Summary
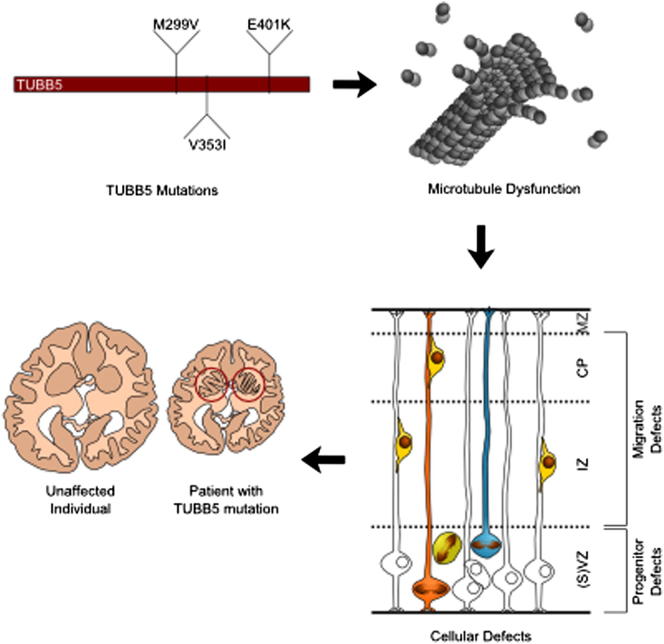
The formation of the mammalian cortex requires the generation, migration, and differentiation of neurons. The vital role that the microtubule cytoskeleton plays in these cellular processes is reflected by the discovery that mutations in various tubulin isotypes cause different neurodevelopmental diseases, including lissencephaly (TUBA1A), polymicrogyria (TUBA1A, TUBB2B, TUBB3), and an ocular motility disorder (TUBB3). Here, we show that Tubb5 is expressed in neurogenic progenitors in the mouse and that its depletion in vivo perturbs the cell cycle of progenitors and alters the position of migrating neurons. We report the occurrence of three microcephalic patients with structural brain abnormalities harboring de novo mutations in TUBB5 (M299V, V353I, and E401K). These mutant proteins, which affect the chaperone-dependent assembly of tubulin heterodimers in different ways, disrupt neurogenic division and/or migration in vivo. Our results provide insight into the functional repertoire of the tubulin gene family, specifically implicating TUBB5 in embryonic neurogenesis and microcephaly.
Graphical Abstract
Highlights
► The β-tubulin Tubb5 is highly expressed in the developing mouse and human cortex ► In vivo knockdown of Tubb5 perturbs the cell cycle and alters neuronal positioning ► Mutations in TUBB5 cause microcephaly with dysmorphic basal ganglia in humans ► TUBB5 mutations affect chaperone-mediated tubulin folding in different ways
The formation of the cortex requires the generation, migration, and differentiation of neurons. While specific tubulin isotypes have been implicated in postmitotic events, those that mediate neurogenesis remain unknown. Here, Keays and colleagues report that mutations in the β-tubulin gene, TUBB5, cause microcephaly. They show that this gene is highly expressed in neuronal progenitors, and its depletion in vivo perturbs the cell cycle and alters neuronal migration. This work provides insight into the functional repertoire of the tubulin gene family.
Introduction
The formation of the mammalian cortex, a complex multilayered structure, requires the birth of neurons in the ventricular and subventricular zones followed by phases of bipolar and multipolar migration before newly born neurons arrive at their final destination in the cortical plate (Feng and Walsh, 2001). This sophisticated cellular journey relies on a myriad of intracellular and intercellular signaling factors that converge on the cytoskeleton (Ayala et al., 2007). The importance of the microtubule cytoskeleton in the sequential steps involved in migration and differentiation is reflected in the finding that mutations in various tubulin genes cause a range of structural brain abnormalities. Mutations in the α-tubulin subunit TUBA1A have been shown to result in lissencephaly (Keays et al., 2007); mutations in the β-tubulin subunit TUBB2B cause asymmetric polymicrogyria (Jaglin et al., 2009); and it has been shown that mutations in TUBB3 cause an ocular motility disorder (Tischfield et al., 2010), as well as a broad range of cortical abnormalities (Poirier et al., 2010). Most recently, the tubulin genes TUBA1A and TUBB2B have also been implicated in autism spectrum disorders (Neale et al., 2012; Pinto et al., 2010). Together, these studies have implicated specific tubulin isotypes in postmitotic cellular events, but those isotypes that mediate the generation of neurons, a process that requires the assembly of microtubules into a highly organized mitotic spindle, remain unknown (Ohnuma and Harris, 2003). Here, we set out to identify those tubulin genes required for cortical neurogenesis. We report that the β-tubulin gene TUBB5 is highly expressed in the developing cortex and that mutations in this gene cause microcephaly, with a range of structural brain abnormalities that include dysmorphic basal ganglia, dysgenesis of the corpus callosum, brainstem hypoplasia, and focal polymicrogyria.
Results and Discussion
Tubb5 Is Expressed at High Levels in the Developing Brain
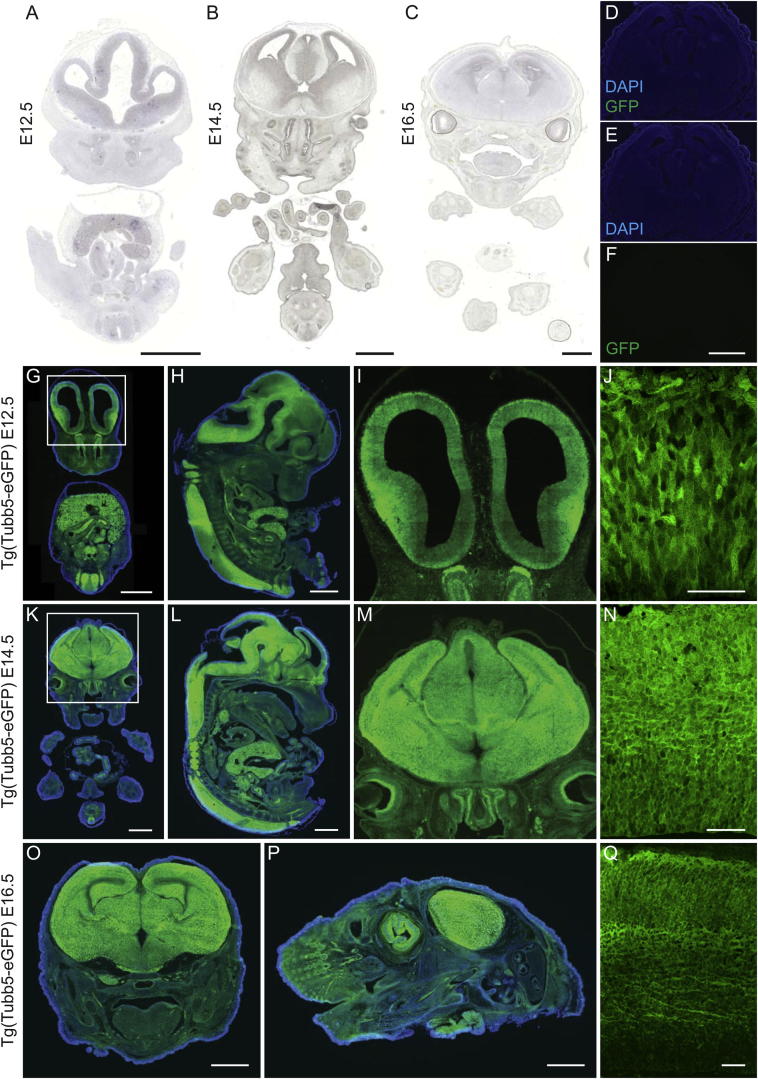
We surveyed the expression of all known β-tubulin genes in the developing mouse (E10.5, E12.5, E14.5, E16.5, P0) and human (gestational week [GW] 13, GW22) cortex by quantitative real-time PCR (Braun et al., 2010). We observed consistently high expression levels of Tubb2b, Tubb3, and Tubb5, with Tubb5 the highest expressed isotype in the embryonic mouse brain (Figures 1A and 1B). We investigated the spatial expression of Tubb5 by in situ hybridization at embryonic day (E) 12.5, E14.5, and E16.5 (Figures 1C–1H). This revealed robust expression throughout the developing cortex, particularly in the subventricular zone (SVZ) at E14.5, which was absent in sense controls (Figures S1A–S1C). Because there are no validated antibodies that are specific for Tubb5, we created a transgenic mouse line that drives enhanced green fluorescent protein (EGFP) from the endogenous Tubb5 promoter to determine which cell types express Tubb5 (Figures S1D–S1Q). This mouse recapitulated our in situ hybridization results and demonstrated that Tubb5 is expressed in radial glial cells (Pax6 positive), intermediate progenitors (Tbr2 positive), migrating neurons (Dcx positive), and postmitotic neurons (Tuj positive; Figures 1I–1X).
Figure 1.
Tubb5 Is Highly Expressed in the Developing Mouse and Human Brain
(A) Relative expression levels of β-tubulin genes in the developing mouse brain determined by quantitative real-time PCR at E10.5, E12.5, E14.5, E16.5, and E18.5 and postnatal days zero (P0) and six (P6) (n = 3). Note the early onset and consistently high expression of Tubb5.
(B) Relative expression levels of all the human β-tubulin genes in the developing brain at GW13 and GW22.
(C–H) In situ hybridization results obtained with an antisense probe specific for Tubb5 at E12.5 (C and D), E14.5 (E and F), and E16.5 (G and H). (D), (F), and (H) show higher magnifications of (C), (E), and (G), respectively. Tubb5 is detected throughout the developing cortex with strong expression in the preplate at E12.5, and in the SVZ at E14.5.
(I–X) Antibody staining for Dcx (I–L), Tuj (M-P), Tbr2 (Q–T), and Pax6 (U–X) performed on coronal sections of the Tg (Tubb5-EGFP) mouse line at E14.5. Grey scale images of (J), (N), (R), and (V) are shown in (K) and (L), (O) and (P), (S) and (T), and (W) and (X). All markers were found to colocalize with or in GFP-positive cells.
Scale bars show 500 μm in (C), (E), and (G); 50 μm in (D), (F), and (H); 50 μm in (U); and 10 μm in (V). Error bars in (A) and (B) show SEM. See also Figure S1.
Figure S1.
Negative Controls for Figure 1 and Analysis of the Tg(Tubb5-EGFP) Mouse Line
(A–C) Negative controls (sense probe) for the in situ hybridization experiments shown in Figure 1 for the indicated time points (E12.5, E14.5, E16.5).
(D–F) Littermate control of the Tg(Tubb5-EGFP) embryo shown in Figure 1, negative for the GFP transgene. Images were captured employing the same settings as those shown in (G)-(Q); no background fluorescence can be detected.
(G–Q) Representative coronal (G, I-K, M-O, Q) and sagittal (H, L, P) sections of Tg(Tubb5-EGFP) embryos at the indicated time points (E12.5, E14.5, E16.5). (I) and (M) show magnifications of the boxed regions shown in (G) and (K). High magnification confocal images of the developing cortex are shown in (J), (N), (Q). Note the robust expression of EGFP throughout the developing cortex, consistent with our in situ studies. Scale bars show 1000 μm for (A–C), (G), (H), (K), (L), (O) and (P), 500 μm in (F), 50 μm for (J), (N) and (Q). DAPI staining (shown in blue) is visible in (G), (H), (K), (L), (O) and (P).
Depletion of Tubb5 Perturbs the Progenitor Cell Cycle and the Migration of Neurons
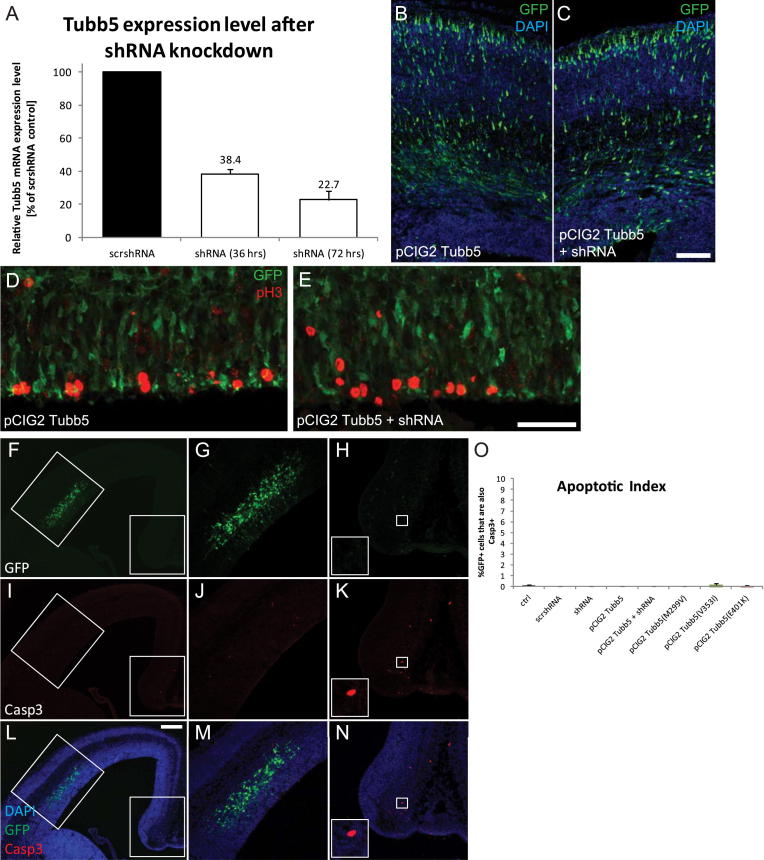
We investigated the effect of Tubb5 depletion on cortical development in mice by employing a small hairpin RNA (shRNA) that targets its 3′UTR. Following validation of this shRNA in Neuro-2a cells (Figure S2A), we electroporated it into progenitor cells of the ventricular zone (VZ) at E14.5, together with a GFP expressing vector (Figure 2A). Quantification of the fraction of GFP-positive cells that coexpress pH3 (mitotic index) after 36 hr of knockdown revealed an increase in the percentage of these mitotic cells in the VZ/SVZ when Tubb5 was depleted (n = 6; p < 0.001). This effect could be rescued by codelivering a Tubb5 expression construct that lacked the shRNA targeting sequence (Figures 2B–2D; Figures S2D and S2E; Table S2) and replicated when employing an alternative shRNA (data not shown). Because mutations in Tuba1a have been shown to induce apoptosis, we studied cell survival by staining for cleaved caspase-3 at E16, following electroporation at E14.5 (Edwards et al., 2011). This revealed no statistically significant difference between conditions, but we cannot exclude the possibility of increased apoptosis following long-term depletion of Tubb5 (Figures S2F–S2O). Next, we investigated the effect of Tubb5 depletion on neuronal migration by electroporating our shRNA at E14.5 and harvesting embryos 72 hr later (migration index). In comparison to controls, we observed a significant decrease in the percentage of GFP-labeled cells in the cortical plate (CP) when Tubb5 was depleted, concomitant with an accumulation of cells within the VZ and intermediate zone (IZ) (n ≥ 5; VZ: p < 0.05; IZ: p < 0.05; CP: p < 0.01; Figures 2E–2G; Figures S2B and S2C; Table S2). To assess whether Tubb5 depletion affects the final positioning of neurons, we harvested brains at postnatal day (P) 17, following electroporation at E14.5. Quantification of the percentage of GFP positive cells in the six layers of the P17 cortex showed that Tubb5 knockdown results in a long-term positioning defect (n = 4; layer VI: p < 0.05; layers II–IV: p < 0.01) (Figure 2H). We conclude that Tubb5 depletion perturbs the neurogenic cell cycle and alters the positioning of migrating neurons.
Figure S2.
Supplemental Information to Figure 2 and Assessment of Apoptosis
(A) Relative expression level of Tubb5 mRNA in Neuro2a cells following transfection with a pSuper vector driving expression of a shRNA targeted to the 3′ UTR of this gene. This results in a ∼62% knockdown (36 hr) and ∼77% knockdown (72 hr) of Tubb5 mRNA levels. The error bars show the SEM (n = 3 independent experiments for each time point).
(B and C) Representative images for the assessment of neuronal positioning following electroporation of pCIG2 Tubb5 and pCIG2 Tubb5 + shRNA.
(D and E) Representative images for the mitotic index assessment when electroporating with pCIG2 Tubb5 and pCIG2 Tubb5 + shRNA.
(F–N) Representative image of the apoptosis experiments showing the GFP channel (F-H), activated caspase-3 staining (I-K) and the merged image (L-N). Boxed regions in F, I and L are shown in the adjacent panels (G, J, M) and (H, K, N). Panels H, K and N highlight the mediodorsal region of the cortex as an internal positive control for caspase-3 staining. The magnified region in H, K and N show an apoptotic cell positive for caspase-3.
(O) Quantification of GFP+ cells co-localizing with activated capase-3. All conditions show less than 1% of co-localization. An ANOVA followed by a multiple comparison test revealed no significant difference between the relevant experiments (scrshRNA, shRNA, pCIG2 Tubb5 and pCIG2 Tubb5 + shRNA; ctrl, pCIG2 Tubb5, pCIG2 Tubb5(M299V), pCIG2 Tubb5(V353I) and pCIG2 Tubb5(E401K)).
Scale bars show 50 μm in (C), 100 μm in (E), and 200 μm in (L).
Figure 2.
Tubb5 Depletion Perturbs Progenitor Mitosis and Alters the Positioning of Postmitotic Neurons
(A) Schematic illustrating the three different in utero electroporation experiments. After electroporation at E14.5, embryos were harvested 36 hr later to assess the mitotic index, harvested 72 hr later to assess the migration index, or harvested at P17 to assess the positioning index.
(B) Quantitation of the mitotic index, showing the relative proportion of GFP positive cells that are also pH3 positive in the VZ and SVZ, IZ, and across all zones (Total) for the following conditions: scrambled shRNA (scrshRNA); shRNA targeting Tubb5 (shRNA); overexpression of Tubb5 (pCIG2 Tubb5); and the rescue experiment (pCIG2-Tubb5 + shRNA). Note that Tubb5 depletion results in a significant increase in the mitotic index in comparison to the scrambled control, overexpression, and rescue experiments (n ≥ 5; VZ and SVZ: p < 0.001; Total: p < 0.05).
(C and D) Representative images for the mitotic index experiment. Arrowheads highlight colocalization of GFP-expressing cells (green) with pH3 staining (red).
(E and F) Representative images showing the migration assessment following Tubb5 depletion. Note the conspicuous reduction in the proportion of GFP-positive cells reaching the CP.
(G) Quantification of the percentage of GFP-positive cells in the VZ, IZ, CP, and marginal zone (MZ) for the migration index following treatment with a scrshRNA; an shRNA targeting Tubb5 (shRNA); a Tubb5 expression construct (pCIG2 Tubb5); and the rescue experiment (shRNA + pCIG2 Tubb5). Tubb5 depletion results in a moderate but significant increase in the percentage of GFP-positive cells located in the VZ and IZ and a decrease in the percentage of GFP-positive cells in the CP (n ≥ 5, VZ: p < 0.05; IZ: p < 0.05; CP: p < 0.01). This phenotype was partially rescued by coexpression of a Tubb5 isotype lacking the shRNA targeting sequence (pCIG2 Tubb5 + shRNA). Note that overexpression of Tubb5 by itself has no effect on migration (pCIG2 Tubb5).
(H) Quantification of the percentage of GFP-positive cells in the six layers of the P17 cortex following treatment with an scrshRNA and a shRNA targeting Tubb5. Tubb5 depletion results in a significant increase in the percentage of neurons in layer VI (n = 4; p < 0.05), with a concomitant decrease in layers II–IV (n = 4; p < 0.01).
(I and J) Representative images for the positioning index in (H). The red channel shows Cux1 staining in layers II–IV.
Scale bars show 50 μm in (D), 100 μm in (F), and 200 μm in (J). Error bars in (B), (G), and (H) show mean ± SEM.∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. MZ, marginal zone; scrshRNA, scrambled shRNA. See also Figure S2.
Mutations in TUBB5 Cause Microcephaly in Humans
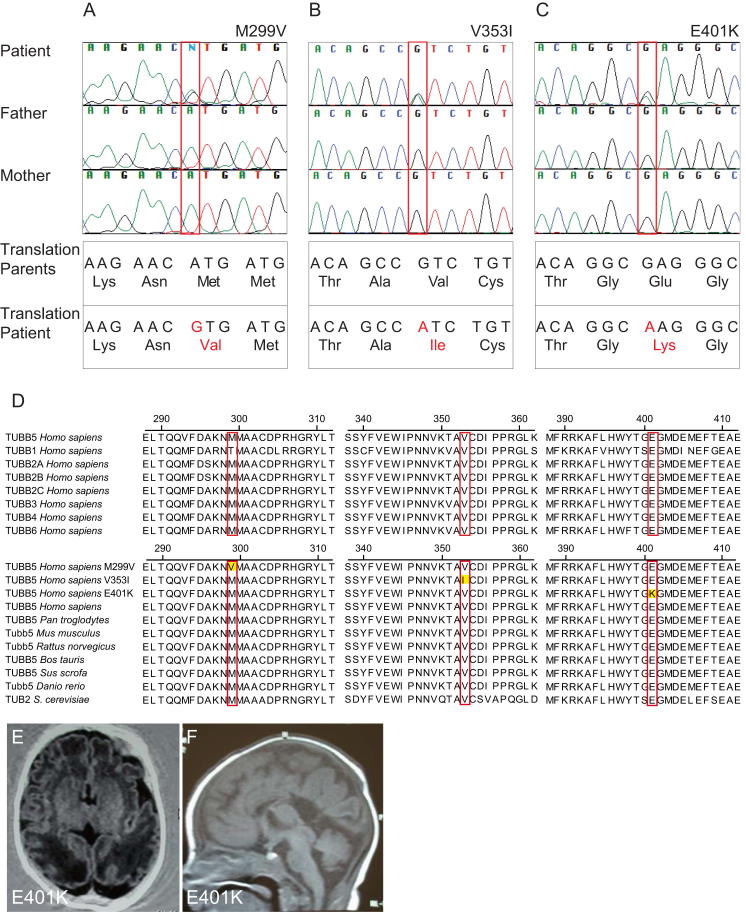
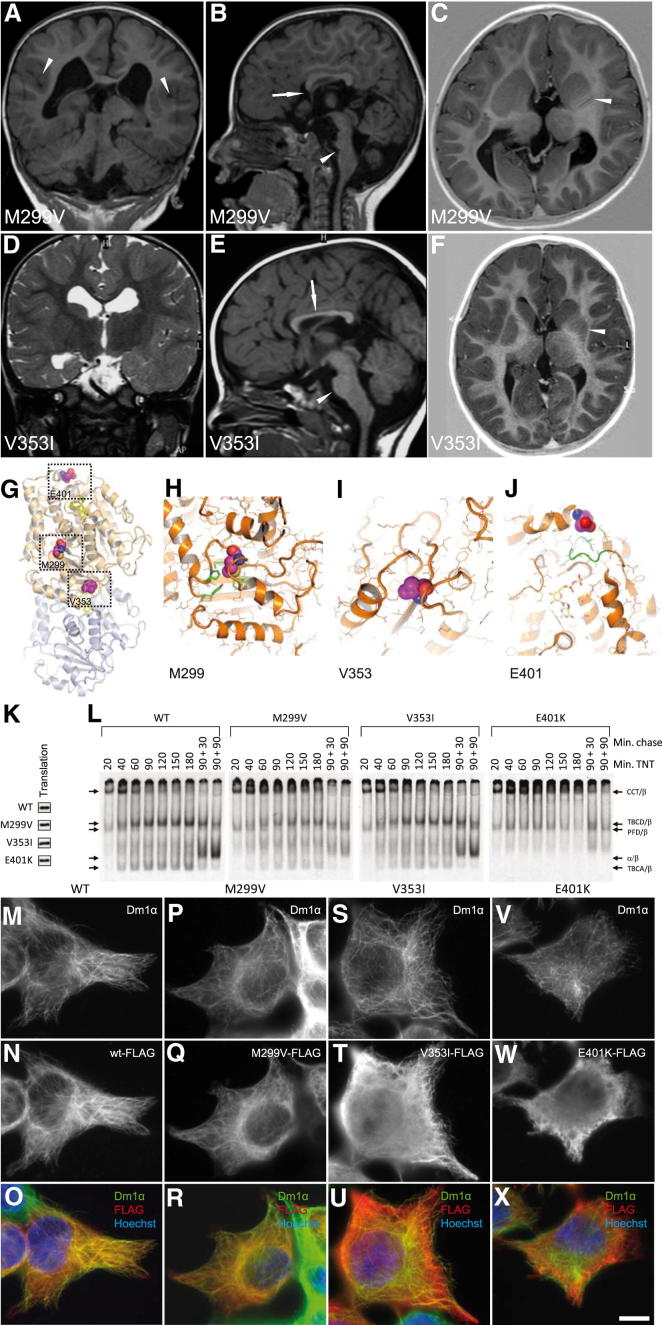
On the basis of these results, we surmised that mutations in TUBB5 might cause neurodevelopmental disease in humans. We therefore screened a panel of patients with a range of brain malformations, resulting in the identification of three unrelated microcephalic individuals harboring de novo mutations in TUBB5 (M299V, V353I, and E401K) (Figures S3A–S3C). Each individual presented with microcephaly (with an orbitofrontal cortex [OFC] ranging from −2.5 SD to −4 SD), dysmorphic basal ganglia, corpus callosum abnormalities, and cognitive impairment with motor and language delay (Table 1; Figures 3A–3F; Figures S3E and S3F). Two of the patients (M299V and V353I) shared unusual white matter streaks with a radial orientation through the lenticular nucleus, as well as brainstem hypoplasia (Figures 3A–3F). The child harboring the V353I mutation originates from a family with a history of microcephaly (without mental retardation), raising the prospect that another genetic factor contributes to his disease. Sequencing of known microcephaly genes (MCPH1, CD5KRAP2, WDR62, ASPM) and the known disease causing tubulins (TUBA1A, TUBB2B, and TUBB3) did not identify any pathogenic variants in the patients described. A search of publicly available genomic databases showed that the M229V, V353I, and E401K mutations are not present in the general population. Moreover, screening of ethnically relevant controls (French/Caucasian, 238; Vietnamese, 115; Indian subcontinent, 252) failed to identify these variants. Homology analysis showed that these residues are highly conserved in all human β-tubulins and related tubulin isotypes from yeast to humans (Figure S3D).
Figure S3.
Supplemental Information to Figure 3
(A–C) Sequencing traces for patients and parents showing the mutations in TUBB5 for M299V, V353I, and E401K together with the amino acid conversions. All mutations are de novo. (D) Protein sequence alignment of β-tubulins present in humans (upper panel). Note the high conservation between the isoforms for all three loci, with the exception of T299 in TUBB1, the most distinct of the human isoforms. The lower panel shows an alignment of TUBB5 homologs in a variety of species. Note that the three disease causing residues are conserved from yeast to man. (E and F) Horizontal (E) and sagittal (F) MRI images taken at 4 months of age for the patient harboring the E401K mutation. This individual presented with microcephaly (−4 SD OFC), partial posterior agenesis of the corpus callosum, and dysmorphic basal ganglia.
Table 1.
Summary of Clinical, Neurological, Biochemical, Cellular and In Vivo Data Associated with TUBB5 Mutations
| M299V | V353I | E401K | |
|---|---|---|---|
| Ethnicity/Origin | Caucasian | Sri Lanka | Vietnam/Caucasian |
| Sex | Male | Male | Female |
| Age at last evaluation | 2 years 6 months | 4 years 10 months | 2 years 8 months |
| OFC at birth | 32 cm (−3 SD) | 31 cm (−3 SD) | 28.5 cm (−4 SD) |
| OFC at last evaluation | −2.5 SD | 46.5 cm (−4 SD) | 42 cm (−4 SD) |
| Cortical dysgenesis | Focal polymicrogyria, localized band heterotopia | None apparent | None apparent at 4 months |
| Basal ganglia | Dysmorphic White matter streaks |
Dysmorphic White matter streaks |
Dysmorphic |
| Cerebellum | Hypoplastic and dysplastic cerebellar vermis | Possible white matter abnormalities. | Large 4th ventricle |
| Corpus callosum | Partial agenesis | Thin and short but complete | Partial posterior agenesis |
| Cognitive abilities | Severe MR | MR (global DQ:48) | Mild developmental delay |
| Verbal abilities | Severe language delay | Limited language Speech delay. |
Delayed |
| Motor abilities | Severe motor delay, ataxia | Motor delay. Walked at 21 months. | Motor delay. Walked at 25 months |
| Other | Retina dysplasia and micro-ophthalmia | None apparent. | Prenatal diagnosis of cardiopathy |
| Family history | None | Mother’s OFC is −4 SD Brother’s OFC is −2 SD Sister’s OFC is −1 SD |
None |
| Heterodimer folding | Slightly impaired | Not affected | Severely impaired |
| Microtubule lattice incorporation | Not affected | Not affected | No incorporation |
| Mitotic index | n.s. | Significantly increased | Significantly increased |
| Migration index | Significantly decreased | Significantly decreased | Significantly decreased |
| Positioning index | Affected | n.s. | Affected |
| Ectopic clusters at P17 | Yes | Yes | Yes |
OFC, orbitofrontal cortex; MR, mental retardation; DQ, development quotient; n.s., nonsignificant.
Figure 3.
Mutations in TUBB5 Cause Microcephaly and Affect the Generation of Tubulin Heterodimers in Different Ways
(A–F) Coronal (A and D), sagittal (B and E), and horizontal (C and F) MRIs of two patients with TUBB5-associated microcephaly (M299V and V353I). (A–C) The patient with the M299V mutation has microcephaly (OFC of −2.5 SD), focal polymicrogyria (shown with arrowheads in A), severe brainstem hypoplasia (shown with an arrowhead in B), partial agenesis of the corpus callosum (shown with an arrow in B), and dysmorphic basal ganglia with streaks of white matter (highlighted with an arrowhead in C). (D–F) The patient with the V353I mutation has microcephaly (OFC of −4.0 SD), a hypoplastic corpus callosum (shown with an arrow in E), and a dysmorphic basal ganglia with streaks of white matter running through the lenticular nucleus (shown with an arrowhead in F).
(G) Structural representation of a tubulin heterodimer highlighting the positions of the mutated residues. The M299 residue is centrally located and is associated with a deep hydrophobic pocket. V353 lies on the intradimer interface, in contrast to E401, which is located at the interdimer interface.
(H–J) Higher-resolution images showing the mutated residues within the three-dimensional structure of the tubulin heterodimer. (H) The M299 side chain is surrounded by hydrophobic residues (M267, P305, Y310, F367, shown in green) that could be disrupted by replacement with valine. (J) E401 lies in proximity to a loop (98–104, shown in green) that is critical for the binding of GTP (shown in yellow).
(K) Denaturing gel of in vitro 35S-methionine-labeled transcription/translation reaction products for wild-type and TUBB5 mutants showing similar translational efficiencies.
(L) Kinetic analysis on nondenaturing gels of the products of in vitro transcription/translation reactions for wild-type and TUBB5 mutants. Arrows (top to bottom) denote the migration positions of the chaperonin (CCT)/β-tubulin binary complex (CCT/β), the TBCD/β-tubulin cocomplex, the prefoldin (PFD)/β-tubulin binary complex (PFD/β), the native tubulin heterodimer (α/β), and the TBCA/β-tubulin cocomplex (TBCA/β), each assigned on the basis of their characteristic electrophoretic mobilities. Note that the V353I mutant polypeptide behaved similarly to wild-type controls, whereas there was a diminished heterodimer yield in the case of M299V and little or no detectable heterodimer in the case of E401K. Note also the absence of TBCA/β-tubulin and TBCD/β-tubulin cocomplexes and a relatively long persistence of the PFD/β-tubulin cocomplex in the case of reactions performed with the E401K mutation. Min. chase indicates that reaction products generated after 90 min were further chased with added native bovine brain tubulin so as to drive the generation of tubulin heterodimers for the times shown.
(M–X) Expression of FLAG-tagged wild-type and mutant (M299V, V353I, and E401K) TUBB5 in cultured Neuro-2a cells. Staining with the anti-FLAG antibody is shown in red and the microtubule cytoskeleton visualized using an anti-α-tubulin antibody (shown in green). Note that wild-type FLAG-tagged TUBB5, as well as the corresponding M299V and V353I mutants, incorporated into the microtubule lattice (M–O, P–R, and S–U, respectively). This contrasts with the E401K protein, which is distributed throughout the cytoplasm and failed to incorporate into the cytoskeletal network (V–X). Scale bar in (X) is 10 μm.
See also Figure S3.
Functional Analysis of Disease-Causing Mutations
We mapped the location of the affected residues onto the known structure of the α/β heterodimer (Figures 3G–3J). M299 lies in a loop following helix 9, with its side chain protruding into a deep hydrophobic pocket. V353 lies on the intradimer interface, whereas E401, which precedes helix 12, lies on the surface of the interdimer interface. This residue is in close proximity to a loop (αα98–104) that is critical for nucleotide binding (Löwe et al., 2001) and that may be reoriented by substitution with a lysine residue. All three mutations have the potential to compromise either the complex chaperone-dependent reactions that are essential for tubulin heterodimer assembly or the dynamic behavior of microtubules (Tian et al., 2010). To address these issues, we monitored the expression and folding of the mutant proteins kinetically in rabbit reticulocyte lysate. Analysis of the products under denaturing conditions showed that the translation efficiency of all three mutants was similar to the wild-type control (Figure 3K). However, analysis of the same reaction products under native conditions revealed quantitative and qualitative differences (Figure 3L). The wild-type protein and V353I and M299V mutants produced the expected folding intermediates (CCT/β, TBCD/β, PFD/β, and TBCA/β) (Cowan and Lewis, 2001) before generating α/β heterodimers when chased with native bovine tubulin, but with a notable reduction in yield for the M299V mutation. In the case of the E401K mutation, the assembly pathway was arrested, with a conspicuous retention of the mutant polypeptide complexed with prefoldin (PFD) and the cytosolic chaperonin (CCT), and a dearth of native α/β heterodimers. We also examined the capacity of the TUBB5 mutants to incorporate into the microtubule network in Neuro-2a cells (Figures 3M–3X). Whereas the wild-type M299V and V353I proteins coassembled into interphase microtubules, we found that the E401K mutant protein failed to do so and was diffusely distributed throughout the cytoplasm. We conclude that the V353I and M299V mutations do not affect the ability of the mutant polypeptides to assemble into heterodimers and incorporate into microtubules, although the yield in the case of M299V may be compromised. In contrast, the E401K mutation results in a severe ablation of TUBB5 function, as it results in a massive failure of chaperone-mediated heterodimer assembly with a consequent inability of the mutant protein to incorporate into microtubules.
Effects of Human Mutations on Murine Neuronal Development
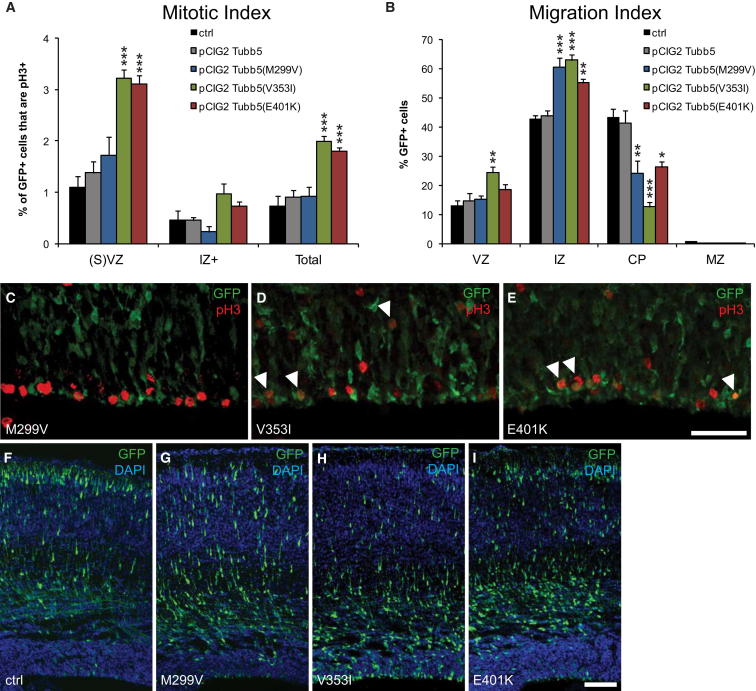
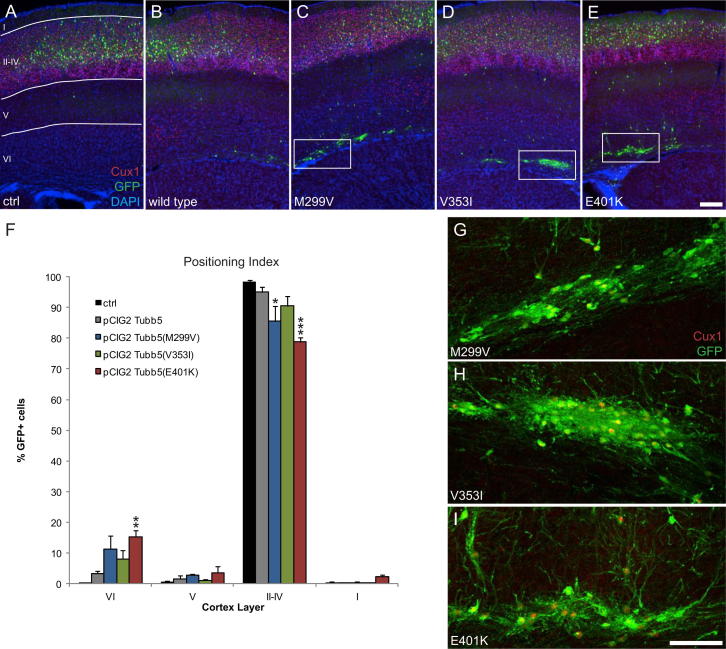
To examine the effect of these mutations in vivo, we used in utero electroporation to overexpress the mutant forms of Tubb5 in the developing mouse brain. While overexpression of wild-type Tubb5 had no effect on the mitotic index (n = 5; p > 0.5), we found a large increase in the percentage of GFP/pH3-colabeled cells in the VZ and SVZ when expressing the E401K and V353I mutants, similar to our Tubb5 depletion experiments (n = 5; p < 0.001 for both mutants; Figures 4A, 4D, and 4E). Electroporation of the M299V expression construct also resulted in a modest increase in the mitotic index within the cortical germinal zone (VZ + SVZ), although this increase was not statistically significant (n = 5; p > 0.05; Figures 4A and 4C). We also examined the migratory index following expression of the Tubb5 mutants. In comparison to controls, we found that expression of all three mutants resulted in a significant increase in the percentage of GFP-positive cells in the IZ and a corresponding decrease in the CP that was most severe in the case of the V353I mutant (n = 5; M299V: IZ: p < 0.001; CP: p < 0.01; V353I: VZ: p < 0.01; IZ: p < 0.001; CP: p < 0.001; E401K: IZ: p < 0.001; CP: p < 0.01; Figures 4B and 4G–4I). These changes were not observed upon overexpression of wild-type Tubb5 alone (n = 5; p > 0.05; Figure 4F). Analysis of neurons at P17 following the overexpression of Tubb5 mutants revealed aberrant clusters of ectopic cells in deep layers of the cortex (Figure S4). Quantification showed that in comparison to controls, this results in fewer cells in superficial layers of the cortex (n ≥ 2; M299V: II–IV: p < 0.05; E401K: II–IV: p < 0.001) and an accumulation of GFP-positive cells in the deeper layers (E401K: n = 3; VI: p < 0.01), but this was not significant in the case of the V353I mutation (n = 3; VI: p > 0.05). This result demonstrates that the migratory defects described above have a long-term effect on neuronal position, and consequently on the structural architecture of the brain. Together, these data support our conclusion that the mutations are pathogenic, with an effect on both the generation and subsequent migration of neurons.
Figure 4.
Expression of Disease-Causing Tubb5 Mutations In Vivo
(A) Quantification of the mitotic index for cells electroporated with control vectors (ctrl), a Tubb5 expression vector (pCIG2-Tubb5; note that this control is also presented in Figure 2B), and the mutants identified (pCIG2-Tubb5(M299V), pCIG2-Tubb5(V353I), pCIG2-Tubb5(E401K)). Tubb5 overexpression resulted in a mitotic index that was comparable to controls. In contrast, there was a large increase in the percentage of GFP-positive cells that are also pH3 positive in the VZ and SVZ when expressing the E401K and V353I mutant constructs (n ≥ 5; E401K and V353I: VZ + SVZ: p < 0.001; Total: p < 0.001). This increase in the mitotic index was also apparent when overexpressing the M299V mutation, although the effect was not statistically significant (n ≥ 5; p > 0.05).
(B) Quantification of the migration index for all five conditions (note that the Tubb5 overexpression control is also presented in Figure 2G). Overexpression of the mutants results in an accumulation of GFP-positive cells in the IZ with a concomitant decrease in the CP (n = 5; M299V: IZ: p < 0.001; CP: p < 0.01; V353I: VZ: p < 0.01; IZ: p < 0.001; CP: p < 0.001; E401K: IZ: p < 0.01; CP: p < 0.05) that was not observed when overexpressing Tubb5 alone (n = 5, p > 0.05).
(C–E) Representative images used for mitotic index assessment with arrowheads showing colocalization of GFP-expressing cells (green) with pH3 staining (red).
(F–I) Representative images used for migration assessment. Note the decrease in the relative number of cells reaching the upper layers of the cortex when expressing the mutant constructs in comparison to controls (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Scale bars show 50 μm in (E) and 100 μm in (I). (A) and (B) show mean ± SEM. See also Figure S4.
Figure S4.
Positioning Index following Expression of Mutant Tubb5 In Vivo, Related to Figure 4
(A–E) Representative images of P17 mouse brains following in utero electroporation at E14.5 with plasmids expressing: (A) scrambled shRNA; (B) Tubb5 wt; (C) Tubb5 M299V, (D) Tubb5 V353I, and (E) Tubb5 E401K. The cortical regions analyzed are indicated in A. The red channel shows Cux1 staining in layers II-IV. Note the increased number of ectopic cells in deep cortical layers when overexpressing the Tubb5 mutants (M299V, V353I, E401K).
(F) Quantification of the percentage of GFP positive cells in layers I-VI of the P17 cortex for all five conditions (note that the control is also presented in Figure 2H; see also Table S2). Overexpression of the Tubb5 mutants results in fewer cells in superficial layers of the cortex (M299V; II-IV: p < 0.05, E401K; II-IV: p < 0.001), and an accumulation of GFP positive cells in the deeper layers (E401K; n = 3; VI: p < 0.01), but this was not significant in the case of the V353I mutation (n = 3; p > 0.05). Magnification of the boxed regions in (C), (D), and (E), showing ectopic clusters of GFP positive cells. Note that a number of these cells are also positive for the post-mitotic marker Cux 1. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are mean ± SEM.
Scale bars show 200 μm in (E) and 100 μm in (I).
The three mutations we identified (M299V, V353I, and E401K) are likely to act by different molecular mechanisms (Walsh and Engle, 2010). The impairment in heterodimer assembly observed in the case of the E401K and M299V mutations suggests that they act by loss-of-function; however, the marked phenotype that results from heterologous expression of all mutants in vivo raises the prospect of a dominant-negative effect. It is conceivable that kinetically trapped E401K and M299V polypeptides have a deleterious effect on the entire chaperone-mediated tubulin folding and assembly pathway, while the V353I mutation may alter the dynamic properties of microtubules or compromise the binding of one or more essential microtubule associated proteins (Kumar et al., 2010; Tischfield et al., 2010; Walsh and Engle, 2010). The underlying cellular pathology is likely to be equally as complex. We have shown that both knockdown of Tubb5 and overexpression of Tubb5 mutants result in a paradoxical increase in pH3 reactivity. There are a number of explanations for this unexpected result, which implies an increase in mitotic output. First, it is conceivable that there is an elongation of M-phase, which delays progression of the cell cycle and the generation of neurons (Lizarraga et al., 2010). Second, it is possible that hyperproliferating apical progenitors result in fewer intermediate progenitors, and consequently an overall reduction in postmitotic neurons (Kim et al., 2009). Third, M-phase arrest may, at a later point in time, result in increased apoptosis, accounting for the incongruity between our in vivo pH3 results and the human phenotype. Further studies focused on the timing and output of the cell cycle will be necessary to establish which of the aforementioned mechanisms are responsible.
It is apparent from this study that patients with mutations in TUBB5 share structural brain abnormalities in common with the previously described tubulinopathies (TUBA1A, TUBB2B, TUBB3), including dysmorphic basal ganglia, abnormalities of the corpus callosum, and brainstem phenotypes (Tischfield et al., 2011). The distinguishing feature of TUBB5-associated disease is microcephaly, which we attribute to the high level of TUBB5 expression in neuronal progenitors. This contrasts with TUBA1A, TUBB2B, and TUBB3, all of which are largely restricted in their expression to postmitotic neurons in the developing brain (Gloster et al., 1999; Jaglin et al., 2009; Liu et al., 2007). TUBB5 may have a specific function in mitotically active cells, or alternatively make a critical contribution to the supply of tubulin heterodimers. In either event, our work provides insight into the functional repertoire of the tubulin gene family, specifically implicating TUBB5 in embryonic neurogenesis and microcephaly.
Experimental Procedures
Animals
Animals were maintained within the animal research laboratories of the Institute of Molecular Pathology and Monash University on a 12:12 light:dark cycle with food available ad libitum. All procedures were performed in accordance with existing animal licenses and guidelines (M58-006093-2011-13 B).
Generation of Tg(Tubb5-EGFP) Mice
The Red/ET system (K001, Gene Bridges) was employed to replace the endogenous Tubb5 (RP24-330C1) with EGFP. Following generation of the construct, we proceeded with pronuclear injection and PCR screening to confirm germline transmission before immunostaining.
In Situ Hybridization
C57/BL6 embryos were harvested, postfixed in 4% paraformaldehyde (PFA), dehydrated in a pressure- and temperature-controlled environment, embedded in paraffin, and sectioned (14 μm). We then proceeded with in situ hybridization as previously described (Braun et al., 2010).
Real-Time Quantitative PCR
Embryonic, postnatal, and adult tissues (C57/BL6) were dissected and snap frozen before mRNA extraction and reverse transcription. Quantitative real-time PCR followed employing exon spanning primers and three internal control genes. For human studies, fetal brain cDNA was obtained from Yorkshire Biosciences.
In Utero Electroporation Experiments
Sequence-verified constructs for wild-type and mutant versions of Tubb5 were cloned into pCIG2. shRNAs optimized for specific knockdown of Tubb5 were cloned into the pSuper vector system. In utero electroporation, cryosectioning, and immunostaining were performed as previously described (Heng et al., 2008; Pacary et al., 2011). Cell counting was performed blind to the condition on representative fields of sections of electroporated brains using ImageJ software.
Biochemical and Cellular Studies
Plasmids encoding the Tubb5 mutants were expressed in rabbit reticulocyte lysate (Promega) as described (Tian et al., 2010) before analysis on native polyacrylamide gels. For cellular studies, FLAG-tagged TUBB5 DNA constructs were transfected into Neuro-2a cells before immunostaining with an anti-α-tubulin antibody (Sigma, T6199-200UL) and an anti-FLAG antibody (Abcam, ab1162).
Human Studies
We obtained DNA and informed consent according to the guidelines of local institutional review boards at Cochin Hospital and INSERM. TUBB5 was screened in 120 individuals with sporadic structural brain abnormalities that included lissencephaly, polymicrogyria, microcephaly, and nodular heterotopia. These patients had previously been screened for mutations in the known disease-causing tubulin genes (TUBA1A, TUBB2B, TUBB3), and (depending on their phenotype) for other pathogenic genes (e.g., DCX, LIS1, FLNA). Following the identification of de novo mutations in TUBB5, parental relationships were confirmed using a suite of microsatellite markers.
Statistics
Statistical analysis was performed with the GraphPad Prism 5 software package. For experiments with two conditions, we performed a Student’s t test. For experiments with multiple conditions, we used a one-way ANOVA followed by Bonferroni multiple comparison test.
For additional details, please see the Extended Experimental Procedures.
Extended Experimental Procedures.
Generation and Immunostaining of Tg(Tubb5-EGFP) Mice
A Bacterial Artificial Chromosome (BAC; RP24-330C1) containing the Tubb5 gene was first validated by restriction enzyme (FspI) and PCR analysis. We employed high fidelity PCR to amplify a GFP-FRT-NEO-FRT cassette flanked by 60 base pair sequences homologous to the Tubb5 locus (see Table S1), and employed the Red/ET system (K001, Gene Bridges) to replace the endogenous Tubb5 gene. Following excision of the neomycin resistance cassette with a 707-FLPe plasmid (A104, Gene Bridges), we proceeded with pronuclear injection, and PCR screening for genomic incorporation (see Table S1). Embryos were harvested at E12.5, E14.5 and E16.5 and fixed in 4% PFA overnight before dehydration in 30% sucrose overnight, embedding and sectioning (12 μm). Sections were incubated with the primary antibody overnight in 0.1%–0.3% triton/PBS with 2% of the appropriate serum at the following concentrations: Dcx (1:100, Santa Cruz), Tuj1 (1:1000, Covance), Tbr2 (1:200, Abcam), Pax6 (1:300, Covance). For Tbr2 and Pax6 staining we performed antigen retrieval with citrate buffer (0.01M, pH6.0); slides were heated from room temperature to 90°C in a water bath and then placed at to room temperature for 20 min prior to incubation with the primary antibody overnight (14-16 hr) at 4°C. The next day, sections were washed 3 times in PBS (5 min each), before application of a species specific secondary antibody (Molecular Probes) for 1 hr in 0.1%–0.3% triton/PBS. Sections were then mounted in VectaShield Mounting Medium (hard set with DAPI). Microscopy was performed on a Zeiss LSM 710, with the exception of the low magnification images of the Tg(Tubb5-EGFP) embryos that were captured with a Mirax slide scanner (Zeiss).
In Situ Hybridization
C57/BL6 embryos were harvested at E12.5, E14.5 and E16.5, post-fixed in 4% PFA, dehydrated in a pressure and temperature controlled environment, embedded in paraffin and sectioned (14 μm). Prior to in situ hybridization these sections were de-paraffined employing standard protocols. A 165 base pair probe for in situ hybridization was amplified by PCR (see Table S1), cloned into a pCRII-TOPO vector (Invitrogen), the vector linearized and an in vitro transcription reaction conducted with a DIG labeling mix (Roche). Sections were post-fixed with PFA (4% for 10mins) before pretreatment with proteinase K (1 μg/ml for 10mins), and acetylated (0.1M triethanolamine, 0.25% acetic anhydride, 15mins). Following a prehybridization step (4hrs at RT) in hybridization buffer (10mM Tris pH7.5, 600mM NaCl, 1mM EDTA, 0.25% SDS, 10% Dextran Sulfate, 1x Denhartdt’s, 200 μg/ml yeast tRNA) the denatured probe was applied, and hybridized overnight at 60°C in a custom-built hybridization chamber. Post hybridization washes and RNase treatment were performed as previously described (Murtaugh et al., 1999). Staining was visualized by incubating sections with an alkaline phosphatase conjugated digoxigenin antibody (1:2000, 4°C, overnight), followed by the application of BM-Purple AP.
Real-Time Quantitative PCR
Embryonic (E10.5, E12.5, E14.5, E16.5, E18.5), postnatal (P0, P6) and adult (8 weeks) tissues (C57/BL6) were dissected and snap frozen, before mRNA extraction employing either an RNeasy lipid tissue kit or an RNeasy fibrous tissue kit. RNA was extracted from three independent samples at the same developmental time point, brain region or organ, quantified and then pooled for reverse transcription (SuperScript III First-Strand Synthesis System, Invitrogen, 18080-051). For human studies fetal brain cDNA was obtained from Biochain (C1244051, C1244035). For quantitative real-time PCR we designed intron-spanning primers employing the Primer3 program, and undertook BLAST searches to confirm their specificity (see Table S1). Following efficiency testing, we did quantitative real-time PCR with SYBR green on a Bio-Rad Cycler together with negative controls. To maximize the accuracy of our real-time PCR experiments we used three internal control genes for our mouse (Pgk1, Tfrc, and Hprt) and human studies (HPRT, PGK1, TBP), and calculated the geometric mean as previously described (Vandesompele et al., 2002).
Generation and Cellular Validation of shRNA Constructs
Short hairpin RNAs for Tubb5 knockdown were cloned into the pSuper vector system according to the manufacturer’s instructions (VEC-pBS-0002). The efficiency of the short hairpin (see Table S1) was assessed by quantitative real-time PCR 36 and 72 hr post transfection of the constructs with Lipofectamine 2000 (Invitrogen, 11668-027) into Neuro-2a cells. RNA extraction (Roche, High Pure RNA Isolation Kit) and cDNA synthesis (Roche, Expand Reverse Transcriptase) were performed in accordance with the manufacturers’ protocol. Quantitative real-time PCR utilized the SYBR green dye on a Roche LightCycler. Tubb5 expression levels were normalized relative to Hprt (see Table S1).
Cellular Studies
Neuro-2a cells were kept under standard cell culture conditions in DMEM supplemented with 10% FBS (Sigma, F7524), 1% L-Glu (Sigma, G7513) and 1% PenStrep (Sigma, P0781). DNA constructs were transfected with Oligofectamine (Invitrogen, 12252-011) according to the manufacturer’s protocol. Immunostaining employing an anti-α-tubulin antibody (Sigma, T6199-200UL) and anti-FLAG antibody (Abcam, ab1162) was performed as previously described (Keays et al., 2007).
In Utero Electroporation Experiments
Sequence-verified expression constructs for wild-type and mutant versions of Tubb5 were cloned by PCR amplification of the coding sequence followed by ligation into the EcoRI/XhoI site of pCIG2. In utero electroporation was performed as described previously (Heng et al., 2008; Pacary et al., 2011). High-quality, low-endotoxin plasmid preparations (QIAGEN) of GFP expression vector (pCIG2) and shRNA vectors were injected at 1 μg/μl for each plasmid, together with Fast Green (0.05%, Sigma). Electroporation of telencephalic vesicles of DNA-injected embryos was achieved using platinum electrodes (Nepagene) connected to an electroporator (ECM830, BTX) to deliver five pulses of 30 V (50 ms duration) at 1 s intervals. Control electroporations for knockdown experiments were performed with a scrambled shRNA in the pSuper vector with an empty pCIG2 vector. Control electroporations for Tubb5 overexpression studies were performed with an empty pCIG2 vector together with an empty pSuper vector. Pregnant dams were sacrificed by cervical dislocation, and the embryonic brains dissected and fixed in 4% PFA in PBS overnight. Following three washes in PBS, the brains were incubated in 20% sucrose/PBS overnight before embedding in optimal cutting temperature compound. Cryosectioning (16 μm) was performed on a freezing microtome (Leica). Fluorescence immunostaining was performed as previously described (Heng et al., 2008), and images were captured on an epifluorescence microscope (Olympus) equipped with a CCD camera (SPOT). Subdivisions of the embryonic cortex (VZ, SVZ, IZ, and CP) were identified based on cell density as visualized with DAPI (4′6-diamidino-2-phenylindole) staining. Cell counting was performed blind to the condition on representative fields of sections of electroporated brains using ImageJ software. Immunostaining for activated (cleaved) caspase-3 utilized a rabbit polyclonal antibody (AB3623, Merck Millipore) at a 1:1,000 dilution.
In Vitro Transcription/Translation Reactions
Plasmids engineered for the T7-driven expression of wild-type, M299V, V353I, and E401K TUBB5 were used to drive expression in rabbit reticulocyte lysate (Promega) containing 35S-methionine as described (Tian et al., 2010). At various intervals, aliquots were withdrawn and analyzed on native polyacrylamide gels. In some cases, the reactions were supplemented (to a final concentration of 0.2 mg/ml) with native bovine brain tubulin after 90 min and the incubation continued for a further 30 or 90 min.
Acknowledgments
This study was supported by FWF (P21092, P24367) grants to D.A.K, Fondation pour la Recherche Médicale (Equipe FRM 2007) and ANR (2010 Blanc 1103 01) grants to J.C., and NIH grants (5R01HD057028 and 5R01GM097376) to N.J.C. J.I.H. is supported by an NH and MRC Career Development Fellowship (ID:1011505). The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government. Thanks to Doug Higgs for access to control DNA samples. D.A.K coordinated and designed this study with J.C. and N.J.C. M.B. and A.B. performed the expression studies. J.I.H., M.B., Y.X., M.H., and Z.Q. performed the in vivo murine experiments. D.A.K., T.G., and K.P. performed the genetic screening and sequencing. K.J.H.R., D.S.R., K.T., and M.B. performed the control sequencing. N.B., S.P., M.M., A.V., and P.G. collected and collated the clinical data. T.C. performed the structural modeling. G.T., X.H.J., and N.J.C. undertook the biochemical studies. M.B. conducted the cellular studies with X.H.J. and L.N. M.B. prepared the figures. D.A.K. and M.B. wrote the manuscript, and all authors commented on it.
Published: December 13, 2012
Footnotes
Supplemental Information includes Extended Experimental Procedures, four figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2012.11.017.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Ayala R., Shu T., Tsai L.H. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Braun A., Breuss M., Salzer M.C., Flint J., Cowan N.J., Keays D.A. Tuba8 is expressed at low levels in the developing mouse and human brain. Am. J. Hum. Genet. 2010;86:819–822. doi: 10.1016/j.ajhg.2010.03.019. author reply 822–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N.J., Lewis S.A. Type II chaperonins, prefoldin, and the tubulin-specific chaperones. Adv. Protein Chem. 2001;59:73–104. doi: 10.1016/s0065-3233(01)59003-8. [DOI] [PubMed] [Google Scholar]
- Edwards A., Treiber C.D., Breuss M., Pidsley R., Huang G.J., Cleak J., Oliver P.L., Flint J., Keays D.A. Cytoarchitectural disruption of the superior colliculus and an enlarged acoustic startle response in the Tuba1a mutant mouse. Neuroscience. 2011;195:191–200. doi: 10.1016/j.neuroscience.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Walsh C.A. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat. Rev. Neurosci. 2001;2:408–416. doi: 10.1038/35077559. [DOI] [PubMed] [Google Scholar]
- Gloster A., El-Bizri H., Bamji S.X., Rogers D., Miller F.D. Early induction of Talpha1 alpha-tubulin transcription in neurons of the developing nervous system. J. Comp. Neurol. 1999;405:45–60. doi: 10.1002/(sici)1096-9861(19990301)405:1<45::aid-cne4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Heng J.I., Nguyen L., Castro D.S., Zimmer C., Wildner H., Armant O., Skowronska-Krawczyk D., Bedogni F., Matter J.M., Hevner R., Guillemot F. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455:114–118. doi: 10.1038/nature07198. [DOI] [PubMed] [Google Scholar]
- Jaglin X.H., Poirier K., Saillour Y., Buhler E., Tian G., Bahi-Buisson N., Fallet-Bianco C., Phan-Dinh-Tuy F., Kong X.P., Bomont P. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat. Genet. 2009;41:746–752. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keays D.A., Tian G., Poirier K., Huang G.J., Siebold C., Cleak J., Oliver P.L., Fray M., Harvey R.J., Molnár Z. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.Y., Wang X., Wu Y., Doble B.W., Patel S., Woodgett J.R., Snider W.D. GSK-3 is a master regulator of neural progenitor homeostasis. Nat. Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.A., Pilz D.T., Babatz T.D., Cushion T.D., Harvey K., Topf M., Yates L., Robb S., Uyanik G., Mancini G.M. TUBA1A mutations cause wide spectrum lissencephaly (smooth brain) and suggest that multiple neuronal migration pathways converge on alpha tubulins. Hum. Mol. Genet. 2010;19:2817–2827. doi: 10.1093/hmg/ddq182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Geisert E.E., Frankfurter A., Spano A.J., Jiang C.X., Yue J., Dragatsis I., Goldowitz D. A transgenic mouse class-III beta tubulin reporter using yellow fluorescent protein. Genesis. 2007;45:560–569. doi: 10.1002/dvg.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga S.B., Margossian S.P., Harris M.H., Campagna D.R., Han A.P., Blevins S., Mudbhary R., Barker J.E., Walsh C.A., Fleming M.D. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. 2010;137:1907–1917. doi: 10.1242/dev.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe J., Li H., Downing K.H., Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- Neale B.M., Kou Y., Liu L., Ma’ayan A., Samocha K.E., Sabo A., Lin C.F., Stevens C., Wang L.S., Makarov V. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma S., Harris W.A. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208. doi: 10.1016/s0896-6273(03)00632-9. [DOI] [PubMed] [Google Scholar]
- Pacary E., Heng J., Azzarelli R., Riou P., Castro D., Lebel-Potter M., Parras C., Bell D.M., Ridley A.J., Parsons M., Guillemot F. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron. 2011;69:1069–1084. doi: 10.1016/j.neuron.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Pagnamenta A.T., Klei L., Anney R., Merico D., Regan R., Conroy J., Magalhaes T.R., Correia C., Abrahams B.S. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier K., Saillour Y., Bahi-Buisson N., Jaglin X.H., Fallet-Bianco C., Nabbout R., Castelnau-Ptakhine L., Roubertie A., Attie-Bitach T., Desguerre I. Mutations in the neuronal ß-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet. 2010;19:4462–4473. doi: 10.1093/hmg/ddq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G., Jaglin X.H., Keays D.A., Francis F., Chelly J., Cowan N.J. Disease-associated mutations in TUBA1A result in a spectrum of defects in the tubulin folding and heterodimer assembly pathway. Hum. Mol. Genet. 2010;19:3599–3613. doi: 10.1093/hmg/ddq276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield M.A., Baris H.N., Wu C., Rudolph G., Van Maldergem L., He W., Chan W.M., Andrews C., Demer J.L., Robertson R.L. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield M.A., Cederquist G.Y., Gupta M.L., Jr., Engle E.C. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr. Opin. Genet. Dev. 2011;21:286–294. doi: 10.1016/j.gde.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.A., Engle E.C. Allelic diversity in human developmental neurogenetics: insights into biology and disease. Neuron. 2010;68:245–253. doi: 10.1016/j.neuron.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplemental References
- Murtaugh, L.C., Chyung, J.H., and Lassar, A.B. (1999). Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 13, 225–237. [DOI] [PMC free article] [PubMed]
- Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., and Speleman, F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.