Abstract
Elevated (4 to 7-fold) levels of urinary dolichol and coenzyme Q and substantially longer chain lengths for urinary dolichols have been reported in Smith-Lemli-Opitz Syndrome (SLOS) patients, compared to normal subjects. We investigated the possibility of similar alterations in hepatic, nonsterol isoprenoids in a well-established rat model of SLOS. In this model, the ratio of 7-dehydrocholesterol (7DHC) to cholesterol (Chol) in serum approached 15:1; however, total sterol mass in serum decreased by >80 %. Livers from treated rats had 7DHC/Chol ratios of ~32:1, but the steady-state levels of total sterols were >40 % those of livers from age-matched (3-month-old) control animals. No significant differences in the levels of LDL receptor or HMG-CoA reductase were observed. The levels of dolichol and coenzyme Q were elevated only modestly (by 64 and 31 %, respectively; p < 0.05, N = 6) in the livers of the SLOS rat model compared to controls; moreover, the chain lengths of these isoprenoids were not different in the two groups. We conclude that hepatic isoprenoid synthesis is marginally elevated in this animal model of SLOS, but without preferential shunting to the nonsterol branches (dolichol and coenzyme Q) of the pathway and without alteration of normal dolichol chain lengths.
Keywords: AY9944, Smith-Lemli-Opitz syndrome (SLOS), Sterol, Dolichol, Coenzyme Q, Liver
Introduction
Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive disease characterized by a wide range of physical, behavioral and neurological abnormalities (for a review, see [1]). Its molecular basis lies in mutations (mostly missense) in the terminal enzyme of cholesterol (Chol) synthesis, sterol-Δ7-reductase (DHCR7; EC 1.3.1.21, OMIM #602858) [2]. As a result, levels of the cholesterol precursor 7-dehydrocholesterol (7DHC) are greatly elevated in tissues and fluids of SLOS patients, compared to normal controls, while Chol levels are markedly reduced [3]. The pathological basis of the phenotype has been ascribed to various factors, including lack of sufficient Chol levels, particularly during a critical period in embryonic development [4], an abnormal buildup of Chol precursors such as 7DHC [5], and the generation of oxidation products derived from 7DHC or other sterol intermediates [6]. Clearly, these possibilities are not mutually exclusive. From a metabolic standpoint, it is of interest that the level of total sterols in the serum of SLOS patients is substantially less than that of control subjects [7], raising the possibility that carbon flux into the sterol biosynthetic pathway is below normal. Sterol balance studies [8] and determinations of fractional sterol synthesis rates [9] using deuterated water support the proposed reduced rate of de novo sterol synthesis in SLOS patients compared to normal subjects. However, Steiner’s group has found that urinary mevalonate levels (an indicator of mevalonate production) are not significantly different in SLOS patients compared to normal subjects [10, 11]. These latter findings have raised the question as to the fate of de novo generated mevalonate in SLOS patients.
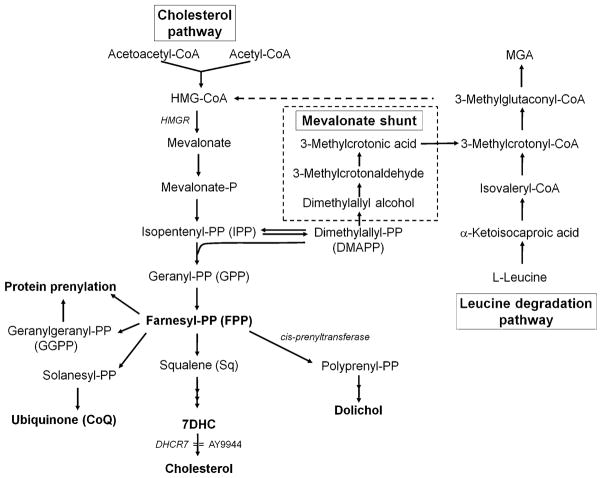
In 1995, Kelley reported that SLOS patients exhibit elevated plasma levels of 3-methylglutaconic acid (MGA), a byproduct of the leucine degradation pathway [12]. Since MGA also can be produced via a shunt from the mevalonate pathway through dimethylallyl pyrophosphate (DMAPP) [13], the possibility exists that some of the mevalonate generated in the tissues of SLOS patients can be shunted to the leucine degradation pathway rather than enter the sterol pathway (see Fig. 1). However, a recent report from Steiner’s group [14] appears to refute this possibility. They demonstrated that feeding Chol to SLOS patients reduced urinary mevalonate levels (as a result of suppression of HMG-CoA reductase (HMGR) activity by Chol), but had no significant effect on urinary MGA levels, thereby arguing against shunting to MGA. Interestingly, this group has also found a 4- to 7-fold elevation in the levels of urinary dolichol and coenzyme Q (a.k.a., ubiquinone) [11, 14] as well as an increase in dolichol chain lengths [14] in SLOS patients, compared to controls. These findings suggested that some of the mevalonate in SLOS patients is shunted not toward leucine degradation, but rather to the nonsterol isoprenoid branches of the pathway.
Fig. 1.
The branched isoprenoid pathway. The major metabolic flux is through the cholesterol pathway, with rate-limiting step at the level of HMG-CoA reductase (HMGR). AY9944 blocks this pathway at the level of sterol-Δ7-reductase (7-dehydrocholesterol reductase, DHCR7), resulting in accumulation of 7DHC and reduced steady-state levels of cholesterol. Farnesyl pyrophosphate (FPP) is the major branch-point intermediate in this pathway, and is an obligate biogenic precursor (through the enzymatic action of cis-prenyltransferase) of dolichol. The mevalonate shunt can divert carbon flux away from this pathway at the level of isopentenyl pyrophosphate (IPP) via its isomer, dimethylallyl pyrophosphate (DMAPP), resulting in formation of 3-methylglutaconic acid (MGA), which is a metabolite of leucine degradation
In the present study, we investigated the possibility of mevalonate shunting to nonsterol isoprenoids under conditions in which DHCR7 activity is impaired, using a pharmacologically-induced animal model of SLOS that recapitulates the developmental and biochemical hallmarks of the human disease, particularly with regard to the abnormal accumulation of 7DHC in bodily tissues and fluids [15–17]. We found that hepatic levels of both dolichol and coenzyme Q were modestly increased in the SLOS rat model, relative to age-matched controls, but there was no shift to longer isoprenoid chain lengths.
Materials and Methods
Materials
Unless otherwise stated, all chemicals and reagents were of the highest purity available and were used as obtained from commercial vendors. Organic solvents were HPLC grade and used as purchased from Fisher Scientific, Atlanta, GA. AY9944 ((trans-1,4-bis [2-dichlorobenzylaminomethyl] cyclohexane dihydrochloride) was custom synthesized and recrystallized to homogeneity (A.H. Fauq, Chemistry Core, Mayo Clinic, Jacksonville, FL). Purity was verified by HPLC and LC–MS, and the structure was verified in comparison to an authentic sample of AY9944 (a gift from Wyeth-Ayerst Research, Princeton, NJ), using NMR, UV–VIS spectroscopy, and MS. C18 SepPak® cartridges were purchased from Waters Corporation, Milford, MA. Authentic chromatographic standards of cholesterol, 7DHC and squalene were obtained from Research Plus (http://www.researchplus.com/). Authentic standards of dolichols and coenzyme Q were from Isoprenoids, LC (http://www.isoprenoids.com/). Rabbit polyclonal whole antisera to LDLR and to HMGR (cross-reactive to human and rat) were generous gifts from Dr. Gene C. Ness (University of South Florida, Tampa, FL). Antibodies to β-tubulin (rabbit IgG, #H-235), with broad cross-species reactivity, were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibodies were obtained from Sigma/Aldrich (St. Louis, MO). All reagents and materials for SDS-PAGE and Western blot analyses were obtained from Bio-Rad Laboratories (Hercules, CA).
SLOS Rat Model
The SLOS animal model was generated as previously described [17], treating Sprague–Dawley rats (Harlan Bioproducts for Science, Indianapolis, IN) with AY9944, a selective inhibitor of DHCR7. All procedures involving animals were approved by the Buffalo VAMC IACUC, and were in accordance with the ARVO Resolution on the Use of Animals in Research and with the NIH Guide for the Care and Use of Laboratory Animals. Rats were fed cholesterol-free chow (Purina Mills Test Diet, Richmond, IN) and water ad lib, and were maintained on a 12 h light/12 h dark cyclic lighting regimen (20–40 lux) at standard room temperature (22–25 °C). Control rats were fed the same diet and maintained under the same ambient conditions, but were given no other treatment.
Tissue Harvesting
Rats (3 months postnatal, AY9944-treated and controls) were euthanized by sodium pentobarbital overdose (i.p.). Tissue harvesting was performed under dim red light, to avoid photoperoxidation of lipids, particularly 7DHC. Livers were then rapidly removed postmortem, blotted, transferred to conical polypropylene screw-top tubes, flash frozen in liquid nitrogen, and stored (wrapped in aluminum foil) at −80 °C until ready for saponification and/or lipid extraction and analysis.
Analysis of Dolichol and Coenzyme Q
Frozen liver specimens (0.5 g each, wet wt.) were thawed and immediately subjected to extraction by homogenization in 10 ml of chloroform/methanol (2:1, v/v) using a Polytron® homogenizer (Kinematica, Model PT 10/35 GT, Thermo Fisher Scientific; 10 s at setting “8”). Internal standards of coenzyme Q7 (14 μg), and dolichol-21 (50 μg) were added to the homogenates, which then were divided into two equal portions. One portion was saponified and the nonsaponifiable lipids (NSLs) were extracted with petroleum ether and redissolved in methanol, essentially as described previously [17]. The NSL samples were then applied to C18 SepPak® cartridges (Waters Corporation, Milford, MA) and eluted with 2 × 5 ml of methanol. The SepPak® cartridges were then eluted with 2 × 5 ml isopropanol, and the pooled eluates (the “dolichol” fraction) were stored at −20 °C until ready for analysis. The other portion of the chloroform/methanol extract was treated with 0.25 volume of 0.9 % (aq.) NaCl and centrifuged. The upper phase was removed, and the lower phase was washed twice with 2 ml of 50 % (v/v) aqueous methanol. The final lower phase, which was slightly turbid, was taken to dryness, dissolved in chloroform/methanol (2:1, v/v), and applied to the application zone of a Whatman K6F preparative thin layer plate (1 mm thickness, 10 × 20 cm; Thermo Fisher Scientific, Inc.). A separate lane containing an authentic standard of coenzyme Q7 was added to aid in detection. The plates were chromatographed in 15 % diethyl ether/hexane (v/v), and the areas representing coenzyme Q were scraped into a glass centrifuge tube and extracted twice with 10 ml diethyl ether. The ether extracts were pooled, taken to dryness, dissolved in 0.5 ml 2-propa-nol/methanol (1:4, v/v), filtered, and stored at −20 °C until ready for HPLC analysis.
The dolichol and coenzyme Q fractions from above were subjected to HPLC analysis as previously described [18]. The total dolichol mass was determined by summing the quantified mass of each peak (Dol-17, Dol-18, Dol-19, and Dol-20) and using the mass of the internal standard (Dol-21) to correct for yield). For the quantification of coenzyme Q, samples eluted from the preparative TLC plate (see above) were subjected to HPLC [18]. The mass of the Q9 and Q10 fractions was determined, and the % recovery of the internal standard (Q7) was used to correct for yield. Data were subjected to statistical analysis using an unpaired Student’s t test, with the cutoff for statistical significance set at p < 0.05 (N = 6 per group).
Saponification and Extraction of Nonsaponifiable Lipids for Sterol Analysis
In parallel to the above, additional frozen liver specimens (0.1 g each, wet wt.) were transferred to glass screw-top tubes with Teflon-lined caps containing 1 ml of 60 % (w/v) KOH in 50 % (v/v) MeOH, flushed with argon, sealed, and subjected to saponification at 95 °C for 1 h, protected from exposure to light. After chilling for 5 min on ice, 3 ml of 50 mM NH4Cl (aqueous) was added followed by 3 ml of petroleum ether, and then mixed by continuous rotary inversion for 1 h at 4 °C (protected from light, under argon atmosphere), followed by centrifugation (10 min at 500×g, refrigerated table-top centrifuge) to separate phases. The upper (petroleum ether) phase containing the nonsaponifiable lipids (NSLs) was transferred with a glass Pasteur pipet to a conical glass tube; the lower (aqueous) phase was extracted twice more with an equivalent volume of petroleum ether, the combined extracts were dried under an argon stream, then redissolved in 200 μl of MeOH and stored under argon at −80 °C until ready for further analysis. The recovery of neutral sterols was >90 % (unpublished results) using these methods.
Quantitative Chromatographic (HPLC) Analysis of Sterols and Squalene
Aliquots (50 μl each) of NSLs were subjected to reversed-phase HPLC using tandem IB-Sil™ and Gemini® RP C18 columns (4.6 × 150 mm, 3 μm; Phenomenex, Torrance, CA), maintained at 37 °C using a column oven, with MeOH as the mobile phase (flow rate 1 ml/min) and flow-through detection at 205 nm (Waters 600E HPLC system, Model 486 detector, Milford, MA). Under the conditions employed, the following retention times were obtained: Chol, 10.6 min; 7DHC, 9.7 min; squalene, 15.4 min. Response factors for authentic standards of cholesterol, 7DHC, and squalene were determined empirically, which then were used to determine the sterol and squalene mass for the rat liver NSL extracts. The integrated areas under the peaks were quantified using Flo-ONE™ software (Packard Instrument Co., Inc., Meriden, CT). Data were subjected to statistical analysis using an unpaired Student’s t test, with the cutoff for statistical significance set at p < 0.05 (N = 6 per group).
Extraction and Quantification of Total Protein from Tissue
Frozen liver specimens (0.1 g each, wet wt.) were thawed in 1 ml of Dulbecco’s phosphate-buffered saline (DPBS), pH 7.2 (Life Technologies, Grand Island, NY), containing Halt™ Protease and Phosphatase Inhibitor Cocktail without EDTA (Thermo Fisher Scientific, Inc.; Waltham, MA). The tissue was homogenized using a Tissuemizer® (SDT-1810; Teledyne Tekmar, Mason, OH) for 30 s on ice, and solubilized by adding 0.5 ml solubilization buffer (6 % SDS, 25 % glycerol, 300 mM DTT), with vortexing. The protein samples then were diluted 1:10 (v/v) with DPBS and the protein concentration of each was determined by absorbance at 280 nm using a Synergy™ HT Multi-Mode Microplate Reader equipped with a Take3 Micro-Volume plate (Biotek, Winooski, VT). Statistical analysis of data was performed using an unpaired Student’s t test, with the cutoff for statistical significance set at p < 0.05 (N = 3 per group).
SDS-PAGE and Western Blot Analysis
Aliquots of liver homogenates (20 μg total protein each) from AY9944-treated and control rats (N = 3) were electrophoretically separated by SDS-PAGE (12 % resolving gel) using a Mini-Protean® II (Bio-Rad) electrophoresis system for 45 min at 200 V in SDS running buffer (25 mM Tris–HCl, 20 mM glycine and 0.1 % (w/v) SDS). The gels were soaked in transfer buffer (25 mM Tris–HCl, 13.5 mM glycine and 20 % (v/v) methanol) for 5 min and subsequently transferred to Immobilon-P® PVDF membrane (Millipore) using a Trans-Blot® SD Semi-Dry Transfer Cell (BioRad) for 60 min at 15 V in transfer buffer. The blots were blocked with 5 % (w/v) blotting grade nonfat dry milk (BioRad) in Tris-buffered saline with Tween-20 (TBST) (25 mM Tris–HCl, 150 mM NaCl and 0.05 % (v/v) Tween-20) for 1 h at room temperature and then probed overnight (at 4 °C) with rabbit anti-LDLR, anti-HMGR, or anti-β-tubulin antibodies, each at 1:1,000 volumetric dilution with 1 % (w/v) nonfat dry milk in TBST. Blots were serially rinsed in TBST and then incubated for 1 h at room temperature with alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibodies at 1:10,000 volumetric dilution with 1 % (w/v) nonfat dry milk in TBST. The blots were developed using ECF™ Substrate (GE Healthcare Bio-Sciences Corp., Piscataway, NY) and imaged with a Storm® 820 PhosphorImager™ (GE Healthcare). Quantitation of the images was performed using ImageQuant™ 5.2 software (GE Healthcare); data were subjected to statistical analysis using an unpaired Student’s t test, with the cutoff for statistical significance set at p < 0.05 (N = 3 per group).
Results
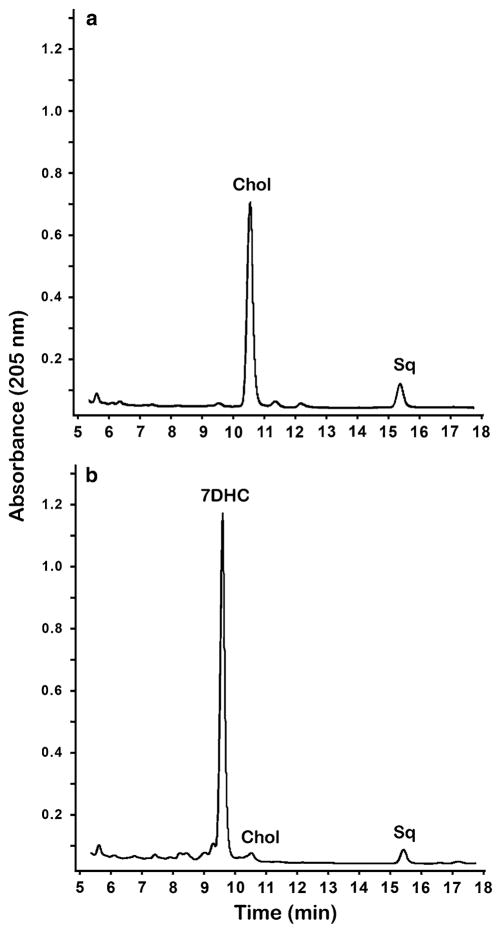
Since the main focus of this study was to evaluate the impact of inhibition of DHCR7 on hepatic dolichol levels and chain lengths, we first needed to confirm that the agent we were using in our animal model of SLOS (AY9944) was, indeed, inhibiting DHCR7. To this end, we analyzed the sterol profiles of serum and liver from AY9944-treated and age-matched (3-month-old) control rats. Figure 2 shows typical reversed phase HPLC profiles of the NSLs derived from the livers of normal and AY9944-treated rats. These profiles confirm the expected effect of the inhibitor, and are in good agreement with the results of prior related studies [15–17, 19, 20]: 7DHC is almost below the limit of detection in control rat liver, while in the AY9944-treated rats, the level of 7DHC greatly surpasses that of Chol. Quantification of the chromatographic data from analysis of serum and liver from control animals and AY9944-treated animals (N = 6 each) is presented in Table 1. Overall, AY9944 treatment brought about a large (>80 %, p < 0.05) decrease in total serum sterols and a significant increase (mean = 143 %, p < 0.05) in the total sterol content of the liver, compared to controls (cf. Table 1, panels a and b). However, the average 7DHC:Chol ratio was ~15 in sera and ~32 in livers from AY9944-treated rats, compared to a ratio of ~0.001 and ~0.003, respectively, in the corresponding specimens from age-matched controls; the former ratio is comparable to, if not greater than, that found in tissues and fluids of even the most severely affected SLOS patients [1, 3]. Thus, these findings validate the use of this animal model as a surrogate for understanding the biochemical alterations in SLOS, particularly as occurs in more severe cases of the disease.
Fig. 2.
HPLC analysis of liver nonsaponifiable lipids from a control and b AY9944-treated rats. Representative reversed phase (C18) HPLC chromatograms are shown, with UV detection at 205 nm. Chromatographic peaks corresponding to retention times of authentic standards of cholesterol (Chol), 7DHC, and squalene (Sq) are indicated
Table 1.
Effect of AY9944 treatment on the steady-state levels of sterols and squalene in rat (a) liver and (b) serum
| Tissue/treatment | Cholesterol (μg g−1) | 7DHC (μg g−1) | Squalene (μg g−1) | Chol + DHC (μg g−1) | Percent of control |
|---|---|---|---|---|---|
| (a) Liver* | |||||
| Control | 1,819 ± 141 | 5.6 ± 0.7 | 29.2 ± 1.8 | 1,825 ± 141 | 100 |
| AY9944 | 78 ± 11a | 2,529 ± 163a | 18.1 ± 3.0b | 2,607 ± 161a | 143a |
| (b) Serum# | |||||
| Control | 105.5 ± 6.8 | 0.17 ± 0.09 | 0.029 ± 0.007 | 105.6 ± 6.9 | 100 |
| AY9944 | 1.1 ± 0.4a | 16.6 ± 2.8a | 0.027 ± 0.011c | 17.6 ± 3.2a | 16a |
Values are mean ± SD (N = 6), as determined by reversed-phase HPLC (corrected for recovery efficiency)
Values expressed as μg per gram wet weight of liver
Values expressed as mg per dl of serum
p < 0.001,
p < 0.05,
not significant
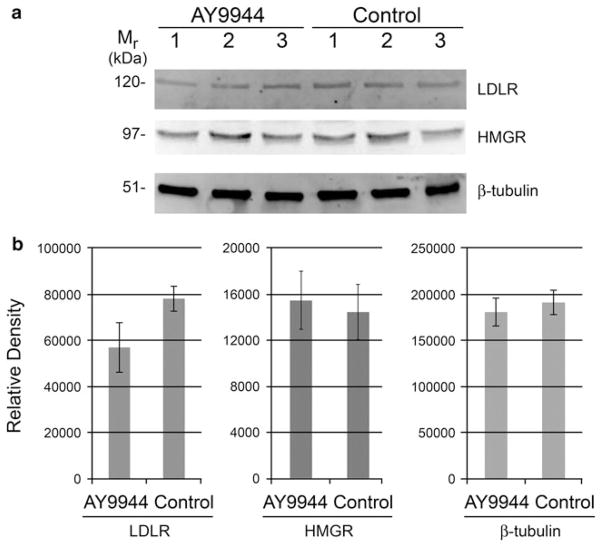
One explanation for the observed increase in steady-state levels of liver sterols in rats treated with AY9944, compared to controls, as noted above might be increased uptake of circulating, lipoprotein-borne sterols from the blood, as a consequence of a homeostatic increase in LDLR levels in liver. Therefore, we examined the levels of LDLR in livers from AY9944-treated and age-matched control rats by Western blot analysis and quantitative densitometry (normalized to β-tubulin). As shown in Fig. 3, AY9944 treatment did not cause any statistically significant change in the levels of LDLR protein (Mr ~ 120 kDa) in the liver. In fact, if anything, there was a trend (not statistically significant; p = 0.067) toward lower, rather than higher, LDLR levels in the AY9944-treated animals, compared to controls.
Fig. 3.
Chronic AY9944 treatment causes no significant changes in the levels of LDLR or HMGR protein in livers of rats, compared to controls. a Western blots of liver homogenates from 3-month-old AY9944-treated and age-matched control rats (N = 3), probed with polyclonal antibodies raised against LDLR, HMGR, and β-tubulin (loading control) followed by detection using an alkaline phosphatase-conjugated secondary antibody and fluorimetric substrates. The apparent molecular weight (Mr, in kDa) of each protein is indicated. b Densitometric quantification of LRLR, HMGR, and β-tubulin protein levels, expressed as mean values ± SD (N = 3). Statistical analysis (unpaired Student’s t test) indicated no significant differences between groups (p = 0.067 for LDLR, p = 0.807 for HMGR, p = 0.502 for β-tubulin)
We also examined the possibility of increased synthesis of sterols due to an induction of HMG-CoA reductase (HMGR), the rate limiting enzyme. We found no significant differences in the levels of HMG-CoA reductase (HMGR; Mr ~ 97 kDa) protein in livers from AY9944-treated animals, compared to controls (Fig. 3). Another approach to determining if treatment with AY9944 caused an increase in de novo sterol biosynthesis is to quantify the levels of sterol pathway intermediates in serum [21]. We measured the serum levels of squalene, since it is an easily and reliably quantifiable intermediate in the cholesterol branch of the biosynthetic pathway and does not require the use of radioisotopically labeled precursors for its determination. Accordingly, squalene was quantified using the area of the peak in reversed phase HPLC chromatograms of the NSL fractions (see Fig. 2 for chromatogram from liver). As shown in Table 1b, the serum squalene values obtained from AY9944-treated rats were nearly identical to those from age-matched controls, suggesting no change in de novo synthesis. However, the squalene steady-state level in liver was found to be decreased by 31 % in AY9944-treated animals compared to age-matched controls (Table 1a). These findings are considered within the context of cholesterol synthesis in the rat model (see “Discussion”).
We also examined the possibility that the chronic treatment of rats over a 3 month postnatal time period may have resulted in systemic dehydration, which potentially could skew the quantitative values normalized to tissue wet weight. However, when we quantified the total protein content of livers from AY9944-treated and age-matched control rats, we found no statistically significant differences between the values (0.338 ± 0.028 vs. 0.337 ± 0.016 mg protein per mg wet wt., respectively; p = 0.391, N = 6).
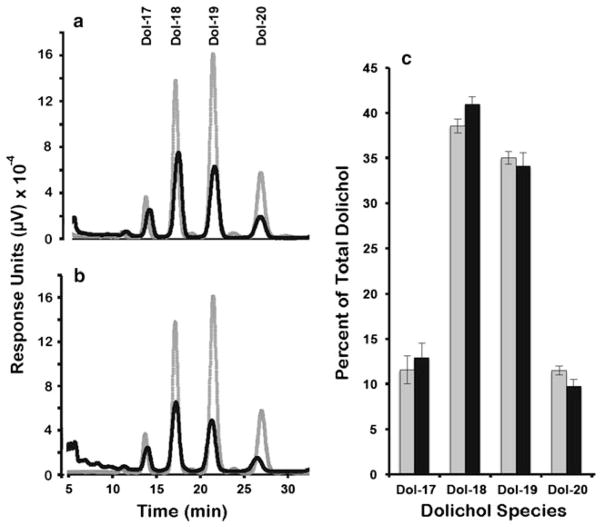
To determine if the inhibition of the sterol pathway at the level of DHCR7 impacted the synthesis of nonsterol isoprenoids, we quantified the levels of both total dolichol and coenzyme Q as well as individual dolichol isoprene species in the chloroform/methanol extracts of livers from control and AY9944-treated rats (see “Materials and Methods”). As shown in Table 2, there was a significant (p < 0.05, N = 6) increase in the levels of both dolichol and coenzyme Q, comparing treated versus control groups. Figure 4 shows representative reversed phase HPLC chromatograms obtained from livers of a control (Fig. 4a) and an AY9944-treated animal (Fig. 4b), where dolichol isoprene species were separated as a function of increasing chain length. The elution profile of authentic dolichol standards (gray tracings, Fig. 4a and b) of differing chain lengths, ranging from 85 carbons (Dol-17) to 100 carbons (Dol-20), is superimposed on each chromatogram, where the number following Dol denotes the number of isoprene (5-carbon) units. As shown, there is no apparent difference in the qualitative isoprene pattern in the livers from the treated rats compared to controls. The corresponding quantitative data for all specimens analyzed (N = 6 per group), showing the relative amounts of each of the major dolichol species in the livers of control (gray bars) and AY9944-treated (black bars) animals, expressed as percent of total, are shown in Fig. 4c. The dominant isoprene species in the livers of both treated and control animals were Dol-18 (90 carbons; 41.0 ± 0.8 % treated, 38.6 ± 0.8 % control) and Dol-19 (95 carbons; 34.1 ± 1.5 % treated, 35.0 ± 0.7 % control). The longest endogenous chain length species measured (Dol-20; 100 carbons) was, by comparison, less prominent in both treated and control animals (12.4 ± 1.2 % treated, 17.6 ± 1.1 % control). Clearly, there was no shift to longer chain length species in the SLOS rat model, compared to untreated controls. We also observed no change in the coenzyme Q9:Q10 ratio (data not shown).
Table 2.
Effect of AY9944 treatment on the steady-state levels of dolichol and coenzyme Q in rat liver
| Treatment | Dolichol (μg g−1) | Percent of control | Coenzyme Q (μg g−1) | Percent of control |
|---|---|---|---|---|
| Control | 28.0 ± 2.7 | 100 | 37.0 ± 7.0 | 100 |
| AY9944 | 45.9 ± 10.9a | 164 | 54.3 ± 8.7b | 131 |
Values are mean ± SD (N = 6), expressed as μg per gram wet weight of liver, as determined by reversed-phase HPLC (for dolichol; corrected for recovery efficiency) or thin-layer chromatography (for coenzyme Q)
p < 0.01,
p < 0.05
Fig. 4.
Chronic AY9944 treatment results in no significant changes in chain lengths of rat liver dolichols, compared to controls. Dolichol isoprene species identification and quantification by HPLC. The panels denote representative reversed phase (C18) HPLC chromatograms monitored at 210 nm for dolichols of increasing isoprene chain lengths from a control rat liver (black line) with dolichol standards (gray line) and b AY9944-treated rat liver (black line) with dolichol standards (gray line). c Relative amounts of liver dolichol species of varying isoprene chain lengths from control (gray bars) and AY9944-treated (black bars) rats as determined empirically from integration of the HPLC chromatograms in (a) and (b). Values represent mean ± S.D. (N = 4)
Discussion
There have been several reports dealing with sterol levels and metabolism in SLOS patients. In all cases, there is a greatly increased level of 7DHC and metabolites in tissues and serum (for a review, see [1]). In addition, it is generally found that total serum sterol levels in SLOS patients are lower than those of control subjects [3, 22, 23], raising the possibility of lowered de novo synthesis of sterols. Indeed, studies by Steiner et al. [8], in which sterol balance was used as an indication of synthesis, showed that whole body sterol synthesis was significantly reduced in SLOS patients, compared to normal human subjects. Although reduced production of total sterols in SLOS is generally accepted, there are conflicting views on the hepatic flux through the rate limiting enzyme, HMGR. Pappu et al. [10] reported that urinary mevalonate excretion (a crude measure of liver HMGR activity) is not significantly reduced in SLOS patients compared to controls. On the other hand, Honda et al. [22] reported that plasma mevalonate levels were significantly less in SLOS patients compared to age-matched control subjects. These workers also reported a decreased level of HMGR in microsomal membranes from a liver biopsy of a single SLOS patient. However, in the same biopsy sample, the activities of HMG-CoA synthase and squalene synthase were substantially elevated. Since changes in the activity of these two enzymes generally parallel changes in HMGR (all of these enzymes are regulated through SREBP [24]), the low value for HMGR found in the single biopsy sample may be spurious. In addition, as noted by Pappu et al. [10], single point measurements of plasma mevalonate—as was performed in the Honda et al. [22] study—may not reliably reflect HMGR activity, due to the diurnal changes in activity of this enzyme. Based on their findings that hepatic mevalonate production is normal in SLOS patients, while sterol synthesis is suppressed, Pappu et al. [11] have proposed that some of the mevalonate produced in SLOS is shunted to nonsterols (see below).
With regard to animal models, inhibition (or knockout) of DHCR7 results in greatly elevated ratios of 7DHC/cholesterol, often much greater than that observed in humans SLOS patients. Part of the explanation for the greater ratio is that humans typically consume large amounts of dietary cholesterol, while rodents are generally maintained on a plant-based (cholesterol-free) diet. Unlike the human situation, when DHCR7 activity is decreased in rats using AY9944, there is a dramatic decrease in total serum sterols and concomitant increase in liver sterols (Table 1). In view of these findings, and since previous work using cultured cells and with a SLOS autopsy specimen [25–27] indicated that reduced DHCR7 activity resulted in elevated levels of LDLR protein, we examined the level of this protein in our rat model. However, in vivo studies employing rats treated with AY9944, either short-term (4 days, postnatally) [19] or chronically (starting in utero and continuing over a 3 month postnatal time course, as in the present study), showed no statistically significant change in the levels of LDLR protein in liver, compared to age-matched controls. Hence, increased uptake of circulating, lipoprotein-borne sterols does not appear to explain the observed increase in the steady-state sterol content of liver in rats treated with DHCR7 inhibitors such as AY9944. A clue to the basis for the loss of serum sterols and gain in liver sterols may be found in early studies employing AY9944. Dvornik and Hill [28] found that long-term treatment of rats with AY9944 resulted in a substantial accumulation of sterols in the lung, consistent with an earlier study by Greselin [29] who showed that such chronic AY9944 treatment caused accumulation of lipid (presumed to be 7DHC) in alveolar macrophages in several animal species, including rats, pigs and dogs. These findings likely represent an off-target (nonspecific) systemic effect of AY9944, which like other amphiphilic drugs is known to promote accumulation of sterols in the lung, particularly in lysosomes and in macrophages [30]. Since ca. 10 % of the cellular mass of the liver is accounted for by resident macrophages (i.e., Kupffer cells) [31]], it is possible that some or all of the increase in total sterols we observed in liver was due to sterol accumulation in these hepatic macrophages. However, since our main focus in the present study was on the nonsterol pathway, we did not pursue this possibility further.
Concerning mevalonate production, we found no significant change in the levels of HMGR protein in the livers of rats treated chronically with AY9944, compared to age-matched controls, by Western blot analysis (Fig. 3). However, we caution against concluding from this observation that there was no change in HMGR enzymatic activity under the conditions employed. HMGR activity is well known to be modulated by a multitude of mechanisms (e.g., phosphorylation-dephosphorylation being just one example), which do not necessarily entail changes in protein level (for a review, see [32]). In fact, short term treatment of rats with DHCR7 inhibitors AY9944 [19] or BM15.766 [20] has been shown to elevate the activity of hepatic HMGR, indicating that the production of mevalonate is likely enhanced under those conditions. Thus, based on previous work and our own findings, it appears that there is no decrease in mevalonate production in the AY9944-treated rat, similar to the findings of Pappu et al. [10] in human SLOS patients.
With regard to quantifying the flux from mevalonate into the sterol branch of the pathway, Miettinen and coworkers have evaluated the use of serum squalene levels as a marker for de novo cholesterol synthesis in humans [33, 34]. They reported that it is a useful marker in the case of Type 2 diabetes [34], but cautioned against the use of cholesterol precursors as metrics in all situations of altered sterol metabolism [33]. To our knowledge, there are no published studies on the use of serum squalene levels to quantify de novo cholesterol synthesis in rats. As shown in Table 1, we found that while there were no significant changes in the level of squalene in the serum of AY9944-treated rats, compared to those in age-matched controls, there was a 50 % decrease in the steady-state level of squalene in liver as a function of AY9944 treatment. While this initially might suggest that flux through squalene may be depressed in the SLOS rat model, apparently not all of the squalene in rat liver is in equilibrium with the pool involved in cholesterol biosynthesis [35]. Given the caveats with regard to the use of squalene levels in either serum or liver or both as a meaningful metric for evaluating de novo sterol synthesis, and the lack of a significant change in serum squalene levels in the present study, we caution against using squalene values to draw firm conclusions regarding alterations in de novo cholesterol synthesis in the rat SLOS model. Further studies involving labeled water (e.g., see [8]) would be required to conclusively determine if the de novo rate of sterol synthesis is altered in this model of SLOS.
Regarding the nonsterol pathways, the Steiner group reported 4 to 7-fold elevations in urinary dolichol and coenzyme Q [11, 14] as well as a large increase in the chain lengths of urinary dolichol [14] in SLOS patients compared to age-matched controls. From these results, along with their finding of normal urinary mevalonate levels and reduced sterol synthesis [8], the Steiner group has proposed that some of the mevalonate is diverted to the above mentioned nonsterol isoprenoids. In their first report on the subject [11], they found that cholesterol supplementation to SLOS patients coordinately decreased urinary mevalonate, dolichol and coenzyme Q levels (suggesting that the urinary nonsterols are derived from liver); however, in the most recent report [14], they found that cholesterol feeding only suppressed the urinary mevalonate level, with no significant effect on dolichol or coenzyme Q levels. Other workers have found that urinary dolichol is associated with cellular debris or membrane fragments [36] and its levels do not correlate well with serum dolichol levels [37]. Thus, it is uncertain whether the dolichol and coenzyme Q measured in the studies reported by Steiner’s group are reflective of hepatic synthesis of these nonsterol isoprenoids. It should be noted at this point that only a very minor amount of shunting could lead to increased production of dolichol or coenzyme Q or both, since the level of cholesterol in the liver is on the order of milligrams, whereas that of dolichol and coenzyme Q is at least two orders of magnitude lower (i.e., tens of micrograms). In rat liver, for example, the flux of mevalonate into cholesterol exceeds that into dolichol by a ratio of >200:1 [38].
In the present work, we found an approximate 50 % increase in the steady-state levels of both dolichol and coenzyme Q in the livers of rats treated with AY9944, but no effect on the chain lengths of these lipids. Previous studies in our laboratory [18] have shown that administering mevalonate to a cholesterol-fed rat (conditions expected to increase the steady-state level of IPP) resulted in greatly increased chain lengths of dolichol and coenzyme Q, but with no effect on the total levels of these nonsterol isoprenoids (see Table 2 and Fig. 4). Sagami et al. [39] also showed in vitro that increasing IPP concentration causes an increase in dolichol chain lengths. In contrast, when a squalene synthase inhibitor (Squalastatin-I) is administered to rats, the level of FPP has been shown to increase ~20-fold and IPP increases ~5-fold, resulting in an increased chain length and a ~3-fold elevation in the level of dolichol and dolichyl phosphate [40]. In fact, when fibroblasts from patients afflicted with congenital disorders of glycosylation were treated with this squalene synthase inhibitor, their Dol-P-mannose levels became substantially elevated and Dol-P chain lengths increased, thereby providing essential proof of principle for therapeutic application of such drugs for diseases involving Dol-P insufficiency [41]. Thus, it seems that FPP, serving as the primer, controls the rate of dolichol synthesis, while IPP, serving as the polymerizing agent, controls the chain length of the final product. Since we observed no effect on chain length of dolichol or coenzyme Q and only a relatively minor change in the steady-state levels of these lipids, it appears doubtful (in this rat model at least) that there is a multi-fold increase in isoprenoid pyrophosphate levels (IPP, FPP) in liver. Furthermore, it is difficult to envision a mechanism by which the cis-prenyltransferase (responsible for dolichol synthesis), and the trans-prenyltransferase (responsible for elaboration of the side chain of coenzyme Q) would both be activated because of suppression of DHCR7 activity. Thus, the most likely scenario in the AY9944-treated rat is that DHCR7 inhibition, by lowering cholesterol levels, activates the SREBP pathway, which modestly induces most of the genes of cholesterol synthesis [24]. As a result of this activation, levels of intermediate isoprenoid pyrophosphates rise slightly, and flux into the nonsterol and sterol pathways increases coordinately. However, to determine conclusively whether or not carbon flux is preferentially diverted to nonsterol branches of the isoprenoid pathway in liver, either in humans or animal models, additional studies will be required using metabolic precursors, such as radioisotopically (or stable isotopically) labeled mevalonate or acetate, and following the rate and extent of their conversion to the various isoprenoid end-products.
Acknowledgments
This work was supported, in part, by U.S.P.H.S. (NEI/NIH) grant EY007361 (SJF), by facilities and resources provided by the Veterans Administration Western New York Healthcare System (SJF), and by an Unrestricted Grant from Research to Prevent Blindness (SJF). We thank Priyanka Patel for technical assistance.
Abbreviations
- 7DHC
7-Dehydrocholesterol (cholesta-5,7-dien-3β-ol)
- AY9944
Trans-1,4-bis[2-dichlorobenzylamino-methyl]cyclohexane dihydrochloride
- Chol
Cholesterol
- DHCR7
3β-Hydroxysterol-Δ7-reductase
- DMAPP
Dimethylallyl pyrophosphate
- Dol
Dolichol
- Dol-P
Dolichyl phosphate
- DTT
Dithiothreitol
- FPP
Farnesyl pyrophosphate
- HMG
3-Hydroxy-3-methyl-glutaryl
- HMGR
HMG-CoA reductase
- IPP
Isopentenyl pyrophosphate
- LDL
Low density lipoprotein
- LDLR
LDL receptor
- MGA
3-Methylglutaconic acid
- NSL
Nonsaponifiable lipid(s)
- SLOS
Smith-Lemli-Opitz Syndrome (OMIM #270400)
- Sq
Squalene
- SREBP
Sterol response element binding protein
Footnotes
Conflict of Interest R. Kennedy Keller has a financial interest in Isoprenoids, LC.
Contributor Information
R. Kennedy Keller, Department of Molecular Medicine, University of South Florida, College of Medicine, Tampa, FL 33612, USA. Isoprenoids, LC, Tampa, FL 33612, USA.
David A. Mitchell, Department of Molecular Medicine, University of South Florida, College of Medicine, Tampa, FL 33612, USA
Christopher C. Goulah, Research Service, Veteran Affairs Western New York Healthcare System, VAWNYHS, 3495 Bailey Avenue, Mail Stop 151 (Bldg. 20 Rm. 202), Buffalo, NY 14215-1129, USA. Departments of Ophthalmology and Biochemistry, The State University of New York at Buffalo (SUNY Buffalo), School of Medicine and Biomedical Sciences, Buffalo, NY 14215, USA. SUNY Eye Institute, Buffalo, NY 14215, USA
Steven J. Fliesler, Email: fliesler@buffalo.edu, Research Service, Veteran Affairs Western New York Healthcare System, VAWNYHS, 3495 Bailey Avenue, Mail Stop 151 (Bldg. 20 Rm. 202), Buffalo, NY 14215-1129, USA. Departments of Ophthalmology and Biochemistry, The State University of New York at Buffalo (SUNY Buffalo), School of Medicine and Biomedical Sciences, Buffalo, NY 14215, USA. SUNY Eye Institute, Buffalo, NY 14215, USA
References
- 1.DeBarber AE, Eroglu Y, Merkens LS, Pappu AS, Steiner RD. Smith-Lemli-Opitz syndrome. Expert Rev Mol Med. 2011;13:24. doi: 10.1017/S146239941100189X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shefer S, Salen G, Batta AK, Honda A, Tint GS, Irons M, Elias ER, Chen TC, Holick MF. Markedly inhibited 7-dehydrocholesterol-delta 7-reductase activity in liver microsomes from Smith-Lemli-Opitz homozygotes. J Clin Invest. 1995;96(4):1779–1785. doi: 10.1172/JCI118223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330(2):107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 4.Gaoua W, Wolf C, Chevy F, Ilien F, Roux C. Cholesterol deficit but not accumulation of aberrant sterols is the major cause of the teratogenic activity in the Smith-Lemli-Opitz syndrome animal model. J Lipid Res. 2000;41(4):637–646. [PubMed] [Google Scholar]
- 5.Engelking LJ, Evers BM, Richardson JA, Goldstein JL, Brown MS, Liang G. Severe facial clefting in Insig-deficient mouse embryos caused by sterol accumulation and reversed by lovastatin. J Clin Invest. 2006;116(9):2356–2365. doi: 10.1172/JCI28988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Mirnics K, Bowman AB, Liu W, Da J, Porter NA, Korade Z. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol Dis. 2012;45(3):923–929. doi: 10.1016/j.nbd.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salen G, Shefer S, Batta AK, Tint GS, Xu G, Honda A, Irons M, Elias ER. Abnormal cholesterol biosynthesis in the Smith-Lemli-Opitz syndrome. J Lipid Res. 1996;37(6):1169–1180. [PubMed] [Google Scholar]
- 8.Steiner RD, Linck LM, Flavell DP, Lin DS, Connor WE. Sterol balance in the Smith-Lemli-Opitz syndrome. Reduction in whole body cholesterol synthesis and normal bile acid production. J Lipid Res. 2000;41(9):1437–1447. [PubMed] [Google Scholar]
- 9.Chan YM, Merkens LS, Connor WE, Roullet JB, Penfield JA, Jordan JM, Steiner RD, Jones PJ. Effects of dietary cholesterol and simvastatin on cholesterol synthesis in Smith-Lemli-Opitz syndrome. Pediatr Res. 2009;65(6):681–685. doi: 10.1203/PDR.0b013e31819ea4eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappu AS, Steiner RD, Connor SL, Flavell DP, Lin DS, Hatcher L, Illingworth DR, Connor WE. Feedback inhibition of the cholesterol biosynthetic pathway in patients with Smith-Lemli-Opitz syndrome as demonstrated by urinary mevalonate excretion. J Lipid Res. 2002;43(10):1661–1669. doi: 10.1194/jlr.m200163-jlr200. [DOI] [PubMed] [Google Scholar]
- 11.Pappu AS, Connor WE, Merkens LS, Jordan JM, Penfield JA, Illingworth DR, Steiner RD. Increased nonsterol isoprenoids, dolichol and ubiquinone, in the Smith-Lemli-Opitz syndrome: effects of dietary cholesterol. J Lipid Res. 2006;47(12):2789–2798. doi: 10.1194/jlr.M600295-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Kelley RI, Kratz L. 3-methylglutaconic acidemia in Smith-Lemli-Opitz syndrome. Pediatr Res. 1995;37(5):671–674. doi: 10.1203/00006450-199505000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Fogelman AM, Edmond J, Popjak G. Metabolism of mevalonate in rats and man not leading to sterols. J Biol Chem. 1975;250(5):1771–1775. [PubMed] [Google Scholar]
- 14.Roullet JB, Merkens LS, Pappu AS, Jacobs MD, Winter R, Connor WE, Steiner RD. No evidence for mevalonate shunting in moderately affected children with Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2012;35:859–869. doi: 10.1007/s10545-012-9453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbu V, Roux C, Lambert D, Dupuis R, Gardette J, Maziere JC, Maziere C, Elefant E, Polonovski J. Cholesterol prevents the teratogenic action of AY 9944: importance of the timing of cholesterol supplementation to rats. J Nutr. 1988;118(6):774–779. doi: 10.1093/jn/118.6.774. [DOI] [PubMed] [Google Scholar]
- 16.Wolf C, Chevy F, Pham J, Kolf-Clauw M, Citadelle D, Mulliez N, Roux C. Changes in serum sterols of rats treated with 7-dehydrocholesterol-delta 7-reductase inhibitors: comparison to levels in humans with Smith-Lemli-Opitz syndrome. J Lipid Res. 1996;37(6):1325–1333. [PubMed] [Google Scholar]
- 17.Fliesler SJ, Vaughan DK, Jenewein EC, Richards MJ, Nagel BA, Peachey NS. Partial rescue of retinal function and sterol steady-state in a rat model of Smith-Lemli-Opitz syndrome. Pediatr Res. 2007;61(3):273–278. doi: 10.1203/pdr.0b013e318030d1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller RK, Vilsaint F. Regulation of isoprenoid metabolism in rat liver: near constant chain lengths of dolichyl phosphate and ubiquinone are maintained during greatly altered rates of cholesterogenesis. Biochim Biophys Acta. 1993;1170(2):204–210. doi: 10.1016/0005-2760(93)90072-h. [DOI] [PubMed] [Google Scholar]
- 19.Chambers CM, McLean MP, Ness GC. Smith-Lemli-Opitz syndrome produced in rats with AY 9944 treated by intravenous injection of lipoprotein cholesterol. Am J Med Genet. 1997;68(3):322–327. doi: 10.1002/(sici)1096-8628(19970131)68:3<322::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Salen G, Shefer S, Ness GC, Chen TS, Zhao Z, Tint GS. Reproducing abnormal cholesterol biosynthesis as seen in the Smith-Lemli-Opitz syndrome by inhibiting the conversion of 7-dehydrocholesterol to cholesterol in rats. J Clin Invest. 1995;95(1):76–81. doi: 10.1172/JCI117678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempen HJ, Glatz JF, Gevers Leuven JA, van der Voort HA, Katan MB. Serum lathosterol concentration is an indicator of whole-body cholesterol synthesis in humans. J Lipid Res. 1988;29(9):1149–1155. [PubMed] [Google Scholar]
- 22.Honda M, Tint GS, Honda A, Salen G, Shefer S, Batta AK, Matsuzaki Y, Tanaka N. Regulation of cholesterol biosynthetic pathway in patients with the Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2000;23(5):464–474. doi: 10.1023/a:1005660130109. [DOI] [PubMed] [Google Scholar]
- 23.Tint GS, Seller M, Hughes-Benzie R, Batta AK, Shefer S, Genest D, Irons M, Elias E, Salen G. Markedly increased tissue concentrations of 7-dehydrocholesterol combined with low levels of cholesterol are characteristic of the Smith-Lemli-Opitz syndrome. J Lipid Res. 1995;36(1):89–95. [PubMed] [Google Scholar]
- 24.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issandou M, Guillard R, Boullay AB, Linhart V, Lopez-Perez E. Up-regulation of low-density lipoprotein receptor in human hepatocytes is induced by sequestration of free cholesterol in the endosomal/lysosomal compartment. Biochem Pharmacol. 2004;67(12):2281–2289. doi: 10.1016/j.bcp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Maziere JC, Maziere C, Mora L, Gardette J, Wolf C, Rainteau D, Barbu V, Roux C, Polonovski J. Effects of AY 9944 on low density lipoprotein metabolism in cultured human fibroblasts. Biochem Biophys Res Commun. 1984;122(3):955–959. doi: 10.1016/0006-291x(84)91184-7. [DOI] [PubMed] [Google Scholar]
- 27.Ness GC, Lopez D, Borrego O, Gilbert-Barness E. Increased expression of low-density lipoprotein receptors in a Smith-Lemli-Opitz infant with elevated bilirubin levels. Am J Med Genet. 1997;68(3):294–299. doi: 10.1002/(sici)1096-8628(19970131)68:3<294::aid-ajmg9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Dvornik D, Hill P. Effect of long-term administration of AY-9944, an inhibitor of 7-dehydrocholesterol delta 7-reductase, on serum and tissue lipids in the rat. J Lipid Res. 1968;9(5):587–595. [PubMed] [Google Scholar]
- 29.Greselin E. An inhibitor of cholesterol biosynthesis and the alveolar macrophages. Can J Comp Med Vet Sci. 1966;30(5):121–126. [PMC free article] [PubMed] [Google Scholar]
- 30.Hruban Z. Pulmonary and generalized lysosomal storage induced by amphiphilic drugs. Environ Health Perspect. 1984;55:53–76. doi: 10.1289/ehp.845553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naito M, Hasegawa G, Ebe Y, Yamamoto T. Differentiation and function of Kupffer cells. Med Electron Microsc. 2004;37(1):16–28. doi: 10.1007/s00795-003-0228-x. [DOI] [PubMed] [Google Scholar]
- 32.Burg JS, Espenshade PJ. Regulation of HMG-CoA reductase in mammals and yeast. Prog Lipid Res. 2011;50(4):403–410. doi: 10.1016/j.plipres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miettinen TA, Gylling H, Nissinen MJ. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr Metab Cardiovasc Dis. 2011;21(10):765–769. doi: 10.1016/j.numecd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Simonen P, Gylling H, Miettinen TA. The validity of serum squalene and non-cholesterol sterols as surrogate markers of cholesterol synthesis and absorption in type 2 diabetes. Atherosclerosis. 2008;197(2):883–888. doi: 10.1016/j.atherosclerosis.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Loud AV, Bucher NL. The turnover of squalene in relation to the biosynthesis of cholesterol. J Biol Chem. 1958;233(1):37–41. [PubMed] [Google Scholar]
- 36.Sakakihara Y, Kamoshita S. Partition of free dolichol in human urine. Biochem Cell Biol. 1992;70(6):518–521. doi: 10.1139/o92-080. [DOI] [PubMed] [Google Scholar]
- 37.Humaloja K, Roine RP, Salmela K, Halmesmaki E, Jokelainen K, Salaspuro M. Serum dolichols in different clinical conditions. Scand J Clin Lab Invest. 1991;51(8):705–709. doi: 10.3109/00365519109104584. [DOI] [PubMed] [Google Scholar]
- 38.Keller RK. The mechanism and regulation of dolichyl phosphate biosynthesis in rat liver. J Biol Chem. 1986;261(26):12053–12059. [PubMed] [Google Scholar]
- 39.Sagami H, Lennarz WJ, Ogura K. The biosynthesis of dehydrodolichyl phosphates by rat liver microsomes. Biochim Biophys Acta. 1989;2:218–224. doi: 10.1016/0005-2760(89)90290-7. [DOI] [PubMed] [Google Scholar]
- 40.Keller RK. Squalene synthase inhibition alters metabolism of nonsterols in rat liver. Biochim Biophys Acta. 1996;1303(3):169–179. doi: 10.1016/0005-2760(96)00081-1. [DOI] [PubMed] [Google Scholar]
- 41.Haeuptle MA, Welti M, Troxler H, Hulsmeier AJ, Imbach T, Hennet T. Improvement of dolichol-linked oligosaccharide biosynthesis by the squalene synthase inhibitor zaragozic acid. J Biol Chem. 2011;286(8):6085–6091. doi: 10.1074/jbc.M110.165795. [DOI] [PMC free article] [PubMed] [Google Scholar]