Abstract
Objective
To assess the effect of prenatal cocaine exposure on mental health symptoms in 9-year old children controlling for potential confounders.
Methods
332 children (170 prenatally cocaine-exposed (PCE), 162 non cocaine-exposed (NCE) were assessed using self (Dominic Interactive; DI) and caregiver report (Child Behavior Checklist; CBCL).
Results
Higher levels of PCE were associated with caregiver report of clinically elevated aggressive and delinquent behavior. With each increased unit of PCE, children were 1.3 times more likely to be rated as aggressive (OR=1.30, 95% CI: 1.02–1.67, p<0.04). For each increased unit of PCE, girls were 2 times more likely to be rated as having delinquent behavior (OR=2.08, 95% CI: 1.46–2.96, p<0.0001). PCE status was also associated with increased odds of delinquent behavior (OR=2.41; 95% CI: 1.16–4.97, p=0.02), primarily due to the increased risk among girls with PCE. While girls with PCE status were 7 times more likely than NCE girls to have delinquent behaviors (OR=7.42; 95% CI: 2.03–27.11, p<0.002) boys with PCE did not demonstrate increased risk (OR=0.98; 95% CI: 0.36–2.65, p>0.97). Foster or adoptive parents were more likely to rate their PCE children as having more thought problems, inattention, delinquent behavior, aggression, externalizing and overall problems (p<0.05) than biologic mothers or relative caregivers. Higher 2nd trimester tobacco exposure was associated with increased odds of caregiver reported anxiety (OR=1.73; 95% CI 1.06–2.81, p<0.03) and marijuana exposure increased the odds of thought problems (OR=1.68; 95% CI 1.01–2.79, p<0.05). Children with PCE self-reported fewer symptoms of oppositional defiant disorder (ODD) compared to NCE children (OR=0.44, 95% CI: 0.21–0.92, p<0.03). Greater tobacco exposure was associated with increased odds of child reported ODD (OR=1.24; 95% CI 1.03–1.78, p<0.03).
Conclusion
Higher PCE was associated with disruptive behaviors including aggression and delinquent behavior among girls by caregiver report, but not child report. These findings highlight the need for early behavioral assessment using multiple informants in multi-risk children.
Keywords: Prenatal cocaine exposure, Mental health, Child Behavior Checklist, Dominic Interactive, Tobacco, Neurobehavioral teratology
1. Introduction
Prenatal cocaine exposure (PCE) became a major focus of research and clinical concern following a significant spike in crack cocaine use in the late 1980s and early 1990s. Among the concerns regarding PCE is the possible long-lasting impact on central nervous system (CNS) functioning, with cocaine assumed to have a negative impact on the developing fetus’ monoaminergic systems (McMurray et al., 2008). Mayes (Mayes, 1999; Mayes et al., 2002) hypothesized that alterations in the monoaminergic (e.g. serotonin, dopamine) systems are likely to have a profound impact on later functioning due to the considerable number of processes that involve monoamines (e.g. arousal, attention). This theory has been supported in studies of infants with PCE (Bendersky and Lewis, 1998; Chiriboga et al., 2006; Eiden et al., 2009; Noland et al., 2003; Singer et al., 2005), as well as preschool and early school aged children (Accornero et al., 2007; Bada et al., 2007; Chaplin et al., 2009; Richardson et al., 2009). Continued investigation into the effects of cocaine on the developing child’s behavioral profile through middle childhood and beyond would further enhance our understanding of the consequences of PCE and the associated environmental and caregiver influences on development.
Important maternal and other environmental variables to consider with respect to developmental outcomes associated with PCE have been identified. First, caregiver functioning and other drug exposures are frequently documented as additional significant risk factors. Several studies with PCE cohorts have found parental psychological distress (Accornero et al., 2002; Bennett et al., 2002), maternal drug use (Accornero et al., 2002), and harsh discipline (Bennett et al., 2002) to negatively impact behavioral outcomes. In utero exposure to other teratogens is common among children who are PCE (Singer et al., 2002b) and is associated with undesirable developmental outcomes. More specifically, attention problems have been associated with prenatal alcohol, tobacco, marijuana, cocaine and lead exposure in infancy and preschool-aged children (Goldschmidt et al., 2000; Linnet et al., 2003; Noland et al., 2003; Plusquellec et al., 2007). Also, disruptive behavior problems including increased rates of substance use have been linked to prenatal tobacco and marijuana exposure (Day et al., 2006; Goldschmidt et al., 2000; Maughan et al., 2004).
Secondly, elevated blood lead levels due to prenatal or environmental exposure are prevalent among inner city children (8.65%< 10 ug/dL) (Meyer et al., 2003), including children who were PCE (Nelson et al., 2004). Given that children with prenatal drug exposure are at increased risk for elevated lead levels and the fact that elevated lead levels appear to predispose children to delinquent behavior (Dietrich et al., 2001; Needleman et al., 1996), the need to monitor and evaluate the effects of elevated blood lead levels in high risk samples seems imperative. Lastly, gender and prenatal exposure to other substances also may moderate the effects of PCE. Sood and colleagues (Sood et al., 2005) found that girls with PCE were at increased risk for aggressive behavior problems after controlling for other prenatal drug exposures and environmental factors. These findings contrast with studies that report that boys with PCE demonstrate more externalizing and aggressive behavior problems than girls with PCE and NCE children (Bendersky et al., 2006; Delaney-Black et al., 2004; Dennis et al., 2006).
Other factors also play an important part in the behavioral outcomes of children with PCE. Research using caregiver report has examined how different caregiver perspectives (i.e. foster or adoptive versus biologic/relative caregivers) influence perception of mental health outcomes for children with PCE. Linares and colleagues (Linares et al., 2006) documented interesting findings for children with PCE in adoptive or foster care, controlling for other potentially confounding variables. Children with PCE in adoptive or foster care self-reported more externalizing behavior problems than NCE children and children with PCE in maternal or relative care. Moreover, using caregiver report, children with PCE in adoptive or foster care were rated as having increased likelihood of aggression, social, thought and attention problems, delinquent behaviors, externalizing behaviors, internalizing and total behavioral problems compared to NCE children and children with PCE in maternal or relative care (Linares et al., 2006; Minnes et al., 2010). Adoptive and non-relative foster caregivers of children with PCE have been found to provide higher quality home environments, have better caregiver educational attainment, vocabulary level and psychological status. Children with PCE in adoptive and non-relative foster care have also been reported to have lower blood lead levels (Accornero et al., 2002; Singer et al., 2008) than those in birth maternal care.
The purpose of the current analyses is to extend the Linares et al., findings (Linares et al., 2006) to nine years of age and to enhance the existing literature on the behavioral effects of PCE by including assessments from multiple informants (i.e. self- and caregiver report). This study will also evaluate important covariates, including other prenatal substance exposure, home environment, child placement, and blood lead levels. It was hypothesized that PCE would be associated with more externalizing behaviors including disruptive behavior and aggression by both self- and caregiver- report. It is further hypothesized that elevated blood lead levels would have an independent negative effect on externalizing behaviors.
2. Methods
2.1. Subjects
The present study is part of a longitudinal, prospective study of 415 children born at a county teaching hospital in the Midwest between September 1994 and June 1996. At delivery, mothers were given a urine toxicology screen if they appeared at-risk for drug use due to lack of prenatal care, behavior suggesting intoxication, a history of involvement with the Department of Human Services, or self-admission of drug use to hospital staff. Maternal urine samples were obtained immediately before or after labor and delivery, with analysis designed to examine the presence of cocaine metabolites (benzoylecgonine; [BZE]), cannabinoids (THC), opiates, PCP, and amphetamines. In addition, infants’ meconium was analyzed for the presence of cocaine and its metabolites (i.e., BZE, meta-hydroxybenzoylecgonine (m-OH-bze), cocaethylene), as well as other drugs of abuse including THC, opiates, PCP, amphetamines, and benzodiazepines. A research nurse approached women screened for substance abuse and obtained informed consent, approved by the affiliate hospital’s Institutional Review Board, from mothers agreeing to participate in the study shortly before or after childbirth. Infants of participating women had meconium specimens collected from consecutive diapers by an obstetric nurse trained in the research protocol for the collection of infant meconium (for a full description of meconium collection and analysis see (Arendt et al., 1999; Singer et al., 2000)). Prenatal cocaine-exposure status was classified as positive if the mother or infant were positive for cocaine on any of the following measures: maternal/infant urine sample, infant meconium, or maternal self-report of cocaine use to hospital or research staff. A negative report on all measures was required to be considered non cocaine-exposed (NCE).
Women who used alcohol, marijuana, or tobacco during pregnancy were included in both groups (refer to Table 1). Participants in both the PCE and NCE groups were predominantly African American and of low socioeconomic status, with all receiving public assistance at the time of infant birth. A writ of confidentiality preventing the release of any research data concerning maternal substance abuse or child drug exposure histories (Writ # DA-04-03) was obtained from the National Institute on Drug Abuse. Forty-nine women who used cocaine and 106 women who did not use cocaine refused to participate, with 54 (20 used cocaine, 34 did not use cocaine) potential participants excluded for a priori specified reasons (i.e., no meconium, Down syndrome, maternal psychiatric history, primary heroin use, HIV positive status, low maternal IQ, fetal alcohol syndrome identified at birth, maternal age <19 years, infant medical illness, maternal chronic illness, and other). Twenty-three women who originally agreed to participate failed to attend their enrollment visit and 11 subjects died up to age 9. Reasons for death among children with PCE included sudden infant death syndrome (SIDS) (4), cardiopulmonary arrest (1), pneumonia (1), accidental asphyxiation (1) and respiratory distress syndrome (1). Reasons for death among NCE children were SIDS (2) and respiratory distress syndrome (1). At nine years of age 368 children, over 90% of the surviving children, completed a self-report and/or a caregiver completed a behavioral assessment. For this study children with IQ less than 70 were excluded (n=36; 19 PCE, 17 NCE) due to the high potential for poor comprehension on the self-reported behavior measure, which would invalidate the assessment. Thus, the final sample size for this study was 329 CBCL (168 PCE, 161 NCE) and 325 DI (166 PCE and 159 NCE). The total number of subjects completing either the CBCL or DI was 332 (170 PCE, 162 NCE).
Table 1.
Maternal characteristics.a
| Cocaine (n=170)
|
Non-Cocaine (n=162)
|
p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Mother’s age at birth | 29.8 | 5.00 | 25.6 | 4.79 | <0.0001 |
| Parity | 3.54 | 1.90 | 2.70 | 1.89 | <0.0001 |
| Number of prenatal visits | 5.24 | 4.65 | 8.78 | 4.83 | <0.0001 |
| Maternal years of education | 11.6 | 1.71 | 11.9 | 1.44 | 0.08 |
| Not complete high school, n (%) | 79 | 46.47 | 53 | 32.72 | |
| High school, n (%) | 56 | 32.94 | 70 | 43.21 | 0.04 |
| Post high school, n (%) | 35 | 20.59 | 39 | 24.07 | |
| PPVT standard score | 74.91 | 15.72 | 78.43 | 14.94 | 0.04 |
| WAIS-R block design scale | 6.93 | 2.07 | 7.11 | 2.17 | 0.45 |
| WAIS-R picture completion | 6.83 | 2.16 | 6.91 | 2.32 | 0.74 |
| Global severity index | 0.80 | 0.73 | 0.51 | 0.54 | <0.0001 |
| Married, n (%) | 14 | 8.28 | 28 | 17.28 | 0.01 |
| Low SES, n (%) | 165 | 97.63 | 158 | 97.53 | 0.95 |
| African-American, n (%) | 141 | 82.94 | 131 | 80.86 | 0.62 |
| Prenatal drug use | |||||
| Cigarettes per day | 11.48 | 11.08 | 4.03 | 7.44 | <0.0001 |
| Drinks per week | 8.46 | 15.20 | 1.23 | 4.04 | <0.0001 |
| Marijuana per week | 1.29 | 3.26 | 0.69 | 3.71 | 0.0003 |
| Cocaine per week | 23.72 | 46.94 | 0.00 | 0.00 | N/A |
Data collected at 1st postnatal visit (average 1 month corrected).
2.2. Instruments and procedure
Children and their caregivers were assessed at multiple time points over 9 years at a university-based child development lab. This report includes data from the post-natal visit conducted on average 1 month post birth (corrected for gestational age) and the follow-up visit at child age 9. Maternal demographic and child birth information were collected either from hospital charts or at the post-natal visit. Mental health, substance use and cognitive data were collected from caregivers at the post-natal visit and mental health, substance use, home environment and child behavior ratings were collected at age 9 years. Research assistants trained in child testing and blind to cocaine-exposure status administered the assessment protocol to the child at age nine. A different research assistant administered maternal interviews privately. Effort was made to keep information about maternal prenatal substance use from child testers to prevent bias in assessment administration and scoring. Subjects were compensated $35 for the participation at the post-natal visit and $100 for their participation in the study at 9 years ($50 each for caregiver and child).
2.3. Measures
2.3.1. Demographics
Maternal and infant demographic and medical characteristics were taken from hospital birth records, including birth weight, height, head circumference, race, age, parity, number of prenatal care visits, family composition, and maternal education and work history. Low socioeconomic status was determined by a Hollingshead score of IV or V (Hollingshead, 1957). At each follow-up interview, information that was subject to change (e.g. education, substance use, child placement, home environment) was updated.
2.3.2. Prenatal cocaine (and other substance) exposure
At the post-natal visit, birth mothers were interviewed using a series of forced choice questions pertaining to timing and frequency of drug use during each trimester of pregnancy and the month prior to birth (Singer et al., 2000, 2002a). Frequency was recorded using a Likert scale ranging from 0 (not at all) to 7 (daily use), with data summarized to reflect the average number of days per week a drug was used. A severity score was calculated for each trimester and the month prior to infant birth by multiplying the frequency of substance use by the amount of the drug used per week. A total average exposure for pregnancy measure was obtained by averaging trimester use scores. This procedure was also used for an average alcohol use per week, joints smoked per week and cigarette use per day across the pregnancy. At age 9 years, a substance use assessment was administered to the current caregiver to determine average weekly drug use of the same drugs over the past 30 days.
2.3.3. Caregiver psychological symptoms
The Brief Symptom Inventory (BSI) (Derogatis and Melisaratos, 1983) is an adult self-report tool consisting of 53 items designed to quickly assess overall psychological distress based on nine psychiatric symptom patterns. The summary measure, the General Severity Index (GSI), reflecting overall psychological distress of the caregiver at the nine year assessment, was used in these analyses.
2.3.4. Caregiver cognitive ability
The Peabody Picture Vocabulary Test-Revised (PPVT-R) (Dunn and Dunn, 1997) and two subscales (Picture Completion and Block Design) of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler, 1989) were administered to the child’s caregiver to assess receptive vocabulary and nonverbal reasoning ability.
2.3.5. Measurement of the home environment
The Middle Childhood Home Observation for the Measurement of the Environment (MC- HOME (Caldwell and Bradley, 1984) was administered in interview format to measure the quality of the caregiving environment. The global score, calculated from summary scores of learning materials, language stimulation, physical environment responsivity, academic stimulation, modeling, variety, and acceptance was used as a measure of care giving quality.
2.3.6. The Dominic Interactive
The Dominic Interactive (DI) (Valla et al., 2000) is a computerized self-report measure that was administered to children. This assessment was designed to tap psychiatric symptoms based on seven DSM-IV (American Psychiatric Association) (Achenbach and Dumenci, 2001) diagnostic categories, including specific phobias, separation anxiety disorder, generalized anxiety disorder, major depression or dysthymia, oppositional defiant disorder (ODD), conduct disorder, and ADHD. All 91 questions were presented with a colorful cartoon picture, with race (Caucasian or African American) and gender appropriate versions employed. This assessment was specifically chosen because questions are read aloud via a voiceover recording that asks the child if they engage in the behavior portrayed by the cartoon image. This presentation is used to enhance understanding of the query. Seven diagnostic category scores and 3 broadband scores (internalizing, externalizing, and total score) were obtained.
Research has supported the psychometric properties of this assessment in children with and without PCE, with moderate to excellent internal consistency on the symptoms scales (Linares et al., 2006). Two French samples assessed the psychometric properties of the DI. Valla (2000) (Valla et al., 2000) indicated that reference and clinical judgment both indicate that the DI is a valid measure of mental health in France. Shojaei et al.(2009) found that the DI had satisfactory psychometric properties. All of the studies indicated that the DI is well accepted and liked (Achenbach, 1991) by children, parents and clinicians. Clinical ranges are only determined for the diagnostic category scales and are coded as no symptoms or clinical concerns, moderate, and severe clinical symptomatology. For these analyses the moderate and severe clinical categories were collapsed into one group reflecting a probable clinical interpretation. Symptom levels falling below these standardized cutoff points were considered non-symptomatic and coded (0).
2.3.7. Caregiver ratings of child behavior
The Child Behavior Checklist (CBCL) is a commonly used 118-item caregiver report measure designed to assess behavioral and emotional problems in children ages 4–16. Research indicates high test-retest reliability of school-age scales (.90 for CBCL empirically based problems scales) and good internal consistency (alpha=0.78–97 for problem scales) (Achenbach and Dumenci, 2001). Various forms of validity have also been demonstrated (Achenbach and Dumenci, 2001). Eight narrowband T scores (withdrawn, somatic complaints, anxious/depressed, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior) and three broadband T scores (internalizing withdrawn, somatic complaints, anxious/depressed, social problems], externalizing [delinquent and aggressive behavior], and total problems [all narrowband subscales]) were obtained. Higher scores indicate maladaptive behavior, with T scores assigned to raw scores. Probable clinical and clinical scores were collapsed into one category labeled probable clinical range. Scores of 67 on the narrowband scales or 60 on the broadband scales were required for classification.
2.3.8. Hematologic assessment
A subset of children was assessed for lead exposure in a separate study (Nelson et al., 2004). Venous blood samples could not be obtained from some children due to lack of parental consent, excessive stress related to the blood draw, child sickness or logistical difficulties. Valid hematologic measures were available for 143 two-year and 274 four-year old children. Measures were averaged for the 122 children seen at both assessment points resulting in 298 valid assessments. The correlation between lead measurements for children seen at both two and four years of age is 0.78. Children with PCE who had a valid lead level were more likely to be African American (p<0.02) and to have had more prenatal care (p<0.008) than children with PCE who did not have lead level assessed. There were no differences in demographic variables among cocaine negative children who had or did not have a valid lead level.
Blood collection and analyses of lead was performed by the affiliate University Hospital Laboratory Services Foundation accredited by The College of American Pathologists and in compliance with Clinical Laboratory Improvement Amendment (CLIA) regulations. The lab is in the Center for Disease Control (CDC)/World Health Organization (WHO) proficiency testing program for blood lead and was approved by the Occupational Safety and Health Organization (OSHA) for blood lead analysis. Approximately 5 mL of venous blood was drawn into a lead free container containing an anticoagulant. Blood lead concentration was determined by atomic absorption spectrophotometry using a graphite furnace and matrix modification to eliminate chemical interferences. Continuous lead values were used in statistical analysis.
2.4. Statistical analyses
The distributions for continuous data were checked for skewness and kurtosis. Positively skewed self-report drug use, BSI (GSI) and lead data were transformed using loge (X+1) to reduce the influence of outlying values prior to analyses. For ease of interpretation, means and standard deviations were reported using the non-transformed variables. Demographic characteristics, prenatal substance use, and birth outcome measures were examined by cocaine status using t-tests for continuous data and Chi-square analyses for dichotomous data. Children with PCE who were in foster or adoptive care and biological maternal or relative care and NCE children were compared on environmental, drug, and caregiver’s characteristics using Analyses of Variance (ANOVA). In the event of a significant group effect, follow-up pairwise tests were performed using the Tukey test to control the overall significance level. This caregiver grouping was established in our earlier studies as an important determinant in cognitive and behavioral outcome due to significantly enhanced environments provided in non-relative foster and adoptive care (Linares et al., 2006; Singer et al., 2008).
The effects of PCE on the odds of DI and CBCL scores above the borderline clinical cut-off were analyzed using logistic regression. Logistic regression evaluating both the continuous cocaine exposure variable, average cocaine exposure in units per week over pregnancy, and the dichotomous grouping variable (PCE vs. NCE) were completed.
Multiple linear regression analyses were used to assess the effects of PCE on continuous broadband scores. Cocaine exposure was considered to be a significant predictor if it remained significant using a p<0.05, 2-tailed test after control for confounding variables. Potential covariates had to meet two statistical criteria in order to be considered for analysis in the regression models for each behavioral outcome. First, the covariate had to differ by cocaine group status at p≤0.20. Second the potential covariate had to be correlated with the behavioral outcome at p≤0.20. Covariates were retained in the model if they were significant at p<0.10 or changed the unstandardized regression estimate (b) for cocaine by 15%. First, HOME score, maternal (birthmother age at infant birth, parity, education, prenatal care, SES) and current (9 years) caregiver characteristics (PPVT-R, Performance IQ and BSI) were entered separately in the model. Second, prenatal exposures and current caregiver cigarette, alcohol, and marijuana use were entered separately. Current cocaine use was not evaluated in the model because almost no current use was reported. Third, after evaluating cocaine status (PCE vs. NCE) current caregiver type (PCE biologic/relative, PCE foster/adoptive care and NCE) was evaluated. Blood lead levels were entered last due to the reduced sample size. Potential moderators included child’s gender and race/ethnicity. Potential mediators, because of their significant relationship to both PCE and behavioral outcomes, included the child’s gestational age, birth length, birth head circumference and birth weight. Mediators were evaluated only if there was a significant effect of cocaine on the dependent variable. Adjusted percentages of children in the probable clinical range and broadband scores were calculated using the mean of covariates. All analyses were performed using SAS (v 8.2, SAS Institute Inc., Cary, NC).
3. Results
3.1. Sample characteristics
Maternal and child birth, health and environmental characteristics by cocaine status (PCE vs. NCE) are presented in Tables 1 and 2. At infant birth, mothers who used cocaine were older (29.8 vs. 25.6; p<0.0001), had more live births (3.54 vs. 2.70; p<0.0001), and received fewer prenatal care visits (5.24 vs. 8.78; p<0.0001). At the post-partum assessment, women who used cocaine had lower PPVT-R scores (74.91 vs. 78.43; p=0.04), greater self-reported psychological symptoms (0.80 vs. 0.51; p<0.0001), and were less likely to be married (14% vs. 28%; p=0.01) than women who did not use cocaine. Women who used cocaine also reported using greater average amounts of cigarettes per day (11.48 vs. 4.03; p<0.0001), drinks of alcohol per week (8.46 vs. 1.23; p<0.0001), and marijuana joints per week (1.29 vs. 0.69; p<0.0003) during pregnancy than women who did not use cocaine. Children with PCE were born earlier (37.75 vs. 38.54; p=0.008), were shorter in length 47.3 vs. 49.18; p<0.0001), had smaller head circumference (32.3 vs. 33.6; p<0.0001) and weighed less (2703 vs. 3100; p<0.0001) than NCE children. At 9 years, children with PCE were more likely to be in adoptive/foster care (39%) compared to NCE children (6%) (p<0.0001). Children with PCE had lower blood lead levels compared to NCE children (6.9 vs. 8.1 ug/dL, p<0.02).
Table 2.
Infant Birth, health and environmental characteristics.
| Cocaine (n=170)
|
Non-cocaine (n=162)
|
p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| HOME score | 42.88 | 7.04 | 43.82 | 6.48 | 0.21 |
| 1 min Apgar | 8.01 | 1.42 | 7.9 | 1.68 | 0.57 |
| 5 min Apgar | 8.78 | 0.64 | 8.8 | 0.59 | 0.86 |
| Gestational age | 37.75 | 2.77 | 38.54 | 2.65 | 0.008 |
| Hobel neonatal risk score | 7.60 | 16.31 | 5.53 | 13.96 | 0.22 |
| Birth length (cm) | 47.3 | 3.94 | 49.18 | 3.54 | <0.0001 |
| Head circumference (cm) | 32.3 | 2.14 | 33.6 | 2.15 | <0.0001 |
| Birth weight (grams) | 2703 | 642 | 3100 | 680 | <0.0001 |
| Male, n (%) | 78 | 45.88 | 79 | 48.77 | 0.60 |
| African-American, n (%) | 140 | 82.35 | 130 | 80.25 | 0.62 |
| Microcephaly, n (%) | 25 | 14.97 | 5 | 3.11 | 0.0002 |
| Small for gestational age, n (%) | 22 | 13.17 | 3 | 1.86 | 0.0001 |
| Currently in adopted/foster care, n (%) | 39 | 22.94 | 6 | 3.70 | <0.0001 |
| 2 yrs and/or 4 yrs lead levela | 6.87 | 4.01 | 8.07 | 4.54 | 0.02 |
| 2 yrs and/or 4 yrs lead level (>10 ug/dL)a, n (%) | 24 | 17.52 | 35 | 26.92 | 0.64 |
Sub-sample size included 137 CE and 130 NCE.
Table 3 shows characteristics of caregiver type by cocaine-exposure status. These categories include children with PCE placed with birth mother/relative, children with PCE placed with an adoptive or foster parent, and NCE children. Women who were adoptive/foster caregivers of children with PCE had higher educational attainment than birth mothers or relatives of PCE children (13.49 years vs. 11.89 years) and caregivers of NCE children (12.53). Receptive language ability (PPVT-R scores) was higher for adoptive or foster caregivers of PCE children (89.11) than for PCE birth mothers/relative caregivers (77.57) and caregivers of NCE children (80.05). Birth mother/relative caregivers of children with PCE used more cigarettes per day on average (6.89) than adoptive/foster parents caring for children with PCE (2.39) or caregivers of NCE children (3.97). Children with PCE and children living in adoptive/foster care also had significantly lower blood lead levels (5.84 ug/dL) than NCE children (8.07 ug/dL). Of those children with PCE placed in foster or adoptive care, 53% had only one placement, 34% had two, 5% had three and 8% had four. Ninety-one percent of NCE children were living with birthmothers, 10 (7%)with biologic fathers or relatives and 4 (2.5%) were adopted.
Table 3.
Caregiver and child characteristics by placement status and cocaine group.
| 9-year current caregiver demographics | PCE: biological/relative (n=131)
|
PCE: adopt/foster (n=39)
|
NCE (n=162)
|
F/Χa | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Home score | 42.52 | 7.53 | 44.08 | 5.00 | 43.82 | 6.38 | 1.61 | 0.20 |
| Education statusa,b.c | 11.89 | 1.77 | 13.49 | 2.45 | 12.53 | 1.68 | 12.18 | <0.0001 |
| PPVT Scoreb.c | 77.57 | 15.04 | 89.11 | 15.80 | 80.05 | 15.92 | 7.15 | 0.0009 |
| Design scale score | 7.01 | 1.90 | 7.06 | 3.15 | 7.34 | 2.13 | 0.84 | 0.43 |
| Picture completion scale score | 7.10 | 2.24 | 6.63 | 2.90 | 7.06 | 2.35 | 0.30 | 0.74 |
| GSI score | 0.36 | 0.38 | 0.24 | 0.29 | 0.39 | 0.51 | 1.92 | 0.15 |
| Cigarettes per daya.b | 6.89 | 7.36 | 2.39 | 5.80 | 3.97 | 6.74 | 8.88 | 0.0002 |
| Alcohol dose per week | 1.27 | 2.69 | 0.26 | 0.71 | 1.07 | 3.31 | 1.67 | 0.19 |
| Marijuana dose per week | 0.43 | 2.73 | 0.00 | 0.00 | 0.32 | 1.90 | 0.58 | 0.56 |
| Cocaine dose per week | 0.01 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.81 | 0.45 |
| Child 2 years and/or 4-years lead levelc.d | 7.11 | 4.09 | 5.84 | 3.54 | 8.07 | 4.54 | 3.59 | 0.03 |
| 2 yrs and/or 4 yrs Lead Level (>10 ug/dL)d, n (%) | 22 | 9.82 | 2 | 7.69 | 35 | 26.92 | 5.227 | 0.073 |
CE: biological/relative vs. NCE, p<0.05.
CE: biological/relative vs. CE: adopted/foster, p<0.05.
CE: adopted/foster vs. NCE, p<0.05.
Sub-sample size included 111 CE: biological/relative, 26 CE: adopt/foster, 130 NCE. 586
Children with IQ less than 70 (n=36) were excluded from the study. These children were compared to children who were included in the study separately by cocaine status. Children with PCE not included in the sample due to IQ <70 (n=19) had significantly lower biologic caregiver PPVT-R standard score s (p<0.00001), higher prenatal average alcohol exposure (month prior and average) and higher PCE (month prior, first trimester and average) (p<0.05) than those PCE children included in the sample. Children with PCE excluded from the study sample due to <70 IQ were also more likely to have a higher percentage of borderline clinical or above total problem scores on the CBCL than those who were maintained in the study sample (p<0.05). Among NCE children, there were no demographic differences between those with IQ≥70 and those with IQ <70 (n=17). Thus, a higher percentage of children with total problem (p<0.04) and externalizing (p<0.05) scores at or above the clinical range were excluded from the study sample, with the majority of these from the PCE group. Gender was equally distributed by those who were retained in the study and those that were excluded for IQ less than 70 in both the PCE and NCE group.
3.2. Effects of cocaine on CBCL and Dominic Interactive
3.2.1. Child Behavior Checklist
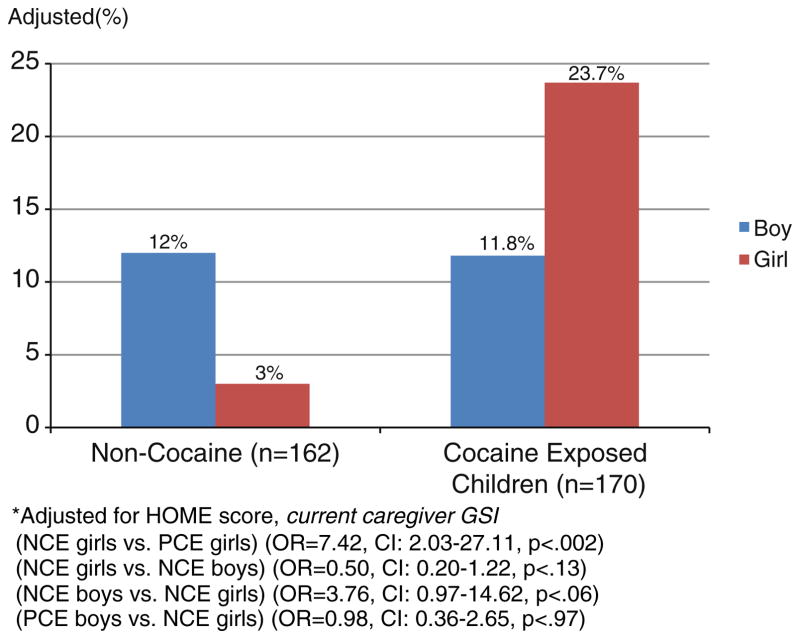
Caregiver report of clinically elevated aggressive behavior increased with higher amounts of PCE (representing average cocaine units per week logged/1.75 average units of cocaine). With each increased unit of PCE, children were 1.3 times more likely to have elevated levels of aggressive behavior on the CBCL (OR=1.30, 95% CI: 1.01–1.67, p<0.04). There was also an effect of higher levels of PCE on reports of delinquent behavior for girls. With each increased unit of PCE, girls were 2 times more likely to be rated as having higher levels of clinically relevant delinquent behavior on the CBCL (OR=2.08, 95% CI: 1.46–2.96, p<0.0001). No such relationship existed for boys (OR=0.74, 95% CI: 0.49–1.11, p=0.146). In addition to using a continuous measure of PCE, PCE was also evaluated by the group status variable PCE vs. NCE. A significant effect of cocaine was found for clinically elevated delinquent behavior. Children with PCE were at significantly greater risk for delinquent behavior than NCE children (OR=2.41; 95% CI: 1.16–4.97, p<0.02). Fig. 1 shows a cocaine by gender interaction indicating that girls with PCE were 7 times more likely than NCE girls to be reported as having clinically relevant delinquent behaviors (3% NCE girls vs. 23.7% PCE girls) (OR=7.42, CI: 2.03–27.11, p<0.002). No such difference existed for boys with PCE or NCE (12% NCE boys vs. 11.8% PCE boys; OR=0.98, CI: 0.36–2.65, p=0.97). When the effect of birth head size was evaluated as a mediator of cocaine, it was found that small birth head size was associated with greater likelihood of delinquent behavior. However, small birth head size did not fully mediate the relationship between cocaine and delinquent behavior. That is, cocaine remained significant in the model even when accounting for the variance in delinquent behavior associated with head size. When evaluating aggressive behavior, small head size fully mediated cocaine’s effect on ratings of aggressive behavior, with cocaine no longer a significant predictor of aggressive behavior with head size in the model.
Fig. 1.
Adjusted percentages of children reaching probable clinical range on CBCL Delinquent Behavior by gender and cocaine*.
3.2.2. Cocaine effects on Dominic Interactive
Prenatal cocaine exposure decreased the odds of self-reported ODD (OR=0.44, 95% CI: 0.21–0.92, p=0.03). PCE was also associated with lower DI continuous broadband internalizing (β=−0.14, p<0.03) and total (β=−0.14, p<0.03) scale scores. Gender did not significantly predict scores on the DI.
3.3. Effects of other drug exposure, environmental factors and elevated lead levels
3.3.1. Child Behavior Checklist
Higher average 2nd trimester tobacco exposure was associated with increased odds of caregiver reported anxiety problems in their child (OR=1.73; CI 1.06–2.81, p<0.03) and higher average marijuana exposure increased the odds of caregiver reported thought problems (OR=1.68; CI 1.01–2.79, p<0.05). Higher average prenatal alcohol exposure was associated with decreased odds of clinically meaningful internalizing symptoms (OR=0.61; CI 0.37–0.99, p<0.04).
Higher caregiver reported psychological distress was associated with more symptoms on every subscale of the CBCL (p<0.01), except for anxiety and attention problems in which there were non-significant trends (ps<0.07). Lead level was not associated with any CBCL caregiver reported scores in the probable clinical range.
3.3.2. Dominic Interactive
Greater prenatal exposure to cigarettes was associated with an increased likelihood of ODD (OR=2.36; CI 1.03–1.78, p<0.03). More optimal HOME scores were related to decreased odds of generalized anxiety (OR=0.94; CI 0.90–0.98, p<0.002), major depression or dysthymia (OR=0.95; CI 0.92–0.99, p<0.01), and conduct disorder (OR=0.95; CI 0.91–0.99, p<0.01). Greater caregiver psychological distress increased the odds of separation anxiety disorder (OR=1.41; CI 1.01–1.98, p<0.05). Higher current caregiver PPVT-R scores were associated with lower odds of separation anxiety disorder (OR=0.98; CI 0.96–1.00, p<0.03). Blood lead level was not associated with self-reported increases of behavioral problems.
3.4. Effects of adoptive/foster care status
3.4.1. CBCL
The adjusted percentages of children with CBCL subscales and broadband mean scores in the probable clinical range are presented by caregiver status in Table 4. Children with PCE in foster or adoptive care were rated by caregivers as being at increased odds of having thought problems (p<0.03), inattention problems (p<0.0005), delinquent behavior (p<0.0004), aggressive behavior (p<0.0003), externalizing symptoms (p<0.005) and total problems (p<0.0007) compared to children with PCE living in biologic relative care or to NCE children. The externalizing CBCL continuous scales were also associated with caregiver status. Adoptive and foster caregivers rated their children with PCE as having more externalizing symptoms compared to biologic mothers or relatives of children with PCE or NCE children (p<0.03).
Table 4.
Adjusted percentage of children reaching probable clinical range and adjusted mean broadband scores based on the CBCL by caregiver status.
| Dichotomous scale | PCE: biologic/relative (n=130) | PCE: adopt/Foster (n=38) | NCE (n=161) | Chi-square* | P** |
|---|---|---|---|---|---|
| Anxietya | 0.85 | 3.40 | 5.20 | 4.54 | .10 |
| Withdrawnb | 3.94 | 6.89 | 3.11 | 0.80 | .67 |
| Somatic Complaintsc | 4.04 | 1.74 | 4.37 | 0.65 | .72 |
| Social Problemsd | 6.48 | 12.95 | 7.34 | 1.74 | .56 |
| Thought Problemse | 3.57 | 16.82 | 3.13 | 7.10 | .029 |
| Attention Problemsd | 7.87 | 44.91 | 8.75 | 15.06 | .0005 |
| Delinquent Behaviorf | 11.48 | 49.60 | 6.98 | 15.78 | .0004 |
| Aggressive Behaviorf | 4.55 | 37.52 | 5.18 | 17.20 | .0003 |
| Internalizingg | 13.58 | 23.76 | 10.94 | 1.44 | .49 |
| Externalizingh | 24.53 | 94.09 | 25.34 | 10.73 | .005 |
| Total scorei | 12.6 | 87.2 | 18.6 | 14.6 | .0007 |
| Continuous broadband scores | M (SE) | M (SE) | M (SE) | F | p |
| Internalizingj | 47.6 (0.95) | 49.01 (1.82) | 48.2 (0.86) | 0.27 | .76 |
| Externalizingk | 50.6 (0.99) | 56.2 (3.01) | 50.3 (0.90) | 3.65 | .027 |
| Total scorel | 48.2 (1.04) | 53.1 (2.07) | 49.7 (0.93) | 2.38 | .09 |
Note. Significant (p<0.05) covariates are listed in italics below.
Chi-square, F and p values represent the overall test of caregiver type.
All significant p-values indicate significant differences of CE Adopt/Foster group from CE: Biologic/Relative and NCE.
Adjusted for current caregiver’s Global Severity Index (GSI) score and birth maternal 2nd trimester cigarettes use.
Adjusted for birth maternal age at birth, current caregiver’s GSI, 2nd trimester alcohol use.
Adjusted for birth maternal age at birth, parity, and PPVT standard score, and birth maternal 2nd trimester cigarette use, and current caregiver’s GSI.
Adjusted for current caregiver’s GSI.
Adjusted for current caregiver’s GSI, birth maternal average use of cigarette and marijuana during pregnancy.
Adjusted for HOME score, current caregiver GSI.
Adjusted for HOME score, birth maternal age at birth, PPVT standard score, average cigarette use during pregnancy and 3rd trimester alcohol use, and current caregiver’s GSI.
Adjusted for HOME score, birth maternal average cigarette use during pregnancy and current caregiver GSI.
Adjusted for HOME score, birth maternal PPVT standard score and average cigarette use during pregnancy, and current caregiver’s GSI.
Adjusted for HOME score, birth maternal age at birth, average cigarette and alcohol use during pregnancy and current caregiver’s PPVT standard score and GSI.
Adjusted for HOME score, birth maternal 2nd trimester cigarette use and current caregiver’s PPVT standard score and GSI.
Adjusted for HOME score, birth maternal average cigarette use during pregnancy and current caregiver’s PPVT standard score and GSI.
3.4.2. Dominic Interactive
The adjusted percentages and means of self-reported behavioral symptoms by cocaine and caregiver status are presented in Table 5. There were significant caregiver effects for broadband externalizing (F=3.13, p<0.04) and total scores (F=3.26, p<0.04). For the total score, there was a significant post-hoc difference between the children with PCE in biologic/relative care versus NCE children (p<0.04). Children with PCE in biologic/relative care reported lower total scores than NCE children. There was a non-significant trend (p<0.09) for children with PCE in foster/adoptive care to report a higher percentage of externalizing symptoms in the probable clinical range compared to the children with PCE in biologic/relative care or NCE (p<0.09).
Table 5.
Adjusted percentage of children reaching probable clinical range and adjusted mean broadband scores based on the DI by caregiver status.
| Dichotomous diagnostic categories | PCE: biological/relative (n=128) | PCE: adopt/foster (n=38) | NCE (n=159) | Chi-square* | p |
|---|---|---|---|---|---|
| Specific phobiasa | 67.16 | 37.36 | 75.35 | 2.60 | .27 |
| Separation anxietyb | 52.93 | 40.82 | 59.97 | 0.74 | .69 |
| Generalized anxietyc | 26.81 | 24.76 | 37.85 | 1.44 | .49 |
| Major depression or dysthymiad | 23.83 | 34.05 | 25.60 | 0.60 | .74 |
| Oppositional defiant disordere | 12.63 | 12.14 | 28.53 | 4.70 | .10 |
| Conduct disorderf | 21.03 | 27.14 | 18.78 | 0.61 | .74 |
| ADHDg | 16.42 | 30.92 | 16.79 | 1.66 | .43 |
| Broadband scores | M (SE) | M (SE) | M (SE) | F | p |
| Internalizingh | 18.3 (0.95) | 19.8 (1.83) | 21.4(0.84) | 2.71 | .07 |
| Externalizingi | 8.8 (0.63) | 11.5 (1.14) | 10.6 (0.56) | 3.13 | .04 |
| Total scorej | 27.1 (1.48) | 31.2 (2.88) | 32.2 (1.31) | 3.26 | .04 |
Note. Significant (p<0.05) covariates are listed in italics.
Chi-square, F and p values represent the overall test of caregiver type.
Adjusted for birth maternal education and alcohol use during pregnancy.
Adjusted for HOME score, birth maternal PPVT standard score and alcohol use during pregnancy, and current caregiver’s Global Severity Index (GSI) score.
Adjusted for HOME score and birth mother’s 1st trimester cigarettes use.
Adjusted for HOME score, parity, and birth mother’s 2nd trimester cigarettes use.
Adjusted for parity and birth maternal average cigarettes use during pregnancy and 1st trimester alcohol use.
Adjusted for HOME score and birth maternal average marijuana use during pregnancy.
Adjusted for HOME score, parity, and birth maternal 2nd trimester marijuana use.
Adjusted for HOME score, birth maternal 1st trimester alcohol use and current caregiver’s PPVT.
Adjusted for HOME score and birth mother’s 1st trimester marijuana use; no significant post hoc differences.
Adjusted for HOME score, birth maternal 1st trimester alcohol use and current caregiver’s PPVT; significant post-hoc difference between CE: Biological/Relative vs. NCE (p=0.035).
4. Discussion
4.1. Summary and interpretation of results
This study investigated the mental health outcomes of children with and without PCE at 9 years of age using caregiver and child self-reported behavior problems. The hypothesized association between PCE and caregiver report of externalizing problems (delinquent and aggressive behavior) was realized, although results from child self-report using the DI, in contrast to findings at 6 years, did not indicate increased externalizing symptoms. Results indicated that as levels of PCE increased there was an increased likelihood of aggressive behavior for all children and increased delinquent behavior among females. Prenatal cocaine exposure (PCE vs. NCE) was associated with increased odds of caregiver reported delinquent behaviors in females after controlling for confounders. Examination of a cocaine by gender interaction indicated that girls with PCE were 7 times more likely to be rated as having clinically relevant delinquent behaviors than girls with NCE. Further analyses using a continuous cocaine variable indicated that increasing levels of PCE were related to increased likelihood of delinquent behavior caregiver ratings for girls. Birth head size (<10th percentile) was associated with increased odds of delinquent behavior suggesting that birth head size partially mediated cocaine’s effect on delinquent behavior. Cocaine effect was reduced from OR 2.41; CI 1.16–4.97, p<0.002 to OR 2.10; CI 1.00–1.44, p<0.05. This finding suggests that the association of cocaine and delinquent behavior can be considered independently from the effects of small head size at birth in this sample. However, small head size was a full mediator of cocaine’s effect on aggressive behavior.
Although an association between PCE and increased odds of delinquent and aggressive behavior ratings using the CBCL at age 9 was found in the current report, it was not present in our previous report at six years of age (Linares et al., 2006). The current findings of increased disruptive behavior ratings are consistent with studies indicating more behavioral problems in PCE animals (Johns et al., 1994; McMurray et al., 2008) and delinquency or oppositional behavior in humans (Bendersky et al., 2006; Bennett et al., 2007). However, the gender by cocaine interaction found in this analysis, and our longitudinal report of CBCL data, are in contrast to other research indicating increased aggressive and hyperactive behavior among boys with PCE (Bendersky et al., 2006; Bennett et al., 2007; Delaney-Black et al., 2004).
Significant effects of caregiver type were found for several of the CBCL subscales and broadband scales, including thought and attention problems, delinquent and aggressive behaviors, and externalizing and total broadband scores. Among children with PCE, those rated by adoptive/foster caregivers had higher odds of behavioral problems compared to children rated by biologic/relative caregivers. This finding was also noted in our previous report of behavior at 6 years of age. However, the finding is in contrast to this research group’s findings indicating benefits of an enriched environment (foster or adoptive care) for cognitive and language outcomes among prenatally cocaine exposed children (Lewis et al., 2011; Singer et al., 2008). Investigation of how the quality and type of caregiving affects outcomes for children with PCE will require further investigation.
At age 9 years, children with PCE self-reported fewer symptoms of ODD and fewer total symptoms than NCE children. This finding is in contrast to the report of this cohort at six years of age in which children with PCE reported more symptoms of ODD, inattention and conduct disorder (Linares et al., 2006). An underestimation of oppositional behavior at age 9 may reflect lack of awareness or denial of problematic behaviors by children with PCE. This finding warrants further investigation.
Other prenatal drug exposures and environmental factors were associated with increased behavioral problems as well. Children prenatally exposed to tobacco reported more symptoms of ODD. This finding is consistent with studies of this cohort and other research indicating that prenatal tobacco exposure is associated with increased odds of externalizing symptoms at 4, 9 and 10 years (Cornelius et al., 2007; Minnes et al., 2010). Second trimester tobacco exposure was also associated with symptoms of anxiety and higher average marijuana exposure was associated with thought problems on the CBCL. Other studies of prenatal marijuana exposure report increases in depression (Gray et al., 2005; Leech et al., 2006) and overall internalizing symptoms (O’Connor and Paley, 2009) but not thought problems. Greater prenatal alcohol exposure was related to fewer caregiver reported internalizing symptoms. This finding is somewhat counterintuitive as O’Connor (O’Connor and Paley, 2009) provides a comprehensive summary of psychiatric conditions (e.g., externalizing, depressive, and anxiety symptoms) associated with prenatal alcohol exposure. Additional studies with adequate control for other drug exposures and environmental factors are needed to clarify the current findings.
One important environmental factor that contributed to both caregiver and self-reported behavioral problems is caregiver psychological distress. Not surprisingly, greater psychological distress among caregivers was associated with an increased report of behavior problems in almost all CBCL subscales, except anxiety and attention problems that approached significance. For caregiver report however, the shared variance of self-reported psychological distress and caregiver rating of the child must be considered. Findings of this study are similar to those reported previously (Friedlander et al., 1986; Linares et al., 2006) and are likely associated with changes in caregiver behavior (e.g. responsiveness) related to psychological distress. Better HOME scores were associated with reduced odds of child reported generalized anxiety, major depression or dysthymia and conduct disorder. Elevated blood lead levels were not associated with increased self- or caregiver report of behavioral problems after control for covariates. This finding does not support the stated hypotheses or previous research focused on the behavioral effects of lead which typically indicates a relationship between higher blood lead levels and externalizing behavior patterns. Nonetheless, lead effects may be less apparent due to the overarching effect of PCE on children’s behavior.
4.2. Strengths and limitations
Results of this study present some yet unanswered questions and highlight some limitations. For example, the consistent finding that foster/adoptive caregivers of children with PCE rate their children as having a higher likelihood of behavioral problems than biologic mother/relative caregivers can be explained in several ways. Adoptive or foster parents have on average higher education and vocabulary levels, and fewer psychological distress symptoms than relative caregivers of either child with PCE or NCE children. They also smoke fewer cigarettes and their children have lower blood lead levels. Higher level of education and vocabulary may enable them to better evaluate the subtleties of child behavior. Alternatively, they may hold particular biases regarding the effects of PCE on behavior, which is then reflected in their reporting. It is also possible that this group of children placed in foster/adoptive care, and who also have the highest average levels of PCE, may actually have more behavioral symptoms. In addition, although caregiver current level of substance use is assessed, these data are often under-reported, particularly related to illegal drug use. These data may be more fully understood if a comparative group of NCE children living in foster/adoptive care were available and there were confirmatory biologic measures of caregiver substance use.
While effects of cocaine were found for aggression and delinquent behavior, it is possible that these results are an underestimation of the level of problem behaviors among children with PCE. The decision to eliminate children with IQs less than 70 from the study sample was a necessary criterion for maintaining reliability on the DI. However, since the cocaine exposed children with <70 IQ had greater total and externalizing problems, a type II error may have been inadvertently introduced. It will be necessary to include additional methods of behavioral assessment for the whole sample at later ages to fully assess the extent of behavioral problems among children with PCE.
The effect of cocaine on disruptive behaviors among girls with PCE in this sample may suggest that the self-regulatory behavior of girls may be more sensitive to the negative effects of PCE on the developing monoaminergic systems. This effect may also be related to a greater ease of reporting disruptive behaviors among girls known to be at biologic risk such as PCE. An additional and/or alternative explanation may be related to advanced pubertal stage among the girls vs. boys in this study. Girls enter puberty on average two years earlier than boys, with puberty associated with increases in behavioral problems (Lynne et al., 2007). It is possible that cocaine’s effect on delinquent behavior is exacerbated by pubertal development and will not be apparent in boys until an older age. In addition, this study did not take into account the effects of violence exposure a factor shown to be important in the presence of mental health symptoms in studies of prenatal drug exposure (Frank et al., 2011; MacDonald et al., 2008).
Elevated blood lead was not an independent predictor of externalizing behavior problems. These findings may be related to timing in the measurement of blood lead. Hematologic assessments were completed when children were two and four years of age and behavioral data was collected at age 9. Concurrently measured lead level and outcome data are more highly correlated than data collected at different ages (Coscia et al., 2003). It is also possible that the reduced sample size and the restricted range of elevated blood lead in our sample (1.75–25.2, with 75% of the sample having blood lead levels less than 9.5) contributed to the lack of an association.
Another potential weakness of this study may be the inability of children to accurately self-monitor their behaviors, particularly undesirable behaviors. Perhaps differences in self-report at 6 and 9 years result from the older children having greater self-awareness and thereby more accurately reporting on their behavior compared to children when they were age 6 who reported more conduct and attention problems. However, a recent study has highlighted promising psychometrics of the DI, including good to excellent internal consistency in the present sample and construct validity (Linares-Scott et al., 2006). At age six there were significant correlations between the Conners’ Teacher Rating Scale (CTRS) scores for aggression and externalizing, ODD and conduct disorders on the DI and CTRS hyperactivity and DI externalizing. Correlations between caregiver CBCL aggression ratings and DI externalizing, ODD and conduct disorder were also reported. These significant correlations indicate, in general, that findings from the DI correlate with more objective (caregiver and teacher) ratings of their behavior. However the DI has a comparatively short history of development compared to the CBCL and relies completely on child self-report. Obtaining teacher report data could augment the research on mental health outcomes of children with prenatal drug exposure. In addition, examination of child and caregiver exposure to violence, which can negatively influence child behavioral outcomes, was not assessed in these analyses presenting a limitation to be addressed in future research.
Despite the noted limitations, this study has several strengths. Among these strengths are the large number of subjects who were studied prospectively and the very low attrition rate <10%) at nine years. The design of the study allowed for a large number of confounders to be examined, increasing confidence in the findings. Further, evaluation of the caregiver status among children with PCE allowed for additional insight into the role of caregiver perspective and the effects of child placement on behavioral outcomes in this sample. An additional strength in the study design was the use of combined biologic and self-report measures which increased the likelihood that subjects were accurately grouped into the prenatally cocaine or non-cocaine exposed groups.
5. Conclusions
It is important to continue to examine the effects of prenatal exposure to cocaine, tobacco, alcohol and marijuana and maternal psychological status to determine how they influence mental health outcomes throughout stages of child development. The findings of this study suggest that, though temporally distal, the role of PCE has a potentially prominent and enduring impact on disruptive behavior during middle childhood, specifically for girls, and that the amount of PCE is an important factor associated with behavioral outcome. Caregiver psychological status and type of caregiver placement may also play an important role in the perception or expression of behavioral problems. Children and their caregivers, particularly those from backgrounds of known physiologic and environmental stressors, should be screened periodically over the course of their routine medical care for potential emotional and behavioral issues. Referrals for behavioral interventions should be made for identified youth. Additionally, caregiver psychological distress should be addressed in therapeutic drug treatments in an effort to alleviate distress and, in turn, potentially enhance behavioral outcomes in children with PCE. Regular assessments by pediatricians and other mental health clinicians for evidence of behavioral problems, as well as referral for intervention and caregiver support, should continue throughout later childhood when risks may increase.
Acknowledgments
Thanks are extended to the participating families and the Cuyahoga County Department of Children and Family Services. We also thank Adelaide Lang, Paul Weishampel, Laurie Ellison, and Terri Lotz-Ganley. This research was supported by the National Institute of Health (NIH) National Institute on Drug Abuse (NIDA) (R01 07957; PI: Lynn T. Singer) and NIDA Research Supplement for Underrepresented Minorities (DA 07957; PI: Lynn T. Singer; A.M. Aguirre). The Study sponsor did not have a role in the study design, collection, analyses or interpretation of these data, the writing of this report, or the decision to submit this paper for publication.
Footnotes
Conflict of interest statement
The authors of this paper have no financial or personal relationship with people or organizations that could inappropriately influence the work submitted.
References
- Accornero VH, Morrow CE, Bandstra ES, Johnson AL, Anthony JC. Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol. 2002;27:259–69. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accornero V, Amado A, Morrow C, Xue L, Anthony J, Bandstra E. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach T, Dumenci L. Advances in empirically based assessment: Revised cross-informant syndromes and new DSM-oriented scales for the CBCL, YSR, and TRF: Comment on Lengua, Sadowksi, Friedrich, and Fischer. J Consult Clin Psychol. 2001;69:699–702. [PubMed] [Google Scholar]
- Arendt RA, Singer LT, Minnes S, Salvator A. Accuracy in detecting prenatal drug exposure. J Drug Issues. 1999;29:203–14. doi: 10.1177/002204269902900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, Lagasse L, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–59. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Dev Psychol. 1998;34:555–64. [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38:648–58. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–72. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Observation for measurement of the environment. Little Rock, AR: University of Arkansas Press; 1984. [Google Scholar]
- Chaplin TM, Fahy T, Sinha R, Mayes LC. Emotional arousal in cocaine exposed toddlers: prediction of behavior problems. Neurotoxicol Teratol. 2009;31:275–82. doi: 10.1016/j.ntt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriboga CA, Kuhn L, Wasserman GA. Prenatal cocaine exposures and dose-related cocaine effects on infant tone and behavior. Neurotoxicol Teratol. 2006;29:323–30. doi: 10.1016/j.ntt.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9:739–50. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscia JM, Ris MD, Succop PA, Dietrich KN. Cognitive development of lead exposed children from ages 6 to 15 years: an application of growth curve analysis. Child Neuropsychol. 2003;9:10–21. doi: 10.1076/chin.9.1.10.14498. [DOI] [PubMed] [Google Scholar]
- Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313–22. doi: 10.1111/j.1360-0443.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, et al. Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25:254–63. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–97. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–8. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test. 3. Circle Pines, MN: AGS Publishing; 1997. (PPVT-III) [Google Scholar]
- Eiden RD, McAuliffe S, Kachadourian L, Coles C, Colder C, Schuetze P. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicol Teratol. 2009;31:60–8. doi: 10.1016/j.ntt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Crooks D, Cabral HJ, Gerteis J, Hacker KA, et al. Adolescent initiation of licit and illicit substance use: impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicol Teratol. 2011;33:100–9. doi: 10.1016/j.ntt.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander S, Weiss DS, Traylor J. Assessing the influence of maternal depression on the validity of the Child Behavior Checklist. J Abnorm Child Psychol. 1986;14:123–33. doi: 10.1007/BF00917228. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325–36. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Gray KA, Day NL, Leech S, Richardson GA. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol. 2005;27:439–48. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. New Haven, CT: Yale University; 1957. [Google Scholar]
- Johns JM, Means MJ, Bass EW, Means LW, Zimmerman LI, McMIllen BA. Prenatal exposure to cocaine: effects on aggression in Sprague–Dawley rats. Dev Psychobiol. 1994;27:227–39. doi: 10.1002/dev.420270405. [DOI] [PubMed] [Google Scholar]
- Leech SL, Larkby CA, Day R, Day NL. Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry. 2006;45:223–30. doi: 10.1097/01.chi.0000184930.18552.4d. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Minnes S, Short EJ, Weishampel P, Satayathum S, Min MO, et al. The effects of prenatal cocaine on language development at 10 years of age. Neurotoxicol Teratol. 2011;33:17–24. doi: 10.1016/j.ntt.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares-Scott TJ, Short EJ, Singer LT, Russ SW, Minnes S. Psychometric properties of the Dominic Interactive Assessment: a computerized self-report for children. Assessment. 2006;13:16–26. doi: 10.1177/1073191105284843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–40. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Lynne SD, Graber JA, Nichols TR, Brooks-Gunn J, Botvin GJ. Links between pubertal timing, peer influences, and externalizing behaviors among urban students followed through middle school. J Adolesc Health. 2007;40:181.e7–181.e13. doi: 10.1016/j.jadohealth.2006.09.008. Epub 2006 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald HZ, Beeghly M, Grant-Knight W, Augustyn M, Woods RW, Cabral H, et al. Longitudinal association between infant disorganized attachment and childhood posttraumatic stress symptoms. Dev Psychopathol. 2008;20:1351. doi: 10.1017/S0954579408000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Arch Gen Psychiatry. 2004;61:836–43. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Grillon C, Granger R, Schottenfeld R. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol. 2002;24:385–95. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- McMurray MS, Joyner PW, Middleton CW, Jarrett TM, Elliott DL, Black MA, et al. Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams. Stress. 2008;11:398–410. doi: 10.1080/10253890701850239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PA, Pivetz T, Dignam TA, Homa DM, Schoonover J, Brody D. CDC, editor. Surveillance for elevated blood lead levels among children —United States, 1997–2001. Centers for Disease Control and Prevention; Atlanta, GA: CDC: 2003. pp. 1–21. [PubMed] [Google Scholar]
- Minnes M, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, et al. The effects of prenatal cocaine-exposure and lead on behavior in children 4–10 years. Neurotoxicol Teratol. 2010;164:518–24. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA. 1996;275:363–9. [PubMed] [Google Scholar]
- Nelson S, Lerner E, Needlman R, Salvator A, Singer LT. Cocaine, anemia, and neurodevelopmental outcomes in children: a longitudinal study. J Dev Behav Pediatr. 2004;25:1–9. doi: 10.1097/00004703-200402000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Short EJ, Arendt RE, Minnes SE, Bearer CF. Attention and executive functioning in pre-school-age children prenatally exposed to alcohol, cigarettes, cocaine, and marijuana. Neurotoxicol Teratol. 2003;25:381–97. doi: 10.1097/01.ALC.0000060525.10536.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15:225–34. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Jacobson SW, Jacobson JL. The relation of low-level prenatal lead exposure to behavioral indicators of attention in Inuit infants in Arctic Quebec. Neurotoxicol Teratol. 2007;29:527–37. doi: 10.1016/j.ntt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. Continued effects of prenatal cocaine use: preschool development. Neurotoxicol Teratol. 2009;31:625–33. doi: 10.1016/j.ntt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei T, Wazana A, Pitrou I, Gilbert F, Bergeron L, Valla JP, et al. Psychometric properties of the Dominic Interactive in a large French sample. Can J Psychiatry. 2009;54:767–76. doi: 10.1177/070674370905401107. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicol Teratol. 2000;22:653–66. doi: 10.1016/s0892-0362(00)00092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, et al. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002a;287:1952–60. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Salvator A, Arendt RE, Minnes S, Farkas K, Kliegman R. Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes. Neurotoxicol Teratol. 2002b;24:127–35. doi: 10.1016/s0892-0362(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Singer LT, Eisengart LJ, Minnes S, Noland J, Jey A, Lane C, et al. Prenatal cocaine exposure and infant cognition. Infant Behav Dev. 2005;28:431–44. doi: 10.1016/j.infbeh.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, et al. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–11. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood BG, Nordstrom Bailey B, Covington C, Sokol RJ, Ager J, Janisse J, et al. Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicol Teratol. 2005;27:191–201. doi: 10.1016/j.ntt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Valla JP, Bergeron L, Smolla N. The Dominic-R: a pictorial interview for 6- to 11-year-old children. J Am Acad Child Adolesc Psychiatry. 2000;39:85–93. doi: 10.1097/00004583-200001000-00020. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Weschler adult intelligence scale- revised. San Antonio, TX: Psychological Corp; 1989. [Google Scholar]