Abstract
Objectives
HIV-infected people have elevated risk for lung cancer and higher mortality following cancer diagnosis than HIV-uninfected individuals. It is unclear whether HIV-infected individuals with lung cancer receive similar cancer treatment as HIV-uninfected individuals.
Design/methods
We studied adults more than 18 years of age with lung cancer reported to the Texas Cancer Registry (N = 156 930) from 1995 to 2009. HIV status was determined by linkage with the Texas enhanced HIV/AIDS Reporting System. For nonsmall cell lung cancer (NSCLC) cases, we identified predictors of cancer treatment using logistic regression. We used Cox regression to evaluate effects of HIV and cancer treatment on mortality.
Results
Compared with HIV-uninfected lung cancer patients (N = 156 593), HIV-infected lung cancer patients (N = 337) were more frequently young, black, men, and with non-Hispanic distant stage disease. HIV-infected NSCLC patients less frequently received cancer treatment than HIV-uninfected patients [60.3 vs. 77.5%; odds ratio 0.39, 95% confidence interval (CI) 0.30–0.52, after adjustment for diagnosis year, age, sex, race, stage, and histologic subtype]. HIV infection was associated with higher lung cancer-specific mortality (hazard ratio 1.34, 95% CI 1.15–1.56, adjusted for demographics and tumor characteristics). Inclusion of cancer treatment in adjusted models slightly attenuated the effect of HIV on lung cancer-specific mortality (hazard ratio 1.25; 95% CI 1.06–1.47). Also, there was a suggestion that HIV was more strongly associated with mortality among untreated than among treated patients (adjusted hazard ratio 1.32 vs. 1.16, P-interaction = 0.34).
Conclusion
HIV-infected NSCLC patients were less frequently treated for lung cancer than HIV-uninfected patients, which may have affected survival.
Keywords: carcinoma, healthcare disparities/statistics, HIV infections/virology, HIV/pathogenicity, lung neoplasms, lung neoplasms/therapy, nonsmall cell lung/therapy
Introduction
In the United States, lung cancer is the third most common malignancy in people infected with HIV, following Kaposi sarcoma and non-Hodgkin lymphoma [1,2]. Among HIV-infected people, lung cancer risk is two to six times that of the general population [2-5]. A high prevalence of tobacco use contributes to this elevated risk [6-8], but other possible risk factors include advanced immunosuppression (i.e., AIDS) and chronic pulmonary inflammation or scarring [2,3,9-11].
Although lung cancer survival is generally poor, appropriate therapy can improve outcomes, and for early stage cancer, be curative. The standard-of-care for treatment of early stage nonsmall cell lung cancer (NSCLC) is surgical resection [12]. For locally advanced lung cancer, acceptable treatments include concurrent chemoradiation, sequential chemoradiation, and, in some cases, surgery with chemotherapy and/or radiation [13-16]. For limited stage small cell lung cancer, the standard-of-care is chemoradiation and prophylactic cranial irradiation (PCI), whereas for extensive disease, chemotherapy with or without PCI is the treatment of choice [17,18].
HIV-infected people who develop lung cancer often present at more advanced stages and have worse survival than HIV-uninfected lung cancer patients [5,10,19-21]. Survival for HIV-infected people with lung cancer is especially poor when CD4 T-lymphocyte (CD4) counts are low or cancer stage is advanced [20-22]. Available data on patterns of cancer care for HIV-infected patients have come from single-institution case series with small numbers of patients [23-31]. Few studies have assessed cancer treatment for HIV-infected people with cancer [22,24,29,30]. Therefore, it is unclear whether worse survival for HIV-infected patients with lung cancer reflects more advanced stage at diagnosis, decreased access to care or referral for treatment, decreased efficacy of therapy, increased treatment toxicity, or death from AIDS-related complications.
With the availability of HAART since 1996, the incidence of AIDS has declined and cancer has increased in importance among HIV-infected people [1,2,32]. A better understanding of disparities in treatments and outcomes for cancer would help improve care of HIV-infected people. In the present study, linked data from the Texas HIV Registry (i.e., the enhanced HIV/AIDS Reporting System, eHARS) and the Texas Cancer Registry were used to evaluate population-level patterns in treatment and outcomes of lung cancer in HIV-infected and HIV-uninfected individuals.
Methods
Study population
The HIV/AIDS Cancer Match (HACM) Study links 14 US population-based HIV and cancer registries (http://hivmatch.cancer.gov/). In each state or metropolitan region, registry staff performed a computerized match of records in the HIV and cancer databases. These matches incorporated a probabilistic algorithm followed by clerical review. Only de-identified data were retained by investigators. The current study was restricted to cases from the most recent HACM linkage in Texas (conducted May 2011), because cancer treatment data were available in this cancer registry and included in the linked data files. Institutional review boards at the National Cancer Institute and Texas registries exempted the study from review.
We selected adults (more than 18 years of age) with invasive lung cancer diagnosed during 1995–2009 and reported to the Texas Cancer Registry. HIV status was determined through linkage with eHARS. A total of 157 004 lung cancer cases occurred in Texas during 1995–2009. Of 411 cases that matched to the HIV registry, we excluded 74 individuals whose lung cancer predated the report of their HIV infection to eHARS.
Lung cancers were grouped by histology codes based on International Classification of Diseases for Oncology (third edition) as follows [33]: NSCLC (see subdivisions below), small cell carcinoma (8041–8045), and carcinoma not otherwise specified (NOS; 8010, 8020–8022, 8030–8033, 8562, 8980). NSCLCs were divided into adenocarcinoma (8140–8145, 8200–8249), squamous cell carcinoma (8050–8084, 8094, 8120–8123, 8130, 8255–8290, 8310–8312, 8320–8330, 8430, 8440–8490, 8510–8560, 8570–8575), bronchioloalveolar carcinoma (8250–8254), large cell carcinoma (8011–8014), and NSCLC NOS (8046). Lung cancer stage (local, regional, distant, or unknown) was obtained from summaries provided in the cancer registry.
Lung cancer treatment and survival
The Texas Cancer Registry provided data only on the first course of cancer treatment. We assessed receipt of any cancer treatment, defined as surgery, radiotherapy, chemotherapy, or any combination of the therapies as a first course. A standard-of-care treatment variable was defined for local stage NSCLC as administration of surgery or radiation. The analysis related to standard-of-care was restricted to local stage NSCLC, wherein approaches are well defined and cure is more likely than for regional or distant stage disease.
Texas Cancer Registry data had been linked previously to Texas Department of State Health Services mortality data through December 2009 to obtain vital status, death date, and underlying cause of death. For lung cancer cases, we assessed all-cause mortality and lung cancer-specific mortality. Lung cancer-related deaths were identified using a recently described classification scheme [34].
Statistical analysis
We compared clinical and demographic characteristics between HIV-infected and HIV-uninfected lung cancer patients. We also compared the proportion of HIV-infected and HIV-uninfected patients who received chemotherapy, radiotherapy, surgery, any therapy, or (for localized NSCLC) standard-of-care therapy. For NSCLC, we used logistic regression to estimate odds ratios (ORs) for treatment across various demographic and clinical characteristics. Multivariate models included year of diagnosis, age, sex, race/ethnicity, stage, histology, and HIV status.
In addition, we created Kaplan–Meier curves to describe overall survival following diagnosis of NSCLC, according to HIV and treatment status. We then used proportional hazards regression to estimate hazards ratios for all-cause mortality and lung cancer-specific mortality, focusing on HIV status with adjustment for cancer stage and treatment. Follow-up was censored after December 2009.
Results
Characteristics of lung cancer patients
Table 1 presents characteristics of the HIV-infected and HIV-uninfected lung cancer patients (N = 337 andN = 156 593, respectively). A greater proportion of HIV-infected patients than HIV-uninfected patients were diagnosed with lung cancer later in the study period (45.7 vs. 34.6% in 2005–2009). Compared with HIV-uninfected patients, HIV-infected patients were younger at diagnosis (median age 53 vs. 69 years), and a greater proportion were men (84.3 vs. 57.2%) and non-Hispanic black (47.8 vs. 11.2%).
Table 1. Lung cancer patients in Texas, by HIV status, 1995–2009.
| Characteristic | HIV-uninfected (N = 156 593) |
HIV-infected (N = 337) |
P value† |
|---|---|---|---|
| Diagnosis year | <0.0001 | ||
| 1995–1999 | 49 401 (31.6%) | 61 (18.1%) | |
| 2000–2004 | 52 943 (33.8%) | 122 (36.2%) | |
| 2005–2009 | 54249 (34.6%) | 154 (45.7%) | |
| Age at cancer diagnosis in years, median (interquartile range) | 69 (61–76) | 53 (47–60) | <0.0001 |
| Sex | <0.0001 | ||
| Male | 89 490 (57.2%) | 284 (84.3%) | |
| Female | 67103 (42.9%) | 53 (15.7%) | |
| Race/ethnicity | <0.0001 | ||
| Non-Hispanic white | 122 335 (78.1%) | 151 (44.8%) | |
| Non-Hispanic black | 17453 (11.2%) | 161 (47.8%) | |
| Hispanic | 14 613 (9.3%) | 23 (6.8%) | |
| Other/unknown | 2192 (1.4%) | 2 (0.6%) | |
| Stage | 0.0001 | ||
| Local | 30493 (19.5%) | 39 (11.6%) | |
| Regional | 38047 (24.3%) | 69 (20.5%) | |
| Distant | 69 614 (44.5%) | 181 (53.7%) | |
| Unknown | 18439 (11.8%) | 48 (14.2%) | |
| Histology | 0.01 | ||
| Small cell | 24152 (15.4%) | 33 (9.8%) | |
| Adenocarcinoma | 44 877 (28.7%) | 110 (32.6%) | |
| Squamous | 41 308 (26.4%) | 90 (26.7%) | |
| Bronchioloalveolar | 4777 (3.1%) | 7 (2.1%) | |
| Large cell | 7239 (4.6%) | 10 (3.0%) | |
| NSCLC, NOS | 18 631 (11.9%) | 53 (15.7%) | |
| Carcinoma, NOS | 15 609 (10.0%) | 34 (10.1%) |
NOS, not otherwise specified; NSCLC, nonsmall cell lung cancer.
P val ues calculated using the χ2 test, except age at cancer diagnosis for which the Wilcoxon rank-sum test was used.
Additionally, HIV-infected patients were more likely than HIV-uninfected patients to present with distant or unknown stage cancer (Table 1). The HIV-infected patients had a smaller percentage of small cell, bronchioloalveolar, and large cell histologic subtypes, and a higher percentage of adenocarcinoma and NSCLC NOS. Squamous cell cancer and carcinoma NOS comprised a similar proportion in both groups. Among HIV-infected lung cancer patients, most had a prior AIDS diagnosis (N = 290, 86.1%).
Predictors of lung cancer treatment
As shown in Table 2, treatment of small cell lung cancer did not differ by HIV status. Due to the small number of HIV-infected small cell lung cancers (N = 33), remaining analyses focused on NSCLC.
Table 2. Treatment of lung cancer, by histologic subtype, stage, and HIV status.
| Small cell lung cancera |
NSCLC - locala |
NSCLC - regionala |
NSCLC - distanta |
|||||
|---|---|---|---|---|---|---|---|---|
| HIV-uninfected (N = 24 152) |
HIV-infected (N = 33) |
HIV-uninfected (N = 26 563) |
HIV-infected (N = 34) |
HIV-uninfected (N = 29 735) |
HIV-infected (N = 58) |
HIV-uninfected (N = 48 511) |
HIV-infected (N = 141) |
|
| Chemotherapy | ||||||||
| Yes | 15 071 (66.9%) | 23 (76.7%) | 3552 (14.9%) | 1 (3.3%) | 11 842 (43.9%) | 20 (35.7%) | 20125 (45.5%) | 41 (31.1%) |
| No | 7458 (33.1%) | 7 (23.3%) | 20 252 (85.1%) | 29 (96.7%) | 15116 (56.1%) | 36 (64.3%) | 24073 (54.5%) | 91 (68.9%) |
| P value† | 0.26 | 0.07 | 0.22 | 0.0009 | ||||
| Radiotherapy | ||||||||
| Yes | 7369 (38.4%) | 8 (25.8%) | 4453 (21.4%) | 4 (12.1%) | 10 502 (43.5%) | 24 (47.1%) | 16 985 (42.0%) | 44 (33.6%) |
| No | 11 824 (61.6%) | 23 (74.2%) | 16 400 (78.7%) | 29 (87.9%) | 13 638 (56.5%) | 27 (52.9%) | 23 452 (58.0%) | 87 (66.4%) |
| P value† | 0.15 | 0.21 | 3 | 0.61 | 0.05 | |||
| Surgery | ||||||||
| Yes | 1672 (7.3%) | 3 (10.0%) | 16 248 (62.5%) | 15 (45.5%) | 11 354 (39.3%) | 18 (31.0%) | 5423 (11.6%) | 10 (7.1%) |
| No | 21 400 (92.8%) | 27 (90.0%) | 9743 (37.5%) | 18 (54.5%) | 17 563 (60.7%) | 40 (69.0%) | 41 1 68 (88.4%) | 130 (92.9%) |
| P value† | 0.56 | 0.04 | 0.20 | 0.10 | ||||
| Any treatment | ||||||||
| Yes | 16 978 (79.9%) | 24 (82.8%) | 20 806 (85.6%) | 18 (58.1%) | 22 655 (84.6%) | 42 (77.8%) | 30497 (72.6%) | 73 (56.6%) |
| No | 4276 (20.1%) | 5 (17.2%) | 3498 (14.4%) | 13 (41.9%) | 4130 (15.4%) | 12 (22.2%) | 11 529 (27.4%) | 56 (43.4%) |
| P value† | 0.70 | <0.0001 | 0.17 | <0.0001 | ||||
NSCLC, nonsmall cell lung cancer.
Numbers in columns do not sum to total, because participants missing treatment data are excluded. Percentages of HIV-uninfected and HIV-infected patients with missing data (and excluded from analysis) were 9.8 and 7.4% for chemotherapy, 21.5 and 13.6% for radiotherapy, and 4.3 and 2.7% for surgery.
χ2 test used to calculate P values.
For local stage NSCLC, HIV-infected individuals were less likely than HIV-uninfected individuals to receive surgery (45.5 vs. 62.5%, P = 0.04), and there was a trend toward a lower likelihood of receiving chemotherapy (P = 0.07, Table 2). As a result, for local stage NSCLC, the HIV-infected group was more frequently untreated (41.9 vs. 14.4%, P < 0.0001). Among people with regional stage NSCLC, HIV-infected individuals were less often treated with chemotherapy or surgery, and more often received no therapy at all, but these differences were not statistically significant (Table 2). For distant stage NSCLC, the HIV-infected group was less frequently treated with chemotherapy (31.1 vs. 45.5%, P = 0.0009) or radiation (33.6 vs. 42.0%, P = 0.05), and thus more likely to receive no treatment (43.4 vs. 27.4%, P < 0.0001).
Among NSCLC patients with treatment data, 78 479 received treatment and 22 789 did not receive treatment of any kind. In multivariate analyses (Table 3), older age was associated with lower likelihood of treatment, and men were slightly more likely to receive lung cancer treatment. Compared with non-Hispanic whites, both non-Hispanic blacks and Hispanics were less likely to receive treatment. With respect to tumor characteristics, treatment was less frequent in lung cancer patients with regional, distant, or unknown stage, compared with local stage. Patients with bronchioloalveolar histology were more frequently treated, and patients with unspecified histologic subtype of NSCLC less frequently treated, compared with adenocarcinoma.
Table 3. Characteristics associated with treatment of nonsmall cell lung cancer.
| Characteristic | Any treatment (N = 78 479) |
No treatment (N = 22 789) |
Odds ratio (95% CI) |
Multivariate odds ratio (95% CI)a |
|---|---|---|---|---|
| Diagnosis year | ||||
| 1995–1999 | 22 633 (81.1%) | 5257 (18.9%) | 1.0 | 1.0 |
| 2000–2004 | 27 796 (80.6%) | 6673 (19.4%) | 0.97 (0.93–1.01) | 1.06 (1.01–1.10) |
| 2005–2009 | 28 050 (72.1%) | 10 859 (27.9%) | 0.60 (0.58–0.62) | 0.65 (0.62–0.68) |
| Age at diagnosis (years) | ||||
| 18–44 | 2461 (86.6%) | 380 (13.4%) | 1.0 | 1.0 |
| 45–64 | 27 808 (82.5%) | 5905 (17.5%) | 0.73 (0.65–0.81) | 0.70 (0.63–0.79) |
| 65+ | 48 210 (74.5%) | 16 504 (25.5%) | 0.45 (0.40–0.50) | 0.41 (0.36–0.45) |
| Sex | ||||
| Female | 32 904 (77.4%) | 9603 (22.6%) | 1.0 | 1.0 |
| Male | 45,575 (77.6%) | 13 186 (22.4%) | 1.01 (0.98–1.04) | 1.04 (1.01–1.07) |
| Race/ethnicity | ||||
| Non-Hispanic white | 61 768 (78.7%) | 16 677 (21.3%) | 1.0 | 1.0 |
| Non-Hispanic black | 8693 (73.5%) | 3132 (26.5%) | 0.75 (0.72–0.78) | 0.74 (0.71–0.78) |
| Hispanic | 6867 (72.6%) | 2597 (27.4%) | 0.71 (0.68–0.75) | 0.76 (0.72–0.80) |
| Other/unknown | 1151 (75.0%) | 383 (25.0%) | 0.81 (0.72–0.91) | 0.82 (0.72–0.92) |
| Stage | ||||
| Local | 20 824 (85.6%) | 3511 (14.4%) | 1.0 | 1.0 |
| Regional | 22 697 (84.6%) | 4142 (15.4%) | 0.92 (0.88–0.97) | 0.93 (0.88–0.98) |
| Distant | 30 570 (72.5%) | 11 585 (27.5%) | 0.45 (0.43–0.46) | 0.46 (0.44–0.48) |
| Unknown | 4388 (55.3%) | 3551 (44.7%) | 0.21 (0.20–0.22) | 0.21 (0.20–0.22) |
| Histology | ||||
| Adenocarcinoma | 30 353 (77.8%) | 8648 (22.2%) | 1.0 | 1.0 |
| Squamous | 28 209 (79.0%) | 7494 (21.0%) | 1.07 (1.04–1.11) | 1.02 (0.98–1.06) |
| Bronchioloalveolar | 3745 (85.8%) | 622 (14.2%) | 1.72 (1.57–1.87) | 1.46 (1.33–1.60) |
| Large cell | 4846 (79.2%) | 1275 (20.8%) | 1.08 (1.01–1.16) | 0.98 (0.91–1.05) |
| NSCLC, NOS | 11 326 (70.4%) | 4750 (29.6%) | 0.68 (0.65–0.71) | 0.79 (0.75–0.82) |
| HIV status | ||||
| No | 78 333 (77.5%) | 22 693 (22.5%) | 1.0 | 1.0 |
| Yes | 146 (60.3%) | 96 (39.7%) | 0.44 (0.34–0.57) | 0.39 (0.30–0.52) |
CI, confidence interval;NOS, not otherwise specified;NSCLC, nonsmall cell lung cancer.
Multivariate odds ratios were derived from a logistic regression model that included the characteristics listed.
HIV-infected NSCLC patients were less likely to receive treatment than HIV-uninfected patients [multivariate OR 0.39, 95% confidence interval (CI) 0.30–0.52; Table 3]. Furthermore, treatment was less likely in people with AIDS than in those with HIV but not AIDS (univariate OR 0.49, 95% CI 0.22–1.10). The proportion of HIV-infected lung cancer patients who received cancer treatment was lower than for HIV-uninfected lung cancer patients in each of the three calendar periods of diagnosis (data not shown, P-interaction = 0.72). Finally, in a separate model limited to local stage NSCLC, HIV-infected patients were less likely than HIV-uninfected patients to receive standard-of-care treatment, defined as surgery or radiation as part of the first treatment course (multivariate OR 0.35, 95% CI 0.17–0.71).
Associations of HIV status and cancer treatment with mortality
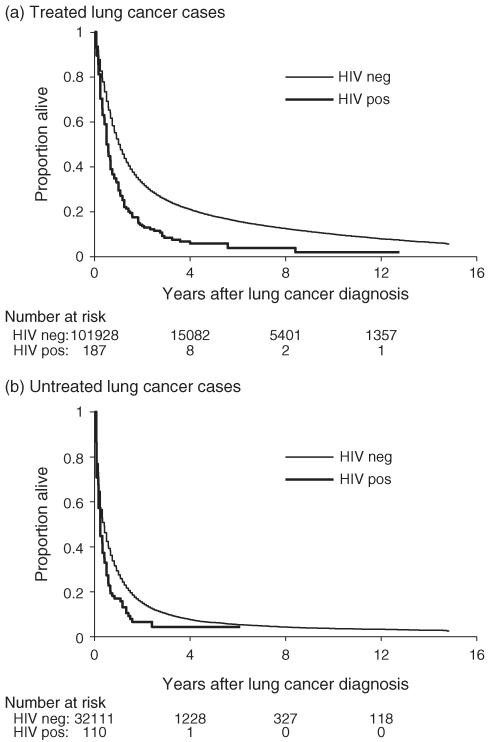
Following NSCLC diagnosis, HIV-infected people had a higher mortality than HIV-uninfected people, both among those who were treated (Fig. 1a) and those who were untreated (Fig. 1b). In univariate proportional hazards regression models, HIV infection was a predictor of increased overall mortality, especially among individuals with local stage cancer (Table 4). The association of HIV infection with higher all-cause mortality remained significant after adjustment for demographics and tumor characteristics, as well as when treatment was added to the multivariate model (Table 4).
Fig. 1. Survival following diagnosis of nonsmall cell lung cancer, according to HIV status and cancer treatment.
(a) Treated lung cancer patients. (b) Untreated lung cancer patients. The figure presents Kaplan–Meier curves illustrating overall survival following lung cancer diagnosis, for patients who received cancer treatment (a) or did not receive cancer treatment (b). The numbers below the panels indicate the number at risk at defined times following lung cancer diagnosis. ‘HIV neg’, HIV-uninfected patients; ‘HIV pos’, HIV-infected patients.
Table 4. Associations of HIV infection with mortality following diagnosis of nonsmall cell lung cancer.
| Unadjusted HR for HIV (95% CI) |
HR (95% CI) for HIV, adjusted for demographics and tumor characteristicsa |
HR (95% CI) for HIV, adjusted for demographics, tumor characteristics, and treatmenta |
|
|---|---|---|---|
| All-cause mortality | |||
| All cancer cases | 1.74 (1.53–1.99) | 1.69 (1.48–1.92) | 1.60 (1.39–1.83) |
| Cancer stage | |||
| Local | 2.12 (1.42–3.16) | 2.52 (1.68–3.76) | 2.39 (1.59–3.61) |
| Regional | 1.75 (1.32–2.33) | 2.02 (1.51–2.68) | 1.89 (1.40–2.55) |
| Distant | 1.35 (1.13–1.61) | 1.45 (1.21–1.73) | 1.34 (1.11–1.61) |
| Unknown stage | 1.77 (1.25–2.52) | 2.10 (1.47–2.99) | 2.56 (1.67–3.91) |
| Calendar year of cancer diagnosis | |||
| 1995–1999 | 2.18 (1.61–2.96) | 2.15 (1.58–2.90) | 2.05 (1.46–2.87) |
| 2000–2004 | 1.72 (1.39–2.11) | 1.60 (1.30–1.98) | 1.57 (1.26–1.97) |
| 2005–2009 | 1.64 (1.34–2.00) | 1.62 (1.32–1.98) | 1.47 (1.19–1.81) |
| HIV disease stage | |||
| HIV-only | 1.15 (0.79–1.67) | 0.97 (0.66–1.41) | 0.87 (0.58–1.31) |
| AIDS | 1.88 (1.63–2.16) | 1.87 (1.63–2.15) | 1.79 (1.54–2.08) |
| Lung cancer-specific mortality | |||
| All cancer cases | 1.43 (1.23–1.67) | 1.34 (1.15–1.56) | 1.25 (1.06–1.47) |
| Cancer stage | |||
| Local | 1.62 (0.96–2.74) | 1.79 (1.06–3.03) | 1.60 (0.93–2.77) |
| Regional | 1.29 (0.90–1.84) | 1.46 (1.02–2.09) | 1.34 (0.92–1.96) |
| Distant | 1.09 (0.89–1.34) | 1.16 (0.94–1.42) | 1.07 (0.86–1.32) |
| Unknown stage | 1.81 (1.24–2.65) | 2.08 (1.42–3.05) | 2.58 (1.65–4.03) |
| Calendar year of cancer diagnosis | |||
| 1995–1999 | 2.07 (1.48–2.88) | 1.92 (1.38–2.67) | 1.77 (1.22–2.57) |
| 2000–2004 | 1.32 (1.03–1.70) | 1.18 (0.92–1.53) | 1.17 (0.89–1.53) |
| 2005–2009 | 1.35 (1.06–1.71) | 1.30 (1.02–1.65) | 1.16 (0.89–1.49) |
| HIV disease stage | |||
| HIV-only | 0.97 (0.62–1.50) | 0.80 (0.52–1.24) | 0.72 (0.45–1.16) |
| AIDS | 1.54 (1.31–1.82) | 1.48 (1.25–1.74) | 1.38 (1.16–1.65) |
CI, confidence interval;HR, hazard ratio. Analyses were restricted to N = 101 268 cases of nonsmall cell lung cancer with data on cancer treatment.
Demographics and tumor characteristics include year of diagnosis, age at diagnosis, sex, race/ethnicity, cancer stage, and histologic subtype. Models stratified on cancer stage and year of diagnosis were adjusted for all demographic and tumor characteristic variables except cancer stage and year of diagnosis, respectively.
HIV infection had a more modest association with lung cancer-specific mortality than with overall mortality, and the associations between HIV status and lung cancer-specific mortality were only significant when all cancer stages were grouped (Table 4). There was a suggestion that adjustment for cancer treatment attenuated the association between HIV infection and lung cancer-specific mortality, but CIs for the hazards ratios were wide and overlapped (hazards ratio 1.34, 95% CI 1.15–1.56 adjusted for demographic characteristics and histologic subtype, vs. hazards ratio 1.25, 95% CI 1.06–1.47 in the full multivariate model including treatment; Table 4).
We also examined the effect of HIV on mortality by period of diagnosis. The effect of HIV on both all-cause mortality and lung cancer-specific mortality was highest in the earlier calendar periods of the study. Within each time period, the addition of treatment to the model attenuated the hazard ratio associated with HIV infection.
In addition, we performed separate analyses for lung cancer patients with HIV-only and those with AIDS. Both all-cause mortality and lung cancer-specific mortality were higher in the AIDS group compared to the HIV-uninfected group. Nonetheless, the addition of cancer treatment to the model again partially attenuated the effect of AIDS (Table 4).
Finally, in an attempt to isolate the effect of treatment on the association between HIV infection and mortality, we performed analyses stratified by cancer treatment status. The association between HIV infection and all-cause mortality tended to be stronger in people who received no treatment (adjusted hazards ratio 1.67, 95% CI 1.34–2.09) than in those who received treatment (adjusted hazards ratio 1.48, 95% CI 1.24–1.77), although this difference was not significant (P-interaction = 0.26). HIV infection also appeared more strongly associated with lung cancer-specific mortality among those who received no treatment (adjusted hazards ratio 1.32, 95% CI 1.01–1.72) than in those who were treated (adjusted hazards ratio 1.16, 95% CI 0.94–1.43), but again the difference was not significant (P-interaction = 0.34).
Discussion
In this population-based study, we found that HIV-infected people were less frequently treated for lung cancer than HIV-uninfected people. Furthermore, survival was substantially worse in HIV-infected people with lung cancer than for their HIV-uninfected counterparts, even after adjusting for demographic factors, cancer stage, and histologic subtype. Although the reasons for the worse survival are not entirely clear, our results highlight the important possibility that the lack of cancer treatment contributed to the high mortality among HIV-infected lung cancer patients.
Demographic differences between HIV-infected and HIV-uninfected lung cancer patients reflect the under-lying characteristics of the Texas and US HIV-infected population [35]. Compared to their HIV-uninfected counterparts, HIV-infected individuals with lung cancer were more frequently men and non-Hispanic black, findings described in previous studies [2,3,21,22]. Like-wise, the relatively young age at lung cancer diagnosis largely reflects the young age distribution of the HIV population [36]. A larger proportion of HIV-infected lung cancer patients were diagnosed in the later time periods of this study, which may be due to aging of the HIV population [1].
Consistent with previous data [3,5,20,21], we found that HIV-infected people were less likely to present with local stage and more likely to present with distant or unknown stage. Reasons for advanced presentation are probably multifactorial, including limited healthcare access, delayed diagnosis, nonadherence with medical evaluation, or more aggressive cancer behavior [21,37]. Most lung cancers in both HIV-infected and HIV-uninfected people were NSCLC, although histologic subtypes differed (Table 1).
Among individuals with NSCLC, HIV was strongly associated with a lower probability of cancer treatment. HIV-infected patients less often received surgery for local stage cancer, even though such therapy is potentially curative. Further, it appears that for distant stage disease, palliative radiotherapy and chemotherapy may be less frequently administered to HIV-infected patients. Other adverse predictors of treatment included older age, black race, and Hispanic ethnicity, as previously documented [38-41]. Lung cancer patients classified as unknown stage or NSCLC NOS were also less likely to receive treatment, perhaps reflecting performance status in patients who were too ill to complete a diagnostic work-up.
Receipt of cancer treatment appeared to decline in the most recent calendar period. We believe that this is an artifact of improved coding of treatment data in the Texas Cancer Registry. Specifically, patients with missing data for any of the three treatment modalities (chemotherapy, radiation, or surgery) were coded as ‘missing data’ and excluded from the analysis, whereas patients who were coded as receiving chemotherapy, radiation, or surgery were coded as ‘treated’ in our analysis (even if there was some missing data). This approach likely led to an overestimate of the proportion of patients who received cancer treatment. With more complete data in later calendar periods, treatment status was more accurately ascertained, leading to an apparent decrease in the proportion of treated patients.
Importantly, we note that HIV-infected individuals less frequently received treatment than HIV-uninfected individuals, even after adjustment for these demographic and tumor characteristics. Furthermore, the subset of HIV-infected patients with local stage NSCLC were even less likely to receive the standard-of-care treatment, defined as either surgery or radiation. To our knowledge, this is the first population-based study of this size to evaluate the association between HIV and treatment of lung cancer. One study of 39 HIV-infected lung cancer patients found comparable treatment rates among HIV-infected and HIV-uninfected individuals, although HIV-infected patients were less likely to have curative surgical resection for early stage disease [29,30]. Two other studies of HIV-infected patients with lung cancer found cancer treatment rates of about 70% but did not have HIV-uninfected controls [22,24].
Among HIV-infected patients, the presence of AIDS was associated with a lower probability of lung cancer treatment. Although reasons for lower cancer treatment rates in HIV-infected people with lung cancer were not identified in this study, several potential explanations may exist. Providers may perceive HIV-infected patients, especially those with AIDS, to have lower performance status, worse cancer treatment toxicity, lower efficacy of cancer treatment, or lower compliance compared with HIV-uninfected patients. Whereas early case reports suggested exaggerated treatment toxicity in HIV-infected patients, and concerns about the interactions of cytotoxic and HIV therapies exist [42-45], currently available data suggest comparable treatment toxicity and completion rates among HIV-infected and HIV-uninfected lung cancer patients [20,24,27,28,30,46-49]. Because HIV-infected patients are often excluded from clinical trial participation [50], provider reluctance to treat some patients may partly arise from the limited information available on the efficacy and safety of cancer treatment in HIV-infected patients. Additional factors such as low socioeconomic status, lack of social support, lack of medical insurance, delays in specialty referral, or provider bias against HIV-infected patients may have played a role in lowering the proportion of HIV-infected lung cancer patients who were treated.
An expected finding was that associations between HIV infection and mortality were stronger for all-cause mortality than for lung cancer-specific mortality, because HIV-infected people are at risk of dying from noncancer causes, such as AIDS-related infections. The effect of HIV on mortality was lower in people with HIV-only than in people with AIDS, and it decreased over time. These results likely reflect differences in mortality from AIDS-associated morbidities, better tolerance of cancer treatment in less immunosuppressed HIV-infected patients, and improvements in HIV care over time. Two sets of results may suggest that lack of appropriate cancer treatment contributed to the poor survival of HIV-infected individuals following a lung cancer diagnosis. First, the association of HIV with lung cancer-related mortality was somewhat attenuated once treatment was added to the regression model (hazard ratio 1.25 vs. 1.34; all stages combined, Table 4). This attenuation was also observed in analyses stratified on calendar year of cancer diagnosis and among people with AIDS (Table 4). Thus, we show that cancer treatment offered to HIV-infected patients in different settings appears to affect postcancer survival. Second, although a test for interaction was not significant, the association between HIV infection and lung cancer-related mortality appeared weaker in people who received cancer treatment (adjusted hazards ratio treatment: 1.16 vs. no treatment: 1.32). Nonetheless, in the absence of clear differences in these analyses, we cannot draw a definitive conclusion, and we note that HIV was associated with elevated cancer-related mortality even among treated patients.
We found that HIV-infected people with distant stage NSCLC were less likely to receive cancer treatment than HIV-uninfected people with distant stage disease. The difference in treatment was smaller for distant stage NSCLC compared with early stage NSCLC (Table 2), perhaps because clinicians recognize that outcomes of distant stage NSCLC are uniformly poor. Nonetheless, palliative cancer treatment in this group of patients may improve quality of life [51] and, therefore, benefit both HIV-infected and HIV-uninfected patients with lung cancer. We could not ascertain quality of life in our dataset. Also, we did not observe significant differences in treatment for HIV-infected individuals with small cell lung cancer. This may be due to the small number of HIV-infected patients with small cell lung cancer and lack of statistical power, or could reflect a more straightforward treatment paradigm, with chemotherapy being the mainstay of treatment.
Our study has several limitations. First, aspects of HIV disease that characterize its severity, including CD4 cell count, use of HAART, and comorbid illnesses, affect candidacy for treatment and survival [20,22,45], but we did not evaluate these factors. It would have been of interest to examine whether treatment and mortality differed among HIV-infected lung cancer patients according to CD4 cell count. Unfortunately, the Texas eHARS did not have these data for the entire study period, and data at the most relevant time points (i.e. lung cancer diagnosis) were not systematically available. Second, we lacked data on socioeconomic status, medical insurance, performance status, and comorbid illness, all of which affect access to and receipt of treatment [22,27,52]. Performance status and comorbidities also affect the likelihood of completing cancer therapy. Additionally, only the first course of therapy was recorded by the cancer registry, and in spite of quality assurance activities by the Texas Cancer Registry to validate treatment data (i.e. linkage with claims databases and chart audits), treatment data were incomplete for 14% of patients with NSCLC. Finally, our study sample was limited to lung cancer patients in Texas, and our results may not be generalizable to other cancers or US regions.
In conclusion, we found that HIV-infected patients with lung cancer were less likely to be treated for their cancer than HIV-uninfected patients, and this disparity may have contributed to poorer survival. The underlying reasons for the observed disparity in cancer treatment are unclear, and further investigation is warranted to identify potential explanations.
Acknowledgements
G.S. and E.A.E. contributed to study design, data collection/analysis, and article preparation.
M.S.S. contributed to data collection/analysis and article preparation.
S.K.M. and M.A.W. contributed to data collection and article preparation.
R.R. contributed to study design and article preparation.
This study was funded by the Intramural Research Program of the National Cancer Institute.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen ML, Farrell KJ, Gunthel CJ. Non-AIDS-defining malignancies in patients with HIV in the HAART era. Curr Infect Dis Rep. 2010;12:46–55. doi: 10.1007/s11908-009-0075-6. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–213. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- 6.Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, Mercie P, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDs. 2007;21:458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
- 7.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 8.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 9.Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavole A, Wislez M, Antoine M, Mayaud C, Milleron B, Cadranel J. Lung cancer, a new challenge in the HIV-infected population. Lung Cancer. 2006;51:1–11. doi: 10.1016/j.lungcan.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–615. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 nonsmall cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. discussion 622-623. [DOI] [PubMed] [Google Scholar]
- 13.Dillman RO, Herndon J, Seagren SL, Eaton WL, Green MR. Improved survival in stage III nonsmall-cell lung cancer: seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210–1215. doi: 10.1093/jnci/88.17.1210. [DOI] [PubMed] [Google Scholar]
- 14.Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III nonsmall-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced nonsmall-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed 4 January 2012];NCCN Clinical Practice Guidelines in Oncology Non-Small-Cell Lung Cancer v 2. 2012. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 17.Turrisi AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 18.Le Péchoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10:467–474. doi: 10.1016/S1470-2045(09)70101-9. [DOI] [PubMed] [Google Scholar]
- 19.Biggar RJ, Engels EA, Ly S, Kahn A, Schymura MJ, Sackoff J, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39:293–299. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 20.Lavole A, Chouaid C, Baudrin L, Wislez M, Raguin G, Pialoux G, et al. Effect of highly active antiretroviral therapy on survival of HIV infected patients with nonsmall-cell lung cancer. Lung Cancer. 2009;65:345–350. doi: 10.1016/j.lungcan.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Brock MV, Hooker CM, Engels EA, Moore RD, Gillison ML, Alberg AJ, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43:47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 22.Pakkala S, Chen Z, Rimland D, Owonikoko TK, Gunthel C, Brandes JR, et al. Human immunodeficiency virus-associated lung cancer in the era of highly active antiretroviral therapy. Cancer. 2012;118:164–172. doi: 10.1002/cncr.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alshafie MT, Donaldson B, Oluwole SF. Human immunodeficiency virus and lung cancer. Br J Surg. 1997;84:1068–1071. [PubMed] [Google Scholar]
- 24.Bertolaccini L, Lybéris P, Soncini S, Perri GD, Manno E. Clinical characteristic lung cancer in HIV-infected patients. Therapy. 2008;6:903–906. [Google Scholar]
- 25.Flores MR, Sridhar KS, Thurer RJ, Saldana M, Raub WA, Jr, Klimas NG. Lung cancer in patients with human immunodeficiency virus infection. Am J Clin Oncol. 1995;18:59–66. doi: 10.1097/00000421-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Karp J, Profeta G, Marantz PR, Karpel JP. Lung cancer in patients with immunodeficiency syndrome. Chest. 1993;103:410–413. doi: 10.1378/chest.103.2.410. [DOI] [PubMed] [Google Scholar]
- 27.Powles T, Thirwell C, Newsom-Davis T, Nelson M, Shah P, Cox S, et al. Does HIV adversely influence the outcome in advanced nonsmall-cell lung cancer in the era of HAART? Br J Cancer. 2003;89:457–459. doi: 10.1038/sj.bjc.6601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spano JP, Massiani MA, Bentata M, Rixe O, Friard S, Bossi P, et al. Lung cancer in patients with HIV infection and review of the literature. Med Oncol. 2004;21:109–115. doi: 10.1385/MO:21:2:109. [DOI] [PubMed] [Google Scholar]
- 29.Spina M, Sandri S, Serraino D, Gobitti C, Fasan M, Sinicco A, et al. Therapy of nonsmall-cell lung cancer (NSCLC) in patients with HIV infection. GICAT. Cooperative Group on AIDS and Tumors. Ann Oncol. 1999;10(Suppl 5):S87–S90. doi: 10.1093/annonc/10.suppl_5.s87. [DOI] [PubMed] [Google Scholar]
- 30.Tirelli U, Spina M, Sandri S, Serraino D, Gobitti C, Fasan M, et al. Lung carcinoma in 36 patients with human immunodeficiency virus infection. The Italian Cooperative Group on AIDS and Tumors. Cancer. 2000;88:563–569. doi: 10.1002/(sici)1097-0142(20000201)88:3<563::aid-cncr11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Vyzula R, Remick SC. Lung cancer in patients with HIV-infection. Lung Cancer. 1996;15:325–339. doi: 10.1016/0169-5002(95)00596-x. [DOI] [PubMed] [Google Scholar]
- 32.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 33.Organization WH. International Classification of Diseases for Oncology. 3rd ed. [[Accessed 4 January 2012]. (ICD-O-3). http://www.who.int/classifications/icd/adaptations/oncology/en/ [Google Scholar]
- 34.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Texas Department of State Health Services . Texas Integrated Epidemiologic Profile for HIV/AIDS Prevention and Services Planning. 2010. Publication No. E13-11937. [Google Scholar]
- 36.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153:452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crespo-Fierro M. Compliance/adherence and care management in HIV disease. J Assoc Nurses AIDS Care: JANAC. 1997;8:43–54. doi: 10.1016/S1055-3290(97)80012-X. [DOI] [PubMed] [Google Scholar]
- 38.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 39.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 40.Brown JS, Eraut D, Trask C, Davison AG. Age and the treatment of lung cancer. Thorax. 1996;51:564–568. doi: 10.1136/thx.51.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potosky AL, Saxman S, Wallace RB, Lynch CF. Population variations in the initial treatment of nonsmall-cell lung cancer. J Clin Oncol. 2004;22:3261–3268. doi: 10.1200/JCO.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 42.Costleigh BJ, Miyamoto CT, Micaily B, Brady LW. Heightened sensitivity of the esophagus to radiation in a patient with AIDS. Am J Gastroenterol. 1995;90:812–814. [PubMed] [Google Scholar]
- 43.Leigh BR, Lau DH. Severe esophageal toxicity after thoracic radiation therapy for lung cancer associated with the human immunodeficiency virus: a case report and review of the literature. Am J Clin Oncol. 1998;21:479–481. doi: 10.1097/00000421-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Mani D, Haigentz M, Aboulafia DM. Lung cancer in HIV infection. Clin Lung Cancer. 2011;13:6–13. doi: 10.1016/j.cllc.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makinson A, Tenon J-C, Eymard-Duvernay S, Pujol J-L, Allavena C, Cuzin L, et al. Human immunodeficiency virus infection and nonsmall cell lung cancer: survival and toxicity of antineoplastic chemotherapy in a cohort study. J Thorac Oncol. 2011;6:1022–1029. doi: 10.1097/JTO.0b013e318217b6e0. [DOI] [PubMed] [Google Scholar]
- 46.Cadranel J, Garfield D, Lavole A, Wislez M, Milleron B, Mayaud C. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax. 2006;61:1000–1008. doi: 10.1136/thx.2005.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makinson A, Pujol JL, Le Moing V, Peyriere H, Reynes J. Interactions between cytotoxic chemotherapy and anti-retroviral treatment in human immunodeficiency virus-infected patients with lung cancer. J Thorac Oncol. 2010;5:562–571. doi: 10.1097/JTO.0b013e3181d3ccf2. [DOI] [PubMed] [Google Scholar]
- 48.Pakkala S, Ramalingam SS. Lung cancer in HIV-positive patients. J Thorac Oncol. 2010;5:1864–1871. doi: 10.1097/JTO.0b013e3181f387fd. [DOI] [PubMed] [Google Scholar]
- 49.See AP, Zeng J, Tran PT, Lim M. Acute toxicity of second generation HIV protease-inhibitors in combination with radiotherapy: a retrospective case series. Radiat Oncol. 2011;6:25. doi: 10.1186/1748-717X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persad GC, Little RF, Grady C. Including persons with HIV infection in cancer clinical trials. J Clin Oncol. 2008;26:1027–1032. doi: 10.1200/JCO.2007.14.5532. [DOI] [PubMed] [Google Scholar]
- 51.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic nonsmall-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 52.Earle CC, Neumann PJ, Gelber RD, Weinstein MC, Weeks JC. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]