Abstract
Retinal vasculature related pathologies account for a large proportion of global blindness. Choroidal neovascularization accompanying age-related macular degeneration is the largest cause of blindness in people over the age of 65 years, proliferative diabetic retinopathy is the main cause of acquired blindness in working adults, and retinopathy of prematurity (ROP) is the leading cause of acquired blindness in children. Given the great success in treating the first category of these conditions with anti-vascular endothelial growth factor (anti-VEGF) therapy, there is understandably considerable interest to employ this strategy to other retinal vascular disorders. Anti-VEGF therapy may not be the optimal course of action, as it may compromise neuronal survival; this is of particular concern when treating ROP where retinal neurogenesis is still not complete. Moreover, retinal neovascularization is preceded by alterations in the vascular wall extracellular matrix with concomitant reduction in mural cell adhesion. This produces vascular instability followed by the pathobiologic process of neovascularization. Thus, stabilizing mural cell-matrix interactions would be a prudent alternative for controlling retinal vascular pathologies. In this review, we will summarize the development of retinal angiogenesis focusing on the role of cell-matrix interaction in each step of the process. Our goal is to identify potential targets for regulating and maintaining normal vascular development and function.
Keywords: Retinal Vascular Disease, Neovascularization, Age-related Macular Degeneration, Cell-Matrix Interface
Introduction
Retinal angiogenesis is a complex process which involves cell-cell and cell-matrix interactions. Any abnormality affecting the retinal vasculature during development or at later stages of life will lead to severe pathologies resulting in blindness. For example, retinopathy of prematurity (ROP) is a retinal vasculature associated pathology occurring in premature infants, proliferative diabetic retinopathy (PDR) occurs in working age adults and wet age-related macular degeneration (AMD) affects older adults.1-4
Together, these retinal vasculature associated pathologies are major causes of global blindness. To treat or to find a novel target for these complex pathologies it is necessary to carefully understand both cell intrinsic and extrinsic players which regulate the development and function of retinal blood vessels. The goal of this review is to summarize the role of extracellular matrix (ECM)-cell interactions during development and their contribution to the pathobiologic events of neovascularization, and to identify potential therapeutic candidates to control disease progression. We will limit this discussion to intra-retinal events leaving aside for the most part issues related to choroidal neovascularization.
Embryonic Blood Supply
Early in development, the inner portion of the eye is nourished by the hyaloid vascular system (HVS) which is a transient intraocular arterial supply attached to the posterior pole of the lens.5 After birth, myeloid cells mediate gradual regression of the HVS.6,7 In human disease conditions such as ROP, familial exudative vitreoretinopathy and Pearson’s syndrome, regression of the HVS is altered, resulting in persistent hyaloid vessels.8-10 Mouse models in which various laminin chains are deleted also show persistent hyaloid vessels, as do mouse mutants in which laminin binding is disrupted and also with other basement membrane mutants.11-15
Regulation of hyaloid vessel regression by laminins is mediated via integrin a6b1. Integrin activation results in changes in chemokines such as tumor necrosis factor (TNF), interferon (IFN)-a, and IFN-g and these chemokines in turn may interact with microglia.16 Hence, the effects of laminin deletion are probably the result of failure to activate or recruit microglia during hyaloid vessel regression. It is of interest to analyze clinical samples where persistent fetal vessels are observed for alterations in ECM pathways, particularly integrin-mediated pathways. If these pathways are deficient, it may then be possible to transiently activate microglia to promote HSV regression.
Retinal Vascular Development
The retinal vascular system consists of three interconnected vascular layers: superficial, intermediate and deep. Development of the superficial layer is the initial step in retinal angiogenesis and is dependent on astrocytes.5 Once the superficial vasculature reaches the periphery of the retina, the deep and intermediate plexiform layers are formed; Muller cells regulate these later stages of retinal vascularization17 (Fig. 1).
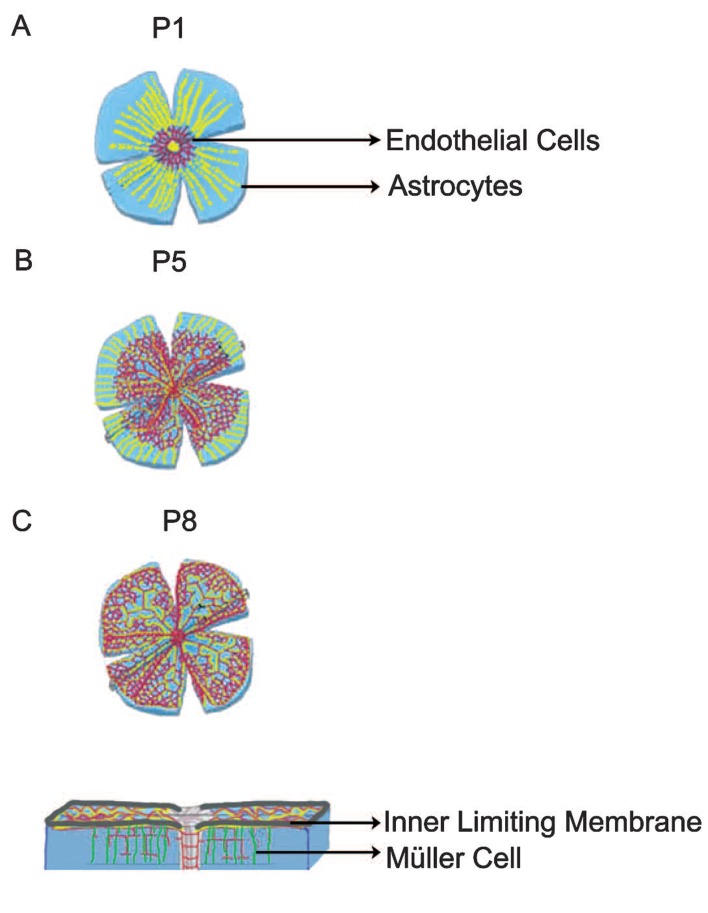
Figure 1.
Astrocytes and Müller cells guide endothelial cells. (A) At postnatal day 1 (P1), astrocytes (yellow) migrate into the retina through the optic nerve head and make a template over which endothelial cells migrate. (B) By postnatal day 5 (P5), endothelial cells cover more than half of the retinal surface. (C) By postnatal day 8 (P8), superficial blood vessel formation is completed. At this point, Müller cells (green) attract the endothelial cells which start to form deep and intermediate vascular layers. This process is completed around postnatal day 15 (not shown). [Modified from Gerhardt et al, 2003. Originally published in The Journal of Cell Biology; doi: 10.1083/jcb.200302047]18
Astrocyte Migration into the Retina
Astrocytes are star-shaped glial cells that regulate a wide variety of functions in the central nervous system.19 One of their most well-characterized functions is the regulation of retinal angiogenesis and participation in the blood-retinal barrier20 (Fig. 2A). Retinas are only vascularized in mammalians in the presence of retinal astrocytes. Retinas lacking astrocytes remain avascular and those in which astrocyte distribution is limited have spatially limited vascular development. For example, in adult possums, astrocytes are distributed only around the optic disc and the optic disc remains the only vascularized region of the possum retina.21 In human and non-human primate retinas, the avascular foveal region also lacks astrocytes.22,23 These data suggest that retinal blood vessel formation is strictly dependent upon astrocytes.
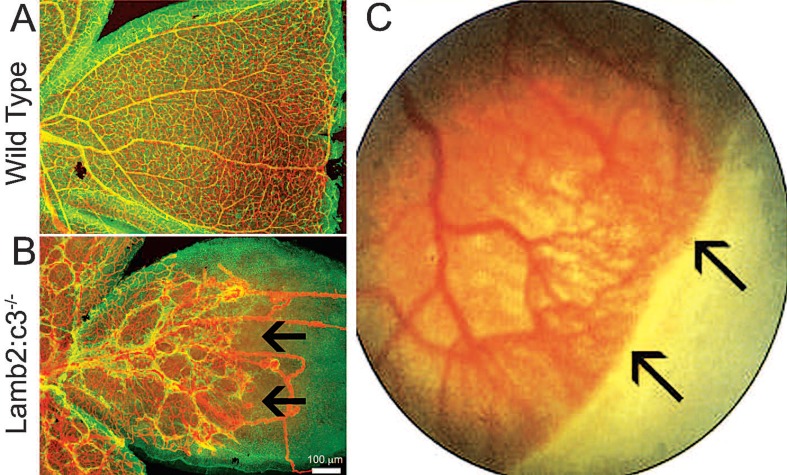
Figure 2.
Vascular growth in peripheral retina is affected in laminin nulls as well as infants with retinopathy of prematurity (ROP). *(A) Postnatal day 15 wild type retina was immunostained for glial fibrillary acidic protein (GFAP) (green) to label astrocytes and CD31 (Red) to label blood vessels. *(B) Postnatal day 15 Lamb2:c3 null retina was immunostained for GFAP (green) to label astrocytes and CD31 (Red) to label blood vessels. Notice abnormal astrocyte patterning and vascular growth. Astrocyte patterning and subsequent vascular growth was affected in the peripheral retina (black arrows). **(C) Black arrows at the peripheral retina show no vascular growth in an infant with ROP. *[Data in A and B are from the author’s laboratory]. **[Reprinted by permission from Nature Publishing Group. Gariano and Gardner, 2005. Originally published in Nature; 438:960-966.]3
Retinal astrocytes are generated from astrocyte precursor cells outside the retina in the optic nerve. The astrocyte precursors express Pax-2 transcription factor which is critical for astrocyte determination and differentiation.24-27 In order to populate the retina, differentiating astrocytes enter the retina through the optic nerve head and migrate over the vitreal surface.28
Astrocytes invade the mouse retina around embryonic days 17-1824,29 (Fig. 1). Upon entering the retina, a proliferating population of astrocytes spreads toward the periphery in a centrifugal fashion.5 The proliferation of astrocytes during this migration is regulated by platelet-derived growth factor (PDGF)-A secreted by ganglion cells.5,30 Overexpression of PDGF-A or neutralization of the PDGF receptor, PDGFR-a, affects astrocyte proliferation and network formation.30 These observations demonstrate that PDGF-A signaling is crucial for astrocyte proliferation.
In addition, the mouse homolog of drosophila tailless, Tlx (Nr2e1), is critical for astrocyte morphogenesis. Mice lacking the Tlx gene display abnormal astrocyte morphology, poor astrocyte scaffold formation and complete failure of assembly in the extracellular matrix.31,32 Although these studies have provided valuable information regarding the regulation of astrocyte proliferation and differentiation, factors that guide astrocytes into the retina remain unknown.
Regulation of Astrocyte Migration by Laminins
Basement membranes (BM) are acellular extracellular matrices which separate tissue compartments and serve as key regulators of differentiation and migration in a variety of tissues including the retina. Classically these structures are composed of laminin, collagen type IV, nidogen and heparin sulfate protoglycans.33 Laminin deposition is the primary and key step in BM formation; the laminin family is a relatively small group of heterotrimeric glycoproteins. A laminin isoform is composed of an a chain, a b chain, and a g chain. There are 5 a, 3 b and 3 g chains identified and a total of 16 laminin isoforms have been isolated and confirmed so far.33 Interactions between laminins and integrins are isoform specific and regulate migration of a variety of cells in different tissues including the retina.33-36 The specificity of laminin’s biological activity is presumed to arise from its differential expression: spatially, in adult tissues and temporally, during development.
Our studies have shown that deletion of the Lamb2 gene (encoding the laminin b2 chain) alone, as well as in combination with the Lamc3 gene (encoding the laminin g3 chain), affects the assembly of one BM in the retina, the inner limiting membrane (ILM).37 Importantly, deletion of these laminin genes, singly or in combination also affects astrocyte migration and patterning12 (compare Figures 2A and 2B). A similar defect in astrocyte patterning and vascular growth is present in Lama1 gene (encoding the laminin a1 chain) mutant retinas.11 These results suggest that laminins in the ILM guide and regulate astrocyte migration. This hypothesis is supported by several ex vivo experiments. Specifically, the addition of exogenous laminin onto the retinal surface of cultured Lamb2:c3 compound null retina rescued both astrocyte migration and spatial patterning.12 Astrocyte spatial patterning is critical for subsequent vascular growth because the astrocyte-derived template guides endothelial migration and patterning.18,32,38,39
Tip Cell Selection and Endothelial Cell Migration
It has been suggested that the astrocyte template secretes a fibronectin-rich matrix, which sequesters vascular endothelial growth factor (VEGF) and subsequently guides endothelial cells.18,32 However, astrocyte-specific deletion of fibronectin only delays vascular growth progression and does not completely abolish endothelial growth suggesting that astrocyte-derived fibronectin is not necessary for endothelial development.40 A critical role for other matrix molecules is likely since the canonical matrix receptors, integrin b1, is expressed by endothelial cells and they have been shown to regulate vascular growth and branching.41,42 Given the data discussed above regarding laminins and the fact that b1 integrins are strong laminin binding molecules, there is little doubt that interaction between ligand (laminin) and receptor (integrins) play a key step in endothelial patterning and constitute an attractive therapeutic target.
The elongating vascular tube consists of two endothelial cell types: leading tip cells perform sensory function responding to cues, and the lagging stalk cells form the more stable vessel wall. Thus, regulation of the number and behavior of both tip and stalk cells determine the rate of vascular growth and the density of branching. Initially, the formation of tip cells is induced by astrocyte-derived VEGF-A gradient. These tip cells express the VEGF receptor (VEGFR)-2,18 which allows tip cells to respond to the astrocyte-derived VEGF-A gradient. In addition to this VEGF-A-VEGFR2 signaling pathway, the VEGF-C-VEGR3 pathway also regulates tip-stalk cell formation in the retina.43,44
Vascular density is regulated by regulating tip cell production; specifically the number of tip cells determines branching points in the elongating vessel. Tip cell selection is regulated by a notch-delta like ligand-4 (Dll4) signaling mechanism.45,46 Deletion of one of the alleles for Dll4 and endothelial specific deletion of notch-1 increases tip cell number.45 Together, these results suggest that activation of the notch-Dll4 pathway is critical for controlled vascular growth. Moreover, laminin-integrin mediated signaling is thought to modulate endothelial tip cell selection through a notch- Dll4 signalling pathway suggesting that the matrix plays an important role in modifying vascular patterning and thereby density.47
In summary, communication between cell extrinsic and intrinsic molecules is necessary to achieve proper vascular growth and patterning. An imbalance in the interactions between cell extrinsic and intrinsic signaling will affect vascular development. Moreover, it is likely that neovascularization events are the result of disruptions anywhere in these pathways. Thus, the most effective treatment of vascular related pathologies will result from careful manipulation of the complex signaling pathways involved. The role of the clinician scientist will be to carefully define the etiology of the vascular defects and construct a rational therapeutic strategy.
Vascular Specification and Branching
Other critical events that occur during vascular growth progression include specification of arteries and veins, and their branching. The exact mechanism by which some endothelial cells take up arterial and venous fate is not completely understood. However, it has been suggested that ephrins and Eph receptors play important roles. The differential expression of ephrin-B2 ligand and the EphB4 receptor segregate endothelial cells from each other. The endothelial cells in arteries express ephrin-B2, whereas endothelial cells in veins express EphB4.29,48,49 These data suggest that specific ephrins including ephrin-B2 play a role in specifying arterial fate, and specific Ephs including EphB4 play a role in specifying venous fate.
The combinatorial action of the specific VEGF-A isoforms 188, 164, and 120 also regulates vascular specification and branching.50,51 For instance, mice solely expressing the heparan sulfate binding isoform, VEGF188 (VEGF188/188), show excessive branching and impaired arterial specification.51 On the other hand, mice expressing only the diffusible VEGF120 (VEGF120/120) show severe defects in vascular branching as well as impairment of arterial specification.50,51 The hypothesis is that matrix bound VEGF serves to orient tip cell extension and thereby vascular branching; the loss of the matrix bound form disorients this process. Importantly, when VEGF188 was ectopically expressed, in VEGF120/120 animals, vascular defects were rescued.50 Mice expressing only VEGF164, which has both diffusible and heparan sulfate binding characteristics, display no vascular branching or specification defects.51 Taken together, these findings suggest that both matrix-bound and diffusible VEGFs are required for vascular growth progression, specification and branching.
Recruitment of Smooth Muscle Actin Expressing Cells and Blood Vessel Maturation
Vessel branching and elongation are initial stages of angiogenesis, following this step the vessel wall must become stabilized. This stabilization is achieved during vascular growth when endothelial cells attract a class of actin-expressing smooth muscle cells (SMCs), the pericyte, into the vascular wall. Endothelial cells are thought to secret PDGF-B, the incorporation of pericytes as mural cells leads to stabilization of the vascular basement membrane and subsequently the vascular wall.28 Intraperitoneal injection of monoclonal antibodies directed against PDGFR-b affects pericyte recruitment and blood vessel integrity, leading to vascular leakage.52
Migration of SMCs over the vascular wall is modulated by interactions between the cellular environment and cell surface receptors including integrins containing the integrin b1 subunit.52 For example, cell specific deletion of integrin b1 affects SMC migration and integration into the vascular wall which destabilizes overall vascular integrity53 (Fig. 3A). Once SMCs are recruited into the vascular wall, they in turn secrete stabilizing factors such as angiopoetin-1 (Ang-1) and transforming growth factor-b (TGF-b). Both Ang-1 and TBG-b are critical for endothelial stabilization of the vascular wall including deposition of basement membrane molecules over the vascular wall52,54,55 (Figures 3A, 3C and 3D). Thus, interaction of SMCs with the vascular wall is critical for vascular stability.
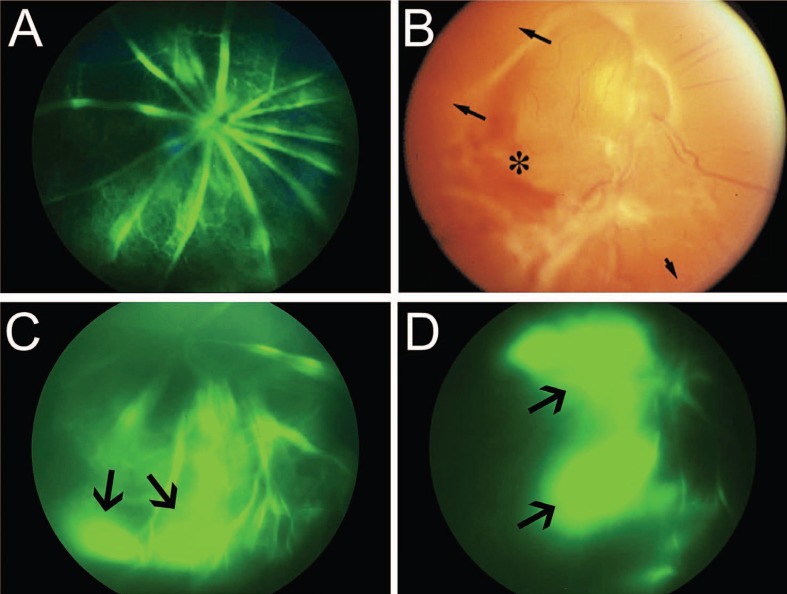
Figure 3.
Defective vascular integrity in laminin null retinas and advanced stage 4 retinopathy of prematurity (ROP). *(A) Fluorescein angiography in wild type P15 mouse retina shows no signs of vascular leakage approximately 120 sec after intra-peritoneal injection of sodium fluorescein. **(B) Advanced stage 4 ROP reveals hemorrhage (asterisk) and avascular peripheral retina (black arrows). *(C) Fluorescein angiography in Lamb2 null retina shows vascular leakage approximately 120 sec after intra-peritoneal injection of sodium fluorescein (black arrow). *(D) Fluorescein angiography in Lamb2:c3 null retina shows vascular leakage approximately 120 sec after intra-peritoneal injection of sodium fluorescein (black arrows). *[Data in A, C and D are from the author’s laboratory]. **[Reprinted with permission from the International Journal of Developmental Biology. Saint-Geniez and D‘Amore, 2004. Originally published in Int J Dev Biol; 48:1045-1058]61
Moreover, the loss of mural cells such as the pericyte is a key step in destabilizing an existing vessel. Interference with pericyte mural adhesion results in the death of the pericyte by anoikis.56 Studies have suggested that manipulation of mural cell-ECM interactions is likely to be important therapeutically.57,58
Formation of Deeper Vascular Plexus
After the formation of the superficial vascular plexus is complete, deep and intermediate vascular plexiform layers start to develop. The development of these layers is complete at approximately postnatal day 15 in mice. These differentiation events occur through several signaling pathways, including those involving Wnts and VEGF.17,59
Wnts expressed by microglia suppress excessive vascular branching through non-canonical Wnt-Flt1 pathway. Microglial specific deletion of the Wnt ligand transporter, Wntless, results in excessive vascular branching in the deep vascular layer.59 Thus, the Wnt pathway is a component during formation of the deep vascular plexus.
The high metabolic demand caused by maturation of cells in the outer retina (particularly with the onset of functional synapses) causes Müller cells to secrete VEGF.5,17 In response to this burst of extracellular VEGF, endothelial cells of veins and venules in the superficial vascular layer extend along Müller cell processes and form the deep and intermediate vascular layers5 (Fig. 1). Unlike superficial capillary branching which was regulated by astrocyte-endothelial interactions, during the formation of these deeper beds, microglial cells regulate vascular branching of deep and intermediate vascular layers.59 Studies on the formation of the deep vascular plexus have provided valuable insight into vascular regulation, but further studies are required to reveal the role of other factors such as delta and notch signaling in this process.
Overall, a major question is how to tackle the complex process of vascular remodeling under pathological conditions, i.e., during neovascularization. As demonstrated above, the molecular processes of angiogenesis during development respond to environmental factors such as metabolic demand to increase vascular supply. These mechanisms remain active throughout adulthood, albeit without the temporal regulation exerted during normal development.
One of the leading causes of neovascularization is hypoxia-driven VEGF expression. Hypoxia-induced, VEGF-mediated signaling promotes abnormal new blood vessel formation.1 However, anti-VEGF therapy may not be appropriate for all conditions, because VEGF-A is an important factor for neuronal survival under ischemia;60 this matter is likely to be particularly important in the premature infant. Thus, finding new targets to stabilize the vasculature would generate a better method to treat pathological neovascularization. The cell-matrix interface is a profoundly important target. Recent studies have reported abnormalities at the cell-matrix interface during pathological conditions (summarized below) and indeed some initial steps have been made in using mural cell-ECM interactions as therapies (above). These successes provide an impetus for further therapeutic developments.
Changes in Cell-Matrix Interactions During Retinal Vasculature Pathologies related to Retinopathy of Prematurity
ROP occurs in two phases: in the first phase, vascular growth becomes attenuated; in the second phase, neovascular sprouts develop in response to VEGF secretion.1 Both phases are likely dependent on cell-matrix interactions. Blood vessel growth in the peripheral retina is severely affected in infants with ROP (Fig. 2C).3 This defective growth could be a result of defective astrocyte migration and template formation in the peripheral retina. As detailed above the template on which astrocytes migrate (Fig. 2A) and vessels form is comprised, in part, of laminins. Deletion of laminin chains contributes to defective astrocyte patterning and blood vessel formation.11,12 Astrocyte distribution is severely affected in Lamb2 null retinas (data not shown) and Lamb2:c3 double null retinas (Fig. 2B). This disruption of astrocyte distribution in laminin null animals is particularly marked in peripheral regions of the retina, where there is poor or no blood vessel formation.12 In addition, infants with ROP also show signs of vascular leakage (Fig. 3B),9 a feature shared with Lamb2 nulls and Lamb2:c3 double null retinas (Figures 3C and 3D; control figure 3A). Because of this similarity, it is of interest to analyze whether the expression and distribution of laminins are affected in ROP models. If there were a loss of laminins in ROP models, rescue of laminin expression may help astrocytes to migrate and pattern properly. This rescue would then allow endothelial cells to re-vascularize in a normal fashion.
The defective vascular growth observed in infants with ROP could also be due to degeneration of astrocytes under hyper/hypoxic conditions. In experimental animal models, astrocytes degenerate under hypoxic conditions.63,64 In addition, brain derived astrocytes lose integrin (a1, a6, b1) and dystroglycan receptors under ischemic conditions.65,66
It is likely that loss of contact with BM causes astrocyte degeneration in ROP. It is also possible that remodeling of BM during cyclic oxygen conditions affects the astrocyte-BM interaction resulting in cell death, because hypoxia has shown to activate matrix metalloproteinases (MMPs). Activation of MMPs affects cellular interaction and causes vascular leakage.67 Maintaining the astrocyte population under hypoxic conditions reduces neovascularization.63 Thus, it is possible that stabilizing astrocyte and endothelial interactions with the BM might reduce the risk of neovascularization in ROP.
Changes in Cell-Matrix Interactions in Diabetic Retinopathy
One of the key clinical features of diabetic retinopathy is BM thickening, with a marked increase in collagen IV deposition.68 Rat models of diabetic retinopathy also have increased levels of fibronectin and experimental down-regulation of fibronectin in these models improves vascular lesions.69 Moreover, laminin b2 chain synthesis is decreased by elevated glucose in both experimental animal models and in vitro studies on the kidney.70,71
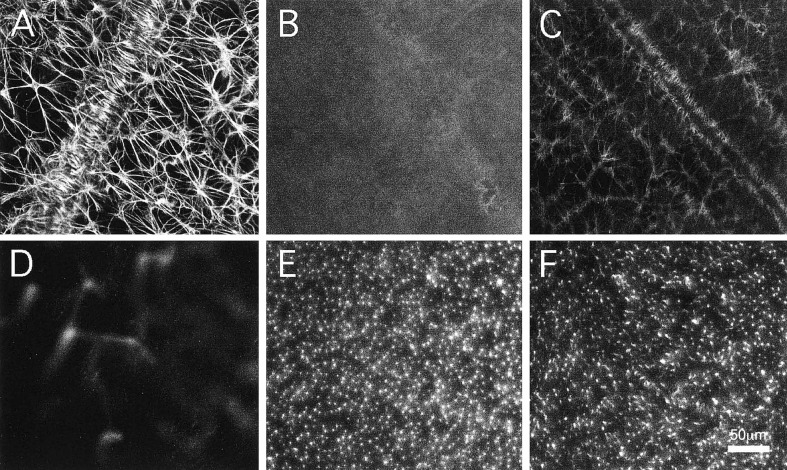
Laminins are critical for astrocyte and Müller cell interaction with the ILM in the retina.12,72 In addition, mutations in the LAMB2 gene lead to Pierson syndrome8,73 and animals with mutations in this gene phenocopy many aspects of the human disease.36,74 It will be of interest to analyze whether the expression of collagens, fibronectin and laminins are altered in the hyperglycemic retina, as hyperglycemia has been shown to cause astrocyte degeneration and induce glial fibrillary acidic protein (GFAP) expression in Müller cells, which is suggestive of reactive gliosis (Fig. 4).62,75 These abnormalities in glial cells could be due to remodelling of the matrix or result from changes involving cell surface receptors. In addition to changes in glial cells, hyperglycemia also causes loss of mural pericytes, which leads to mural instability, vascular leakage and subsequent neovascularization.76,77 As noted above, mural cell adhesion to the vascular wall is mediated through integrins.53
Figure 4.
Experimental rat model for diabetes shows loss of GFAP expression in astrocytes and reactive gliosis of Müller cells. (A) Control flat-mount retina showing glial fibrillary acidic protein (GFAP) expression in astrocytes. (B) Streptozotocin (STZ)-diabetic rats show loss of GFAP expression in astrocytes after 4 months of induction of diabetes. (C) Insulin treatment for 48 hr of STZ-diabetic rats shows partial recovery of GFAP expression in astrocytes. (D) Control rats show no GFAP immunoreactivity in Müller cells (image focused on the outer plexiform layer). (E) STZ-diabetic rats show GFAP expression in Müller cells after 4 months of induction of diabetes (image focused on the outer plexiform layer). (F) Insulin treatment for 48 hr of STZ-diabetic rats shows partial reduction of GFAP in Müller cells (image focused on the outer plexiform layer). [Reprinted with permission from Association for Research in Vision and Ophthalmology as the copyright holder. Barber et al, 2000. Originally published in Investigative Ophthalmology & Visual Science; 4: 3561-3568]62
In conclusion, the data above suggest that coordinated signaling between cell extrinsic ECM molecules and cell intrinsic molecules are necessary for proper retinal vascular development and function. Experimental animal models of ischemia and PDR suggest that the first insult occurs at the mural cell-ECM interface. Therefore, a careful proteomic study accompanied with a microarray analysis will better clarify changes taking place with BM molecules and their interacting cell surface receptors in experimental ROP and diabetic retinopathy models. In addition, the results of these analyses may contribute to the discovery of additional ways to stabilize cell surface receptor interactions with BM molecules, which will ultimately prevent cell death in the retina and reduce neovascularization.
Acknowledgments
Supported by NIH EY12676 and in part by an unrestricted grant from the Research to Prevent Blindness Inc. to the Department of Ophthalmology.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr. 2011;23:173–178. doi: 10.1097/MOP.0b013e3283423f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Smith LE. Protective inflammasome activation in AMD. Nat Med. 2012;18:658–660. doi: 10.1038/nm.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 6.Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–3638. doi: 10.1242/dev.124.18.3633. [DOI] [PubMed] [Google Scholar]
- 7.Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009;16:1395–1407. doi: 10.1038/cdd.2009.78. [DOI] [PubMed] [Google Scholar]
- 8.Bredrup C, Matejas V, Barrow M, Blahova K, Bockenhauer D, Fowler DJ, et al. Ophthalmological aspects of Pierson syndrome. Am J Ophthalmol. 2008;146:602–611. doi: 10.1016/j.ajo.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Nissenkorn I, Kremer I, Ben-Sira I. Association of a persistent hyaloid artery and ROP. Ophthalmology. 1988;95:559–560. doi: 10.1016/s0161-6420(88)33151-9. [DOI] [PubMed] [Google Scholar]
- 10.Chang-Godlinich A, Paysse EA, Coats DK, Holz ER. Familial exudative vitreoretinopathy mimicking persistent hyperplastic primary vitreous. Am J Ophthalmol. 1999;127:469–471. doi: 10.1016/s0002-9394(99)00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Edwards MM, Mammadova-Bach E, Alpy F, Klein A, Hicks WL, Roux M, et al. Mutations in Lama1 disrupt retinal vascular development and inner limiting membrane formation. J Biol Chem. 2010;285:7697–7711. doi: 10.1074/jbc.M109.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnanaguru G, Chew J, Pinzon-Duarte G, Bachay G, Brunken WJ. Astrocyte migration and vascular development in the retina are regulated by laminin mediated signaling mechanisms. ARVO. 2012 [Google Scholar]
- 13.Lee Y, Kameya S, Cox GA, Hsu J, Hicks W, Maddatu TP, et al. Ocular abnormalities in Large (myd) and Large (vls) mice, spontaneous models for muscle, eye, and brain diseases. Mol Cell Neurosci. 2005;30:160–172. doi: 10.1016/j.mcn.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Kanesaki H, Igarashi T, Kameya S, Yamaki K, Mizota A. Reactive gliosis of astrocytes and Muller glial cells in retina of POMGnT1-deficient mice. Mol Cell Neurosci. 2011;47:119–130. doi: 10.1016/j.mcn.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Hurskainen M, Eklund L, Hagg PO, Fruttiger M, Sormunen R, Ilves M. Et al. Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J. 2005;19:1564–1566. doi: 10.1096/fj.04-3101fje. [DOI] [PubMed] [Google Scholar]
- 16.Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the alpha6beta1 integrin. J Neurosci. 2002;22:1562–1572. doi: 10.1523/JNEUROSCI.22-05-01562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner TW, Lieth E, Khin SA, Barber AJ, Bonsall DJ, Lesher T, et al. Astrocytes increase barrier properties and ZO-1 expression in retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 1997;38:2423–2427. [PubMed] [Google Scholar]
- 21.Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987;255:35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- 22.Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol. 2008;126:507–511. doi: 10.1001/archopht.126.4.507. [DOI] [PubMed] [Google Scholar]
- 23.Provis JM, Sandercoe T, Hendrickson AE. Astrocytes and blood vessels define the foveal rim during primate retinal development. Invest Ophthalmol Vis Sci. 2000;41:2827–2836. [PubMed] [Google Scholar]
- 24.Chan-Ling T, Chu Y, Baxter L, Weible Ii, Hughes S. In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia. 2009;57:39–53. doi: 10.1002/glia.20733. [DOI] [PubMed] [Google Scholar]
- 25.Chu Y, Hughes S, Chan-Ling T. Differentiation and migration of astrocyte precursor cells and astrocytes in human fetal retina: relevance to optic nerve coloboma. FASEB J. 2001;15:2013–2015. doi: 10.1096/fj.00-0868fje. [DOI] [PubMed] [Google Scholar]
- 26.Soukkarieh C, Agius E, Soula C, Cochard P. Pax2 regulates neuronal-glial cell fate choice in the embryonic optic nerve. Dev Biol. 2007;303:800–813. doi: 10.1016/j.ydbio.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Sehgal R, Karcavich R, Carlson S, Belecky-Adams TL. Ectopic Pax2 expression in chick ventral optic cup phenocopies loss of Pax2 expression. Dev Biol. 2008;319:23–33. doi: 10.1016/j.ydbio.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988;332:834–837. doi: 10.1038/332834a0. [DOI] [PubMed] [Google Scholar]
- 29.Uemura A, Kusuhara S, Katsuta H, Nishikawa S. Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res. 2006;312:676–683. doi: 10.1016/j.yexcr.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Fruttiger M, Calver AR, Kruger WH, Mudhar HS, Michalovich D, Takakura N, et al. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron. 1996;17:1117–11131. doi: 10.1016/s0896-6273(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 31.Miyawaki T, Uemura A, Dezawa M, Yu RT, Ide C, Nishikawa S, et al. Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J Neurosci. 2004;24:8124–8134. doi: 10.1523/JNEUROSCI.2235-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S. Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J Clin Invest. 2006;116:369–377. doi: 10.1172/JCI25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiwara H, Gu J, Sekiguchi K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp Cell Res. 2004;292:67–77. doi: 10.1016/j.yexcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. doi: 10.1002/jnr.490270319. [DOI] [PubMed] [Google Scholar]
- 36.Desban N, Lissitzky JC, Rousselle P, Duband JL. Alpha1beta1-integrin engagement to distinct laminin-1 domains orchestrates spreading, migration and survival of neural crest cells through independent signaling pathways. J Cell Sci. 2006;119:3206–3218. doi: 10.1242/jcs.03057. [DOI] [PubMed] [Google Scholar]
- 37.Pinzon-Duarte G, Daly G, Li YN, Koch M, Brunken WJ. Defective formation of the inner limiting membrane in laminin beta2- and gamma3-null mice produces retinal dysplasia. Invest Ophthalmol Vis Sci. 2010;51:1773–1782. doi: 10.1167/iovs.09-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- 39.Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43:522–527. [PubMed] [Google Scholar]
- 40.Stenzel D, Lundkvist A, Sauvaget D, Busse M, Graupera M, van der, et al. Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development. 2011;138:4451–4463. doi: 10.1242/dev.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malan D, Wenzel D, Schmidt A, Geisen C, Raible A, Bolck B, et al. Endothelial beta1 integrins regulate sprouting and network formation during vascular development. Development. 2010;137:993–1002. doi: 10.1242/dev.045377. [DOI] [PubMed] [Google Scholar]
- 42.Tanjore H, Zeisberg EM, Gerami-Naini B, Kalluri R. Beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn. 2008;237:75–82. doi: 10.1002/dvdy.21385. [DOI] [PubMed] [Google Scholar]
- 43.Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 44.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 46.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estrach S, Cailleteau L, Franco CA, Gerhardt H, Stefani C, Lemichez E, et al. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res. 2011;109:172–182. doi: 10.1161/CIRCRESAHA.111.240622. [DOI] [PubMed] [Google Scholar]
- 48.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 50.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abraham S, Kogata N, Fassler R, Adams RH. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- 54.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 55.Ramsauer M, D'Amore PA. Contextual role for angiopoietins and TGFbeta1 in blood vessel stabilization. J Cell Sci. 2007;120:1810–1817. doi: 10.1242/jcs.003533. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaquor B. Cysteine-rch protein 61 and connective tissue grouwth factor induce deadhesion and anoikis of retinal pericutes. Endocrinology. 2008;149:1666–1677. doi: 10.1210/en.2007-1415. [DOI] [PubMed] [Google Scholar]
- 57.Caballero S, Yang R, Grant MB, Chaqour B. Selective blockade of cytoskeletal actin remodeling reduces experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2011;52:2490–2496. doi: 10.1167/iovs.10-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasan A, Pokeza N, Shaw L, Lee H, Lazzaro D, Chintala H, et al. The matricellular protein cysteine-rich protein 61 (CCN1/Cyr61) enhances physiological adaptation of retinal vessels and reduces pathological neovascularization associated with ischemic retinopathy. J Biol Chem. 2011;286:9542–9554. doi: 10.1074/jbc.M110.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefater JA, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 62.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;4:3561–3568. [PubMed] [Google Scholar]
- 63.Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, et al. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010;58:43–54. doi: 10.1002/glia.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37:290–299. [PubMed] [Google Scholar]
- 65.Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tagaya M, Haring HP, Stuiver I, Wagner S, Abumiya T, Lucero J, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Bauer AT, Bürgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2010;30:837–848. doi: 10.1038/jcbfm.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roy S, Maiello M, Lorenzi M. Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest. 1994;93:438–442. doi: 10.1172/JCI116979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roy S, Nasser S, Yee M, Graves DT. A long-term siRNA strategy regulates fibronectin overexpression and improves vascular lesions in retinas of diabetic rats. Mol Vis. 2011;17:3166–3174. [PMC free article] [PubMed] [Google Scholar]
- 70.Abrass CK, Spicer D, Berfield AK, St John PL, Abrahamson DR. Diabetes induces changes in glomerular development and laminin-beta 2 (s-laminin) expression. Am J Pathol. 1997;151:1131–1140. [PMC free article] [PubMed] [Google Scholar]
- 71.Schaeffer V, Hansen KM, Morris DR, Abrass CK. Reductions in laminin beta2 mRNA translation are responsible for impaired IGFBP-5-mediated mesangial cell migration in the presence of high glucose. Am J Physiol Renal Physiol. 2010;298:F314–322. doi: 10.1152/ajprenal.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirrlinger PG, Pannicke T, Winkler U, Claudepierre T, Varshney S, Schulze C, et al. Genetic deletion of laminin isoforms beta2 and gamma3 induces a reduction in Kir4.1 and aquaporin-4 expression and function in the retina. PLoS One. 2011;6:e16106. doi: 10.1371/journal.pone.0016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohney BG, Pulido JS, Lindor NM, Hogan MC, Consugar MB, Peters J, et al. A novel mutation of LAMB2 in a multigenerational mennonite family reveals a new phenotypic variant of Pierson syndrome. Ophthalmology. 2011;118:1137–1144. doi: 10.1016/j.ophtha.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, et al. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011;52:9316–9326. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- 76.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 77.Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]