit time of the dark-adapted b-wave was comparable to that of the wild type mouse; however, the implicit time of the light-adapted b-wave was approximately 60 ms, which is similar to that for the untreated eye but is approximately 15 ms longer than for the normal control. Finally, as additional controls, we tested subretinal AAV5-smCBA-GFP and PBS in rd10 eyes. No rescue effects were observed in these eyes, indicating rescue is not a consequence of the injection procedure itself (data not shown).
Figure 2.
Scotopic and photopic ERGs in normal C57 and one eye–treated rd10 mice. (A) Averaged, scotopic b-wave amplitudes at 2.68 cd · s/m2 flash intensity in normal C57 (left), treated (middle), and untreated rd10 (right) eyes. (B) Averaged photopic b-wave amplitudes at 12 cd · s/m2 flash intensity in normal C57 (left), treated (middle), and untreated (right) eyes from rd10 mice 3 weeks after treatment at P14. (C) Representative of scotopic ERG waveforms elicited from a set of input single-flash intensities. (A: 0.1 mcd · s/m2; B: 1.0 mcd · s/m2; C: 10 mcd · s/m2; D: 100 mcd · s/m2; E: 1.0 cd · s/m2; F: 1.5 cd · s/m2; G: 2.68 cd · s/m2) from a normal C57 eye (left) and a rd10 eye 5 weeks after treatment at P14 (middle) compared with the untreated eye (right) from the same 7-week-old rd10 mouse. Y axis: 250 μV/Division; X axis: 25 ms/Div. (D) Representative of photopic ERG waveforms from a normal C57 eye (left), a treated (middle), and an untreated (right) eye of an rd10 mouse 5 weeks after treatment at P14. Y axis: 100 μV /Div ; X axis: 20 ms/Div. Input flash intensities are H: 1 mcd · s/m2; I: 10 mcd · s/m2; J: 100 mcd · s/m2; K: 1 cd · s/m2; L: 5 cd · s/m2; M: 10 cd · s/m2; N: 12 cd · s/m2. Symbols and bars represent mean ± SEM. *Significant difference between rd10-untreated and rd10-treated and between rd10-untreated and normal C57 mice. #Significant difference between rd10-treated and normal C57 mice.
PDEβ Expression
Two days after the final ERG examination, PDEβ expression was assayed by immunohistochemistry of retinal sections in rd10 eyes. Strong PDEβ staining is evident in the outer segments of treated eyes, similar to that seen in the normal C57 retinas (Fig. 3A). Inner segment staining is weak but detectable in treated eyes. In contrast, no PDEβ expression was observed in any portion of the untreated retina from the same rd10 mouse. To confirm the identity of this signal, Western blot analysis showed the presence of PDEβ protein in treated rd10 eyes but not in untreated contralateral eyes (Fig. 3B). We estimated that the level of PDEβ from pooling protein extracts from five treated eyes was slightly less than that seen from a single wild-type eye and, therefore, further estimated that we restored an average of 10% to 15% of the normal level of PDEβ in treated rd10 eyes compared with that in age-matched uninjected normal eyes.
Figure 3.
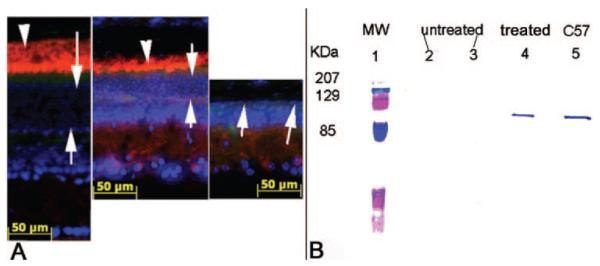
Comparison of PDEβ expression in C57BL/6J and rd10 mice. (A) PDEβ immunostaining (red) in a 5-week-old uninjected normal C57BL/6J eye (left), treated eye (center), and untreated eye (right) from one rd10 mouse. Nuclei were stained with DAPI (blue). Arrows: photoreceptor. Arrowheads: outer segments of photoreceptor. (B) Western blot showing PDEβ from 5-week-old AAV5-smCBA–PDEβ treated and untreated rd10 eyes and an untreated age-matched normal C57BL/6J control eye. Lane 1: molecular weight marker; lanes 2 and 3: five pooled, untreated rd10 retinas; lane 4: five pooled, treated rd10 retinas; lane 5: one normal C57BL/6J retina.
Structural Rescue
Vector-treated rd10 eyes were assessed for the degree of structural rescue that accompanied ERG preservation. In light microscopic images at low magnification, it is apparent that most treated rd10 retinas maintained a relatively normal ONL, whereas in the untreated eye of the same rd10 mouse, the ONL contained few and difficult-to-visualize photoreceptor cell bodies (Fig. 4A). Images at higher magnification showed that a typical treated retina retained approximately 30% of its outer segment length compared with the wild-type retina and approximately 60% of its ONL thickness. The best results showed that up to 90% of ONL nuclei and more than 50% of the outer segment length was preserved by treatment (Fig. 4B). In contrast, in the entire untreated eye from the same mouse, at most only one to three rows of ONL nuclei remained, with no outer segments evident in the central retina and only residual outer segment membrane in the periphery (Fig. 4B). Electron microscopic images confirmed that the treated eye contained shortened but normal-appearing outer segments. In the contralateral untreated rd10 eye, outer segments were absent or only residual structures remained, resulting in the outer limiting membrane (OLM) being nearly opposed to the RPE layer (Fig. 4C). A limited number of electron-dense photoreceptor nuclei remained beneath the OLM in the untreated rd10 retina (Fig. 4C, lower left).
Figure 4.
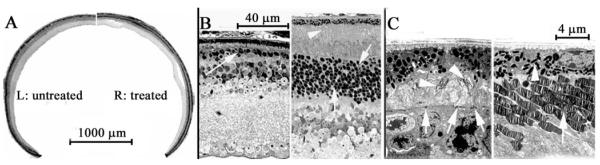
Light microscopic (LM) images of treated and untreated eyes from one rd10 mouse. (A) LM images at low magnification showing an untreated (left) and a treated (right) eye from the same rd10 mouse. (B) LM images from the central retina of both eyes of an rd 10 mouse 3 weeks after P14 injection with rAAV5-smCBA-PDEβ into one eye. Left: untreated eye. Right: treated eye. Arrows: photoreceptor nuclei. Arrowhead: photoreceptor outer segments. (C) Electron microscopic images from a 5-week-old rd10 mouse with one eye treated with AAV5-smCBA-PDEβ at P14. Left: untreated eye. Arrowheads: residues of outer segments. Arrow: outer limiting membrane. Right: treated eye. Arrowhead: RPE cells. Arrow: outer segments.
Rescue of Visually Guided Behavior
To determine whether the observed electrophysiological, biochemical, and structural preservation of the rd10 retina on vector treatment led to improvement in vision that may be useful in a behavioral sense, we tested several rd10 mice in a visually guided water maze task.26 After 4 days of training, analysis of times to find the platform under very dim light conditions showed that normal C57 mice averaged 9.7 ± 0.8 seconds, rd10 mice vector treated in one eye averaged 22.6 ± 4.2 seconds, and untreated rd10 mice averaged 51.5 ± 1.1 seconds (Fig. 5). Statistical analysis showed significant deterioration on the vision-guided performance task in untreated rd10 mice (n = 3) compared with normal C57 mice (n = 3; P < 0.0001). Although not as quick as normal C57 mice (P = 0.0192), rd10 mice (n = 4) treated in just one eye showed significantly improved vision-guided performance compared with untreated littermates (P = 0.0003).
Figure 5.
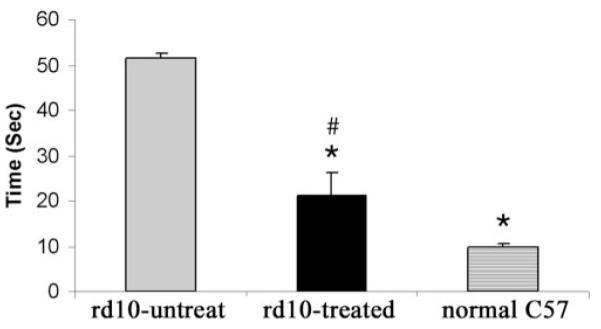
Water maze visually guided behavioral test showed the average times to the platform among untreated rd10 (left, gray bar; n = 3), treated rd10 (middle, black bar; n = 4), and age-matched normal C57 (right, solid diamonds bar; n = 3) mice. Symbols and bars represent mean ± SEM. *Significant difference between rd10-untreated and rd10-treated, and rd10-untreated and normal C57 mice. #Significant difference between rd10-treated and normal C57 mice.
Discussion
The rd10 mouse is a funduscopically identified RD mouse that has a recessive PDEβ mutation similar to one type of human retinitis pigmentosa.18 Although there is an early recordable scotopic a-wave, rod photoreceptor degeneration initiates at about P18 and progresses to only two to three ONL nuclei remaining at P30.19 In fact, photoreceptor outer segments in rd10 mice are never fully developed when animals are reared in a normal cyclic room light environment. This presents a significant challenge for gene therapy because we estimate it takes 1 to 2 weeks for AAV5-smCBA-PDEβ to express sufficient PDEβ protein for physiological demand in the rd10 retina, suggesting that the best timing for vector delivery would be P0 to P5 in rd10 mice. However, mouse eyes at this age have not yet opened and can be easily damaged by any invasive procedure. Transscleral subretinal injection is still possible in neonatal mice. Typically, however, less than 40% of the retina can be transfected, and many injection-related complications have been noted.13,15,17,27 Successful subretinal injection in this sense requires two elements—a relatively large transfected retinal area that can be reached by transcorneal subretinal injection and minimal injection-related damage—both difficult to achieve in very young mice but more easily achieved in older mice. Hence, the need is apparent to slow degeneration in the rd10 retina sufficiently to allow more mature animals to be tested while still allowing time for vector to express therapeutic levels of PDEβ protein before degeneration is too far advanced. Fortunately, we confirmed the observation that retinal degeneration could be slowed if rd10 mice are reared in darkness.19 The effect of light on accelerating retinal degeneration has been noted for the T4R rhodopsin dog30 and the T17M rhodopsin mouse.31 The observation we have made, that light also accelerates degeneration in mutant PDEβ mice, generalizes the phenomenon beyond rhodopsin mutations affecting retinal integrity.
Gene therapy improved dark- and light-adapted ERGs in rd10 mouse. In particular, the light-adapted b-wave amplitudes in the treated rd 10 mice were comparable to those of the normal wild-type mice. However, the timing of the light-adapted ERG b-wave of the treated rd10 eye remains delayed. Further investigation will be necessary to clarify whether the second exposure during ERG examination in P14+5W rd10 mouse accelerated the b-wave peak delay, whether it was related to the cone degeneration in rd10 mouse, or both. The general lack of a rescue effect in previous PDEβ gene therapy attempts using the rd1 mouse13–15 raises several questions about why the rd1 mouse did not respond well to gene replacement therapy though the rd10 mouse, tested here, did. First, is there something specific to recessive PDEβ mutations that confers the property of cryptic dominance when attempting gene replacement therapy, thus preventing more effective gene replacement therapy? Given that we clearly show an upregulation of the PDEβ vector transgene in treated retinas and structural, concomitant with electrophysiological and vision-guided behavioral preservation, this would seem to argue otherwise. However, a cryptically dominant property of the rd1 mutation not shared by the rd10 mutation cannot be eliminated. Second, is it simply that retinal degeneration in the rd1 mouse is too early and too rapid for AAV-vectored gene therapy to be effective? We believe this may be at least partially the case because slowing the degenerative process in the rd10 mouse through dark rearing allowed therapy to become more effective. Third, and related to the first two questions, are relatively well-developed photoreceptor outer segments necessary for PDEβ gene therapy to be successful? This may also be partially true. If what is needed for successful PDEβ therapy is a substantial fraction of the normal amount of PDEβ in the presence of other outer segment proteins—perhaps most important the major binding partners of PDEβ, PDEα, and PDEγ—then the expression of vectored PDEβ in dark-reared rd10 animals with normal outer segments is internally consistent with this hypothesis. Thus, it seems plausible that our ability to preserve photoreceptor structure and function in the rd10 mouse reflects its slower rate of degeneration, which allows vector-expressed PDEβ to be stably integrated into a normal complement of preexisting outer segment phototransduction components.
Although longer term rescue will clearly be required if gene replacement therapy for PDEβ-based RP is a viable therapeutic option, results reported here using the dark-reared rd10 mouse demonstrate that relatively complete rescue is possible. This model, therefore, seems well suited for modified gene therapy approaches that may express wild-type PDEβ more rapidly than current vectors and may offer the potential for longer term functional rescue. If achieved, this would represent a hopeful development for one of the more common and aggressive forms of human RP.
Acknowledgments
The authors thank Bo Lei (Veterinary Medicine and Surgery and Ophthalmology, University of Missouri-Columbia, Columbia, MO) for his suggestions during preparation of the ERG portion of this manuscript.
Supported by National Institutes of Health Grants EY018331, EY13729, EY11123, NS36302, EY08571, EY07758, EY014046, and EY06360 and by grants from the Macular Vision Research Foundation, Foundation Fighting Blindness, Juvenile Diabetes Research Foundation, and Research to Prevent Blindness, Inc., for partial support of this work.
Footnotes
WWH and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
Disclosure: J. Pang, None; S.L. Boye, None; A. Kumar, None; A. Dinculescu, None; W. Deng, None; J. Li, None; Q. Li, None; A. Rani, None; T.C. Foster, None; B. Chang, None; N.L. Hawes, None; J.H. Boatright, None; W.W. Hauswirth, P
References
- 1.Keeler C. Retinal degeneration in the mouse is rodless retina. J Hered. 1966;57:47–50. doi: 10.1093/oxfordjournals.jhered.a107462. [DOI] [PubMed] [Google Scholar]
- 2.Keeler CE. The inheritance of a retinal abnormality in white mice. Proc Natl Acad Sci U S A. 1924;10:329–333. doi: 10.1073/pnas.10.7.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowes C, Li T, Frankel WN, et al. Localization of a retroviral element within the rd gene coding for the beta subunit of cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 1993;90:2955–2959. doi: 10.1073/pnas.90.7.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farber DB, Lolley RN. Enzymatic basis for cyclic GMP accumulation in degenerative photoreceptor cells of mouse retina. J Cyclic Nucleotide Res. 1976;2:139–148. [PubMed] [Google Scholar]
- 6.Danciger M, Blaney J, Gao YQ, et al. Mutations in the PDE6B gene in autosomal recessive retinitis pigmentosa. Genomics. 1995;30:1–7. doi: 10.1006/geno.1995.0001. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin ME, Ehrhart TL, Berson EL, Dryja TP. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995;92:3249–3253. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caley DW, Johnson C, Liebelt RA. The postnatal development of the retina in the normal and rodless CBA mouse: a light and electron microscopic study. Am J Anat. 1972;133:179–212. doi: 10.1002/aja.1001330205. [DOI] [PubMed] [Google Scholar]
- 9.Farber DB, Lolley RN. Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974;186:449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- 10.LaVail MM, Sidman RL. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974;91:394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- 11.LaVail MM, Sidman RL. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974;91:394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- 12.LaVail MM, Matthes MT, Yasumura D, Steinberg RH. Variability in rate of cone degeneration in the retinal degeneration (rd/rd) mouse. Exp Eye Res. 1997;65:45–50. doi: 10.1006/exer.1997.0308. [DOI] [PubMed] [Google Scholar]
- 13.Bennett J, Tanabe T, Sun D, et al. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 14.Jomary C, Vincent KA, Grist J, Neal MJ, Jones SE. Rescue of photoreceptor function by AAV-mediated gene transfer in a mouse model of inherited retinal degeneration. Gene Ther. 1997;4:683–690. doi: 10.1038/sj.gt.3300440. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Miyoshi H, Verma IM, Gage FH. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol. 1999;73:7812–7816. doi: 10.1128/jvi.73.9.7812-7816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar-Singh R, Farber DB. Encapsidated adenovirus mini-chromosome-mediated delivery of genes to the retina: application to the rescue of photoreceptor degeneration. Hum Mol Genet. 1998;7:1893–1900. doi: 10.1093/hmg/7.12.1893. [DOI] [PubMed] [Google Scholar]
- 17.Dejneka NS, Surace EM, Aleman TS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9:182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 19.Chang B, Hawes NL, Pardue MT, et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007;47:624–633. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2006;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boatright JH, Moring AG, McElroy C, et al. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis. 2006;12:1706–1714. [PubMed] [Google Scholar]
- 22.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 23.Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander JJ, Umino Y, Everhart D, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min SH, Molday LL, Seeliger MW, et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of X-linked juvenile retinoschisis. Mol Ther. 2005;12:644–651. doi: 10.1016/j.ymthe.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13:565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Pang JJ, Lauramore A, Deng WT, et al. Comparative analysis of in vivo and in vitro AAV vector transduction in the neonatal mouse retina: effects of serotype and site of administration. Vision Res. 2008;48:377–385. doi: 10.1016/j.visres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Haire SE, Pang J, Boye SL, et al. Light-driven cone arrestin translocation in cones of postnatal guanylate cyclase-1 knockout mouse retina treated with AAV-GC1. Invest Ophthalmol Vis Sci. 2006;47:3745–3753. doi: 10.1167/iovs.06-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang JJ, Chang B, Hawes NL, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- 30.Gu D, Beltran WA, Li Z, Acland GM, Aguirre GD. Clinical light exposure, photoreceptor degeneration, and AP-1 activation: a cell death or cell survival signal in the rhodopsin mutant retina? Invest Ophthalmol Vis Sci. 2007;48:4907–4918. doi: 10.1167/iovs.07-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White DA, Fritz JJ, Hauswirth WW, Kaushal S, Lewin AS. Increased sensitivity to light-induced damage in a mouse model of autosomal dominant retinal disease. Invest Ophthalmol Vis Sci. 2007;48:1942–1951. doi: 10.1167/iovs.06-1131. [DOI] [PubMed] [Google Scholar]