Abstract
Bone responds to supraphysiological mechanical loads by increasing bone formation. Depending on the applied strain magnitude (and other loading parameters) the response can be either adaptive (mostly lamellar bone) or injury (mostly woven bone). Seminal studies of Hert, Lanyon and Rubin originally established the basic 'rules' of bone mechanosensitivity. These were reinforced by subsequent studies using noninvasive rodent loading models, most notably by Turner et al. More recent works with these models have been able to explore the structural, transcriptional and molecular mechanisms which distinguish the two responses (lamellar vs woven). Wnt/Lrp signaling has emerged as a key mechanoresponsive pathway for lamellar bone. However, there is still much to study with regard to effects of ageing, osteocytes, other signaling pathways, and the molecular regulation that modulates lamellar vs woven bone formation. This review summarizes not only the historical findings but also the current data for these topics.
Introduction
The topic of this review is how bone responds to increased mechanical loading. Functional bone adaptation, the relationship linking mechanical loading and bone structure, was recognized by Roux and Wolff well over a century ago.1 However, only since the 1970s and 1980s have advances in animal models and strain measurement techniques allowed researchers to explore this relationship with a controlled experimental approach. The key experimental models for advancing this field have been ones in which controlled external forces are applied to the skeleton of a live animal, and the local mechanical strains engendered by said forces are known. The primary functional outcomes are changes in local bone structure or dynamic indices of bone formation. Generally speaking, when the intensity of applied loading is greater than habitual loading, a bone formation (modeling) response is produced. Recent advances in biological factor detection (for example, gene expression) and genetic manipulation (for example, knockout mice) have facilitated examination of the biological mechanisms underlying the relationship between loading and bone.
An important distinction in all these studies is whether the loading stimulus engenders a lamellar or woven bone response. Increased lamellar bone formation may be considered an adaptive response to mild/moderate overloading, whereas woven bone formation may be considered an overloading/injury response. Although an appreciation of the lamellar-woven dichotomy is not new, it is unfortunate that many reports do not show histology or even state which type of bone formation was stimulated.
Our objectives in this article are to briefly summarize key 'classic' findings related to bone and mechanical loading, and to review recent work on lamellar vs woven bone formation, aging and mechanisms by which bone responds to loading. We focus on increased loading, and do not address disuse/unloading. Moreover, although a wealth of knowledge has been gained from in vitro and in silico experiments, as well as in vivo exercise experiments, their inclusion is beyond the scope of this brief review. We have chosen to mention only results from in vivo animal experiments that used controlled loading parameters.
Key early findings
Seminal studies from Hert, Lanyon and Rubin led to the over arching paradigms of cortical bone mechanoresponsiveness. The following 'rules' relating mechanical loading and cortical bone formation are widely accepted. First, dynamic loading elicits a response but static loading does not.2,3 Second, there exists a minimum strain threshold. Applied loads that produce strains below this threshold induce no change in bone formation whereas loads above this threshold increase bone formation in a dose-dependent manner.4 The exact magnitude of the threshold is context-dependent and may vary based on factors such as species, age, sex and loading model. Third, the anabolic effects of adaptive loading plateau after a relatively low number of cycles (<100 cycles per day).5
Trabecular bone has received less attention because most loading models target cortical bone only. However, Chambers et al.6 developed a vertebral compression model in the 1990s, and several groups have used this model in recent years.7 Similar to cortical bone there is reported to be a context-dependent minimum force threshold for trabecular bone formation and a linear increase above the threshold.8 In contrast to cortical bone, there is conflicting/insufficient evidence as to whether static forces are effective and whether there is a plateau effect with respect to cycles and trabecular bone formation.
Noninvasive loading models
A limitation of the models used for the studies cited above is that they were surgically invasive, and thus may have introduced unintended side effects related to inflammation and soft tissue damage, as well as making them technically challenging.2,3,4,5,6,7,8 To address these concerns noninvasive loading models have been developed and implemented in rodents (Table 1). For more extensive descriptions of both invasive and noninvasive loading models see Robling et al.18 Results from the noninvasive models provided further support for the three rules described above.19,20,21,22 The noninvasive models have also facilitated investigation of other loading variables, given insights into what mechanisms differentiate adaptive vs injury responses (that is, lamellar vs woven bone), and provided evidence supporting the role of osteocytes as load-responsive cells. We have focused the remainder of this review on studies utilizing rats and mice because a multitude of reagents for and genetic manipulations of these animals are currently available. Furthermore, we excluded some studies where the type of bone formed was not adequately documented.
Table 1. Summary of the three noninvasive loading models.
| Loading model | Limb tested | Stimulated bone types | Animals used | Physiological? | Complications | References |
|---|---|---|---|---|---|---|
| Four-point bending | Tibia | Cortical | Rat and mouse | No | Woven bone at loading points | Akhter et al.9; Silva and Brodt10 |
| Cantilever bending | Tibia | Cortical | Mouse | No | Limited strain range | Gross et al.11 |
| Axial compression | Tibia | Cortical and trabecular | Mouse | Yes | De Souza et al.12 Fritton et al.13 | |
| Fibula | Mouse | Moustafa et al.14 | ||||
| Ulna | Rat and mouse | Kotha et al.15; Lee et al.16; Torrance et al.17 |
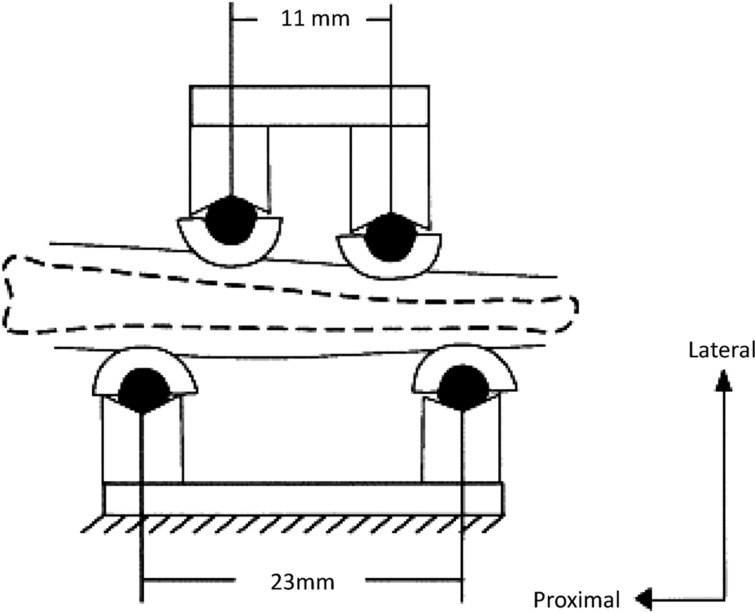
A noninvasive four-point bending model was created by Turner et al.23 for use with rats, and later adapted for use with mice9 (Figure 1). The tibia rests on two stationary pads; two movable pads apply a transverse force to the lateral side of the limb such that a bending moment is created in the central portion of the diaphysis. Features of this loading method are that a defined strain gradient can be produced in cortical bone over a known area and that loading is applied in a non-physiological direction. The main limitation of this model is that direct pressure on the periosteal surfaces and overlying soft tissues often triggers a woven bone response, which occurs as an 'all or nothing' phenomenon,19 suggesting it is not proportional to the loading stimulus. The pressure-induced bone formation complicates interpretation of periosteal results19,10 especially in smaller animals such as mice. For this reason, use of this model has declined and we do not recommend it.
Figure 1.
Schematic of four-point tibial bending. Bottom fixed points support the leg; top contact points displace downward. Reprinted from Turner et al.79 with permission.
Another model that generates tibial bending is the cantilever model developed by Gross et al.11 for use in mice. In this model, the knee is held rigid while a transverse load is applied to the distal end of the tibia, generating strains large enough to induce a periosteal bone formation response. A practical limitation of this model is that it is difficult to grip the knee and thus the peak force (and strain) is limited in magnitude and does not stimulate an endocortical response. Like the four-point bending model, the loading mode (direction) is non-physiological, which means that the applied loads generate a strain stimulus that is non-habitual in distribution/direction. It is unclear how the response to this novel stimulus differs from the response when loads are applied in a habitual direction, although these differences are likely to be in the degree of response rather than its fundamental nature.
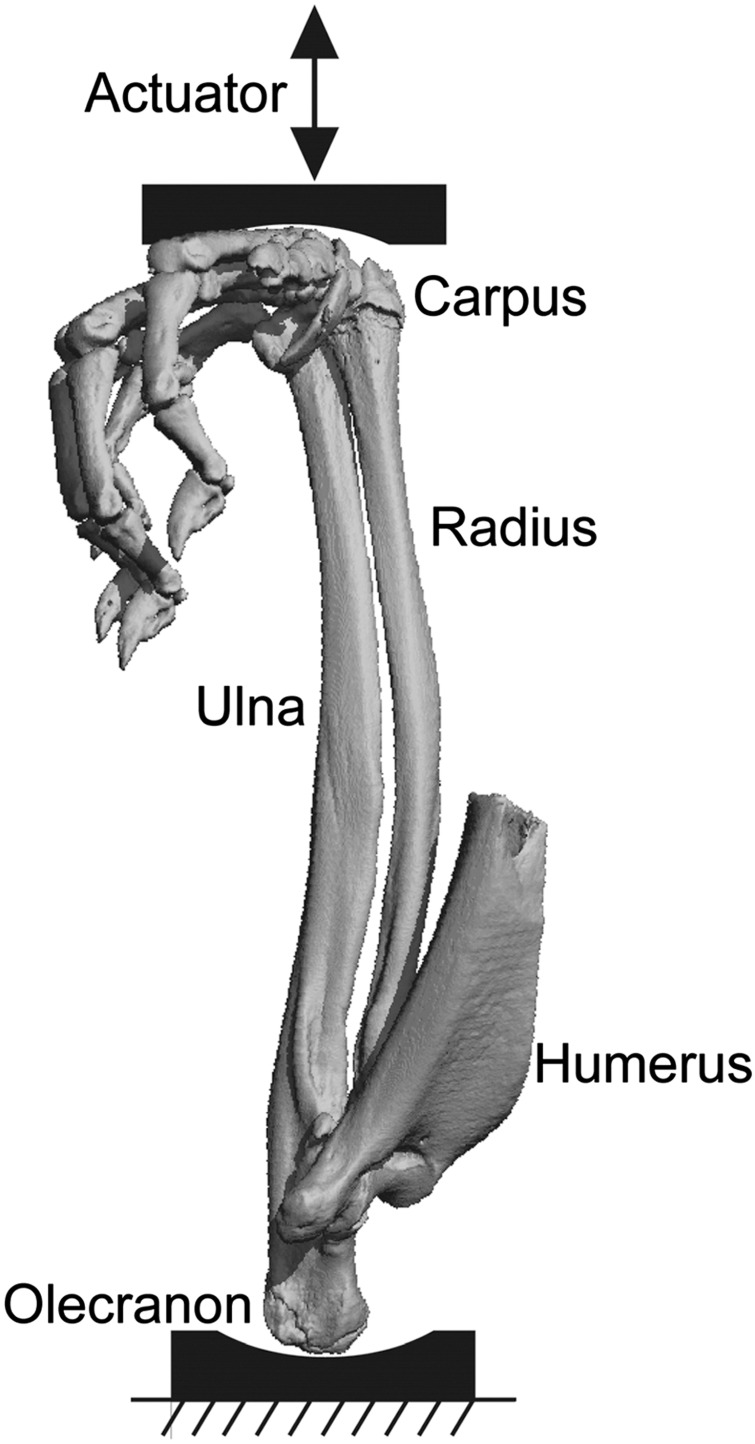
Another noninvasive loading approach, axial compression, has become the gold standard for studying mechanically induced bone formation in rats and mice. Axial compression is meant to mimic physiological loading through the joints. Its first application by Torrance et al.17 on rat forelimbs successfully utilized the natural curvature of the ulna to create bone-stimulating strain on the medial and lateral periosteal surfaces without soft tissue damage or ectopic periosteal reaction (Figure 2). Approximately two-thirds of the total forelimb force is carried by the ulna.15 The forelimb compression model has since been applied to mouse ulna.16 In both mice and rats, forelimb compression is used to study cortical responses in the central diaphysis of the ulna (although the radius could be examined as well).
Figure 2.
Micro computed tomography of rat forelimb positioned for axial compression loading. The olecranon sits in a fixed cup, whereas the flexed wrist is displaced downward with each load cycle. Reprinted from Uthgenannt et al.36 with permission.
The axial compression approach was later developed for the mouse hindlimb, with a focus on the tibial response12,13 (although the fibular response can also be examined14). The greatest bone formation is on the periosteal surface experiencing maximum strain, although depending on the force applied, endocortical responses are also seen. Axial compression of the tibia also permits examination of the trabecular bone response because loads are transmitted through the proximal metaphysis. We are not aware of axial tibial compression being used in rats.
Key mechanical parameters affecting the loading response
For all noninvasive models three independent parameters of dynamic loading are commonly modulated, each of which may influence the amount of new bone formation: magnitude, frequency and rest-insertion.
Magnitude refers to the peak applied force or strain, as they are linearly coupled (that is, increasing loading force linearly increases strain). Typically, peak strain is controlled as this tightly correlates to the amount of bone production.22,24,25 Also, application of the same force magnitude to all animals may result in different bone strains across experimental groups, depending on differences in bone geometry and material properties. However, once the force required to produce a target strain is established for an individual animal, the same force can safely be applied to species-, sex-, age-, weight- and genotype-matched animals and can be assumed to impart similar strains. For details on this procedure see Saxon and Lanyon.26 The range of strain magnitudes that stimulate a bone formation response depends on the animal variables listed above as well as the other loading variables. Generally, local peak strains in the range 1200–2000 microstrain (μɛ) have been shown to elicit a lamellar bone formation response.22,26 Turner et al.19 noted a switch from lamellar to woven when the applied peak force (and hence peak strain) exceeded a threshold, although the particular strain threshold they reported (1900 μɛ) was for the rat tibial bending model and may not apply to other models. An important caveat in all loading studies is that the strain magnitude is only controlled for at the start of the study. Over time, as adaptation occurs, the values of strain may differ.
The second parameter, frequency, also has a threshold response. Similar to static loading, very low frequencies (<1 Hz) produce little response. With increasing frequency bone formation increases until a peak is reached around 10–20 Hz.17,27,28,29 There is some evidence that frequencies over ∼20 Hz are dampened by the overlying soft tissues and joints, thus resulting in lower applied strain on the bone surface and no additional benefit.27 A frequency of 2–4 Hz is commonly used, which matches the range of stride frequency reported for rats during locomotion.24
The third parameter, rest insertion, is based on the observation that increased lamellar bone formation in response to loading was saturated after relatively few cycles.5 Essentially, cells become desensitized to any additional stimulation. Two methods of restoring mechanosensitivity are to either break the total cycles up into shorter bouts separated by several hours25,30 or insert a short (∼10 s) rest in between each cycle.31 For example, Robling et al.25 saw a >50% increase in bone formation if 360 cycles of axial compressive loading were divided into four 90-cycle bouts separated by as few as 3 h rather than administered in one bout. Likewise, Srinivasan et al.31 enhanced the response to tibial cantilever loading by adding a 10-s hold between each cycle.
Strain rate is another parameter shown to modulate bone formation.32,33 But if loading magnitude and frequency are prescribed, then strain rate is not an independent factor. In practice, it is difficult to decouple frequency and strain rate effects. Moreover, to achieve different strain rates while keeping number of cycles and total loading time similar across study groups, a dwell or rest has to be inserted between cycles. The dwell effectively mimics a rest insertion and introduces another variable.
Depending on the combination of the three independent loading parameters and the end point (for example, number of cycles, loss of stiffness and increase in displacement) you can not only 'switch on' quiescent bone cells in as little as one bout of mechanical loading but also control whether the response is lamellar or woven bone.34 The concept of a single bout being sufficient to induce lamellar bone formation was established by Forwood et al.35 They noted that new bone formation is histologically evident 5–8 days after a bout of loading, and that subsequent loading bouts incrementally increase the magnitude of the bone formation response in a 'quantum' manner, a finding consistent with the later rest insertion studies.
Damage as a stimulus for woven bone formation and intracortical remodeling
Woven bone formation is triggered under a variety of loading conditions. It may occur after relatively few loading cycles when the applied strain magnitude exceeds some threshold;19 it is unclear if this reflects a damage response or an extreme on the strain-adaptive continuum. More clearly, under conditions where loading produces discrete bone damage (for example, a stress fracture) robust woven bone formation ensues.34,36,37,38,39 Although it looks disorganized, woven bone formation is a well-regulated response to certain extreme conditions. A woven bone response occurs when there is a 'need' to accrue bone at a faster rate than can be accomplished by lamellar bone formation.
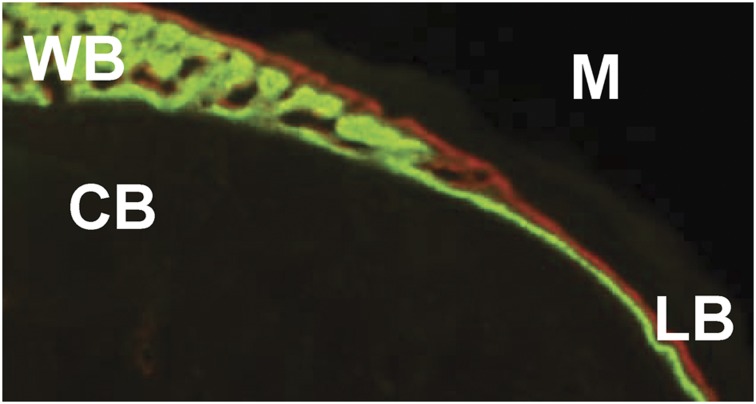
Despite the obvious differences in histological organization, lamellar and woven bone can be forming at the same time on contiguous segments of the bone surface. To estimate the maximal rate for lamellar bone apposition, we analyzed bone sections where a lamellar–woven transition was evident (Figure 3). Transverse, undecalcified sections (n=100) were obtained from a previous study in which damaging fatigue loading of the rat ulna was used to stimulate woven bone formation.36 Samples were included from different timepoints, damage levels and longitudinal locations. The average mineral apposition rate for lamellar bone just adjacent to woven bone was 3.5±1.1 μm per day (data not previously reported). This 'upper bound' for lamellar mineral apposition rate likely depends on a number of factors, but these observations indicate that there is a maximal rate at which a single team of osteoblasts can deposit lamellar bone.
Figure 3.
Woven–lamellar transition. Transverse section of the periosteal surface of a rat ulna, collected 14 days after fatigue loading.36 Calcein green was administered on day 7 and alizarin red on day 12.CB, original cortical bone; LB, new lamellar bone; M, muscle; WB, new woven bone.
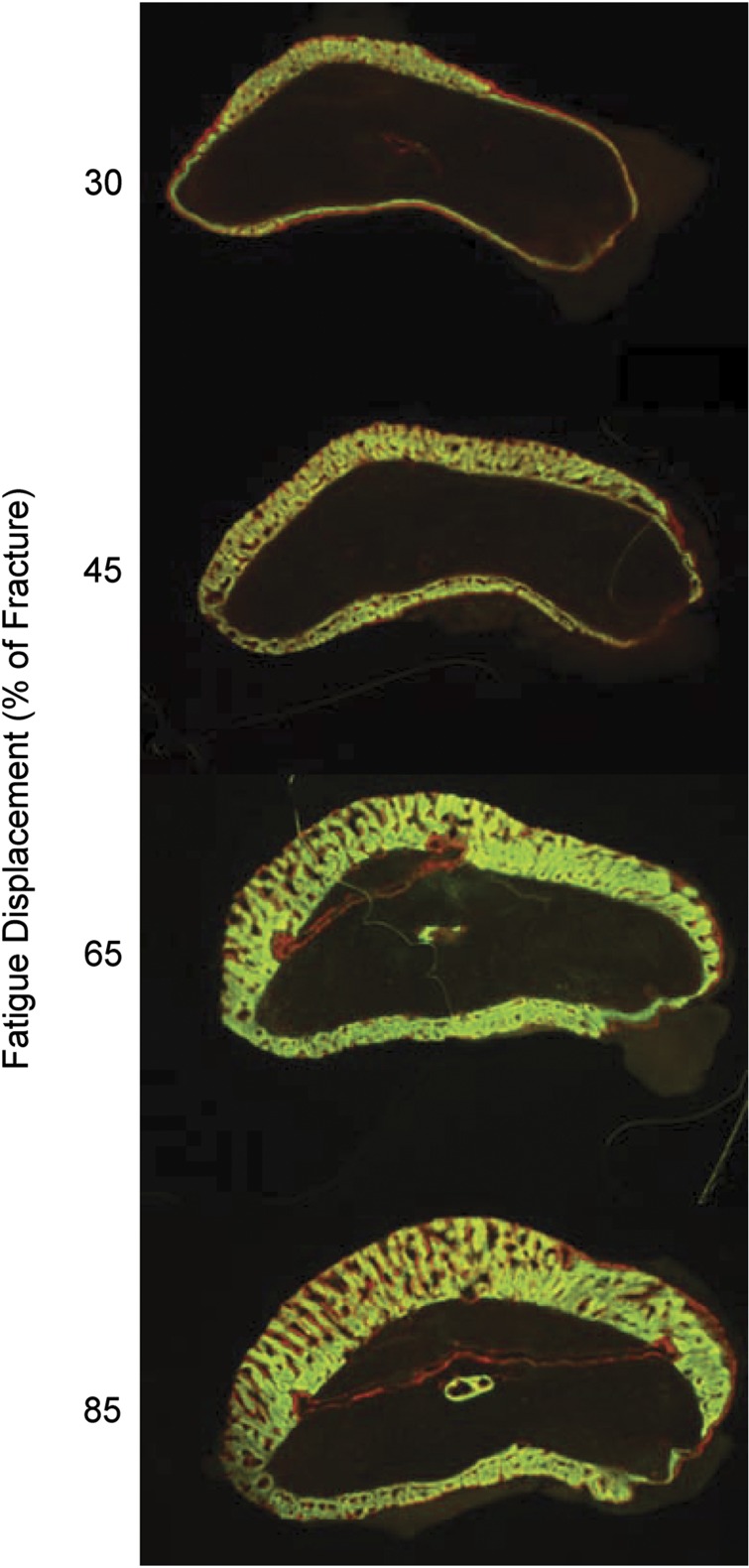
To better understand bone's responses to damaging loading, the ulnar axial loading protocol of Torrance et al.17 was adapted to create a model of controlled, fatigue damage.37,38,40 Basically, by cyclically loading the forelimb until a certain loss of stiffness or increase in displacement is reached, one can control the amount of bone damage (for example, loss of strength, micro- and macro-cracks) and examine both the periosteal modeling (formation) response and the intracortical remodeling response. Using this model, we have shown that the amount of periosteal woven bone formation directly parallels the amount of induced fatigue damage36 (Figure 4). In this setting, woven bone formation is clearly not an 'all or nothing' response but is well modulated in location and extent. Additional support for the concept that bone damage induces a proportional woven bone response came from a follow-up experiment.41 In this study, creep loading (that is, progressive displacement under a static force) was applied until prescribed levels of bone damage were produced. Even in the absence of dynamic loading, damage alone resulted in significant woven bone formation in a dose-dependent manner.
Figure 4.
Woven bone-dose response. Transverse sections of the periosteal surface of rat ulnas, collected 14 days a\fter fatigue loading. Calcein green was administered on day 7 and alizarin red on day 12.The amount of periosteal woven bone increases with increasing bone damage. Damage is controlled by the applied fatigue displacement. Modified from Uthgenannt et al.36 with permission.
Apart from the periosteal response, microdamage produced by fatigue loading triggers intracortical osteoclastic remodeling.37,39,40 Kennedy et al.42 have demonstrated that microcracks cause local osteocyte apoptosis and that the adjacent, non-apoptotic osteocytes produce osteoclastogenic factors such as RANKL. Additional support for this mechanism of targeted bone remodeling came from experiments wherein apoptosis was inhibited and local resorption was diminished.43 Recently Herman et al.44 demonstrated that damage severity governs osteocyte apoptosis, osteoclast recruitment and resorption. Increased apoptosis and osteoclast resorption were found in the areas containing and proximal to microcracks, but not areas with less severe (diffuse) damage.
Effect of aging
Does age alter the mechanoresponsiveness of the skeleton? If so, this may be one factor contributing to age-related bone loss. However, the few published studies that directly address this question have not reached consensus (Table 2). Two earlier studies reported that aged animals had a negligible response to loading protocols that were previously shown to be strongly anabolic in younger animals.45,46 However, these studies used loading models with some of the previously noted limitations (invasive,45 periosteal contact46). More recently, Srinivasan et al.47,48 reported that aged (22 month) C57Bl/6 mice have a markedly diminished periosteal response to cantilever tibial bending compared with young–adult (4 month) mice loaded to the same peak periosteal strain. We have examined this question using axial tibial compression in BALB/c mice at different ages, with strain matching across the age groups. We recently reported that 4-month-old mice added more cortical bone than 2-, 7- and 12-month-old mice after 6 weeks of daily tibial loading.49 Interestingly, loading increased the expression of osteogenic genes in older mice, offsetting the normal declines that occurred with aging (Figure 5). In a separate experiment comparing 7-month- (adult) vs 22-month-(old) mice, we found no deficit in the bone formation response to loading in the old mice.50
Table 2. In vivo studies comparing young and old animals using direct loading models.
| Authors | Loading model | Animals | Age | Findings |
|---|---|---|---|---|
| Rubin et al.45 | Isolated ulnar compression | Turkey | 1 and 3 years | Aged animals unresponsive to protocol that is anabolic in younger animals |
| Turner et al.46 | Tibial four-point bending | Rat | 9 and 19 months | Markedly diminished endocortical response in older animals |
| Srinivasan et al.47,48 | Tibial cantilever bending | C57Bl/6 mouse | 4 and 22 months | Diminished response in older animals; rescued by concurrent Cyclosporin A treatment |
| Silva et al.49 | Tibial axial compression | BALB/c mouse | 2, 4, 7 and 12 months | All ages responsive; bone accrual moderately greater at 4 months |
| Brodt and Silva50 | Tibial axial compression | BALB/c mouse | 7 and 22 months | Aged animals have equivalent periosteal response, greater endocortical response |
Figure 5.
Relative expression of type I collagen mRNA in tibial samples from control and loaded limbs of mice aged 2–12 months. Right hindlimbs were loaded daily by axial tibial compression for 1 week. With aging there is a natural decline in expression of this bone matrix gene (relative to the reference gene cyclophilin), indicating reduced bone formation. Mechanical loading increases expression in older animals, offsetting the age-related decline. (Modified from Silva et al.49) *loaded vs control, P<0.05.
Based on available evidence, our current view is that under some conditions old animals can respond robustly to loading with a re-activation of bone modeling. Although the magnitude of the response may be diminished compared with younger animals, old bones are clearly mechanoresponsive. Additional work is needed to better define the loading and animal factors that might contribute to age effects, and to determine a mechanistic basis for any differences between young and old animals.
Osteocytes as mechanosensors
Osteocytes are widely believed to be the primary mechanosensing cell in bone. Their abundance and interconnectivity make them prime candidates for this function, although direct in vivo evidence is still quite limited.51,52 Tatsumi et al.53 reported that mice in which osteocytes were acutely ablated were relatively resistant to bone loss with hindlimb unloading, although the absence of osteocytes did not affect bone gain during reloading. Recently, Kwon et al.54 used the same transgenic mice and reported diminished loss of cortical bone with unloading when compared with wild type. However, when mice with osteocyte ablation were subjected to an anabolic, intramedullary pressure stimulus, they had no deficit in their response. We are unaware of comparable experiments performed using a direct loading model (for example, axial compression). Perhaps the best evidence for the role of osteocytes in mechanoresponse comes from studies on the role of osteocytes in Wnt signaling (reviewed below).
It is thought that osteocytes indirectly sense strain via the increased fluid movement through the lacunar/canalicular system that occurs when pressure gradients are created by functional loading, although it is also possible that osteocytes sense the bone strain directly.51,52 Although not the focus of this review, a wealth of in vitro information exists that attests to the mechanosensitivity of osteocytes to fluid movement and substrate strain. For recent and detailed reviews see Chen et al.51 and Jacobs et al.52
Owing to the tight correlation between local strains experienced by osteocytes and formation of new bone, conventional wisdom says that osteocyte signaling remains local. Thus, adaptation is limited to the highly strained regions of loaded bones. However, arguments have arisen suggesting a systemic response to loading regulated by the nervous system whereby loading stimulates neuronal signaling causing systemic bone formation and also affecting local bone formation.55,56 Although there is compelling evidence to support this claim, it has only been shown in the setting of loading-induced woven bone formation, which, as will be discussed below, involves many more biological processes and pathways than lamellar bone formation. Furthermore, this claim has been specifically refuted by other studies.34,57 The importance of systemic effects in loading-induced bone formation remains to be determined, although most evidence indicates that local effects dominate the response.
Mechanoresponsive pathways
Early efforts to identify the pathways activated by mechanical loading focused on prostaglandin E2,58 nitric oxide59 and cyclooxygenase-2.60 All three factors were noted to be released within minutes following loading, and pharmacological inhibition diminished bone formation. Many in vitro and ex vivo studies have supported these findings and investigated other pathways (for example, Papachristou et al.61). However, in vitro and ex vivo studies will never truly replicate the complex in vivo scenario. Unfortunately, interpretation of many early in vivo studies is challenging because they failed to clearly differentiate between lamellar and woven bone formation.
Recent technical advances provide many opportunities for extending our knowledge of in vivo bone mechanobiology. Quantitative PCR and microarray technologies allow extensive probing of gene responses, while genetically modified mice allow studies into biological mechanism. In vivo microarray studies have shown that during lamellar bone formation, genes relating to cell signaling, movement, proliferation and metabolism have a modest peak in transcriptional activity shortly after loading (4–8 h),62,63,64 most of which return to basal levels by 24 h.63,64,65 Somewhat surprisingly, another peak was reported to occur around 12–16 days for genes related to solute carrying, matrix production, transforming growth factor-β signaling and Wnt/β-catenin signaling.64 The importance of estrogen signaling in mechanoresponsiveness of bone has also been established by a number of in vivo studies (recently reviewed elsewhere Melville et al.66). A key result is that loss of circulating estrogen does not appear to strongly alter loading responses, whereas loss of signaling through the estrogen receptor alpha diminishes responses to loading.
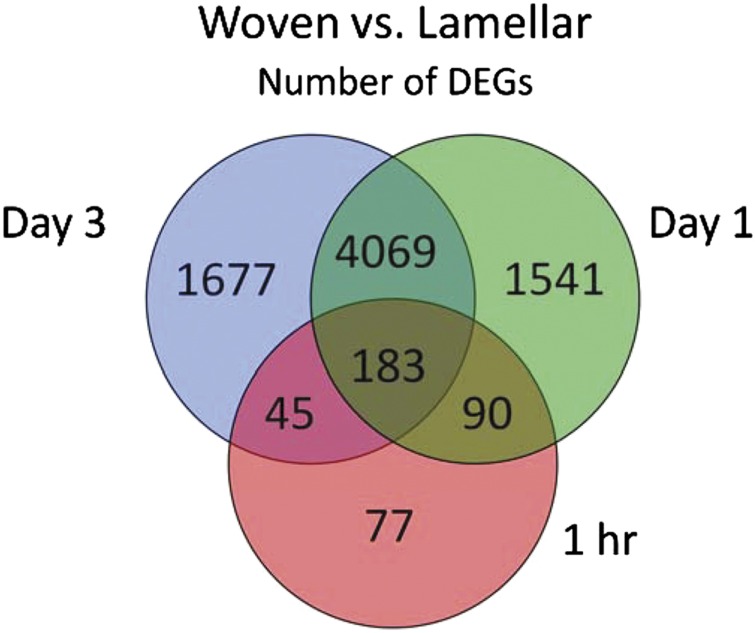
Compared with lamellar bone formation, the number of differentially regulated genes occurring in the context of loading-induced woven bone formation is markedly greater.65 Inflammation, cytoskeletal remodeling, cell adhesion and developmental pathways are all affected, with inflammatory genes being particularly notable 1 and 24 h after loading (Figure 6). Angiogenesis occurs with woven but not lamellar bone formation.34 Processes associated with injury and anabolism are dominant early after damaging loading, while the expression of factors related to bone remodeling/resorption are activated later, showing increases from 1 to 7 days.34,39,65,67
Figure 6.
Gene transcription profiles are greatly different for woven bone formation compared with lamellar bone formation. Venn diagram shows the number of differentially expressed genes (DEGs) in woven vs lamellar loading groups based on microarray analysis of rat ulnae collected 1 h, 1 and 3 days after mechanical loading. From McKenzie et al.65
Importance of wnt/lrp5 pathway
The WNT/Lrp pathway has emerged as a key regulator of skeletal anabolism, and has been implicated as an important mechanoresponsive pathway in bone (reviewed in Bonewald and Johnson68). In lamellar bone formation, WNT signaling has recently attracted attention due to: (1) the complete lack of response to loading in mice lacking LRP5 (a key receptor of WNT ligands)69 and (2) an increased, dose-dependent response in constitutively active LRP5 mice.70 Sclerostin, the protein product of the Sost gene, is an LRP5 antagonist and has been identified as mechanoresponsive. Sost expression in osteocytes decreases with increased mechanical strain71,72,73 Alternatively, if SOST levels cannot decrease, which has been accomplished in mice by periostin knockout72 or SOST over expression,73 new lamellar bone will not form.
The effect of WNT/Lrp signaling on bone formation is thought to be through downstream effects on β-catenin. Canonical WNT signaling blocks β-catenin degradation allowing increased translocation to the nucleus and transcription of osteogenic target genes. But, in vitro assays have shown multiple ways to affect β-catenin levels without modulating WNT signaling.74 More recently, in vivo knockout of factors such as Stat3,75 midkine76 and HIF-1 α77 have significantly modulated load-induced lamellar bone formation. It is hypothesized that each of these factors affects β-catenin levels through non-WNT mechanisms.
The role of WNT/Lrp/β-catenin signaling on bone responses to damaging loading remains to be determined. One recent study has shown a decrease in osteocytic sclerostin levels in bones loaded with a protocol that induced woven bone formation.78 Lastly, we reported marked downregulation of Sost expression after damaging fatigue loading and before woven bone formation,65 suggesting that osteocytes might be orchestrating the woven bone response to bone damage thru the Wnt/Lrp pathway.
Conclusions
In conclusion, several loading models can be used to apply precisely controlled mechanical loads to bone to study adaptive and injury responses. Noninvasive models are necessary to study the molecular mechanisms and resulting gene regulation. Three such models have been developed and are commonly used: four point bending, cantilever bending and axial compression. All three types of loading stimulate osteogenesis, but not all mimic physiological loading and thus may cause off target effects. Furthermore, all the models are limited to appendicular long bones. This precludes or limits the study of mechanical loading on flat bones and trabecular bone. The most physiological model is compressive axial loading, which can be applied to either the ulna or the tibia. Depending on how parameters such as strain magnitude, cycle frequency, rest insertion and test end point are applied, the response outcome can be tuned from adaptive (mostly lamellar) to damage/injury (mostly woven). Strikingly, both are dose-dependent and can be provoked in as little as one bout of loading with few cycles.
The mechanisms elicited in lamellar and woven bone formation pathways are still not fully understood. Nonetheless, controlled in vivo studies of gene expression and knock out have indicated a role for cell signaling, cell metabolism and WNT/Lrp signaling in lamellar and woven bone formation, with the addition of inflammation and angiogenesis in woven bone formation. At this time, it is unclear if the importance of these pathways is universal to all loading-induced bone formation, or if different pathways will be more/less important as a function of loading method, animal age and other factors. Nonetheless, with current understanding of how to control formation outcomes and advancement of genetic models, researchers are poised to clarify known mechanisms and discover new ones.
Acknowledgments
The analysis of mineral apposition rate at the lamellar–woven transition was performed by Manolis Zampiakis, MD. This study is financially supported from the NIH/NIAMS (R01AR047867, R01AR050211 and P30AR057235). SHM is supported by NIH/NIAMS T32AR060719.
Footnotes
The authors declare no conflict of interest.
References
- Cowin S. The false premise of Wolff's law. In: Cowin SC (ed.) Bone Mechanics Handbook. CRC Press: Boca Raton,2001,pp 30-1–30-15. [Google Scholar]
- Hert J, Lisková M, Landa J. Reaction of bone to mechanical stimuli. 1. Continuous and intermittent loading of tibia in rabbit. Folia Morphol 1971;19:290–300. [PubMed] [Google Scholar]
- Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech 1984;17:897–905. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 1985;37:411–417. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 1984;66:397–402. [PubMed] [Google Scholar]
- Chambers TJ, Evans M, Gardner TN, Turner-Smith A, Chow JW. Induction of bone formation in rat tail vertebrae by mechanical loading. Bone Miner 1993;20:167–178. [DOI] [PubMed] [Google Scholar]
- Webster D, Wasserman E, Ehrbar M, Weber F, Bab I, Müller R. Mechanical loading of mouse caudal vertebrae increases trabecular and cortical bone mass-dependence on dose and genotype. Biomech Model Mechanobiol 2010;9:737–747. [DOI] [PubMed] [Google Scholar]
- Chow JW, Jagger CJ, Chambers TJ. Characterization of osteogenic response to mechanical stimulation in cancellous bone of rat caudal vertebrae. Am J Physiol 1993;265:E340–E347. [DOI] [PubMed] [Google Scholar]
- Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int 1998;63:442–449. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Brodt MD. Mechanical stimulation of bone formation is normal in the SAMP6 mouse. Calc Tissue Int 2008;82:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res 2002;17:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza RL, Matsuura M, Eckstein F, Rawlinson SCF, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 2005;37:810–818. [DOI] [PubMed] [Google Scholar]
- Fritton JC, Myers ER, Wright TM, van der Meulen MCH. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone 2005;36:1030–1038. [DOI] [PubMed] [Google Scholar]
- Moustafa A, Sugiyama T, Saxon LK, Zaman G, Sunters A, Armstrong VJ et al. The mouse fibula as a suitable bone for the study of functional adaptation to mechanical loading. Bone 2009;44:930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha SP, Hsieh Y-F, Strigel RM, Müller R, Silva MJ. Experimental and finite element analysis of the rat ulnar loading model-correlations between strain and bone formation following fatigue loading. J Biomech 2004;37:541–548. [DOI] [PubMed] [Google Scholar]
- Lee KCL, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone 2002;31:407–412. [DOI] [PubMed] [Google Scholar]
- Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int 1994;54:241–247. [DOI] [PubMed] [Google Scholar]
- Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact 2001;1:249–262. [PubMed] [Google Scholar]
- Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res 1994;9:87–97. [DOI] [PubMed] [Google Scholar]
- Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone 1998;23:399–407. [DOI] [PubMed] [Google Scholar]
- Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone 2001;29:105–113. [DOI] [PubMed] [Google Scholar]
- Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo: the strain threshold for an osteogenic response varies with location. J Bone Miner Res 2001;16:2291–2297. [DOI] [PubMed] [Google Scholar]
- Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling. Bone 1991;12:73–79. [DOI] [PubMed] [Google Scholar]
- Mosley JR, March BM, Lynch J, Lanyon LE. Strain magnitude related changes in whole bone architecture in growing rats. Bone 1997;20:191–198. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res 2002;17:1545–1554. [DOI] [PubMed] [Google Scholar]
- Saxon LK, Lanyon LE. Assessment of the in vivo adaptive response to mechanical loading. Meth Mol Biol 2008;455:307–322. [DOI] [PubMed] [Google Scholar]
- Hsieh YF, Wang T, Turner CH. Viscoelastic response of the rat loading model: implications for studies of strain-adaptive bone formation. Bone 1999;25:379–382. [DOI] [PubMed] [Google Scholar]
- Hsieh YF, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res 2001;16:918–924. [DOI] [PubMed] [Google Scholar]
- Warden SJ, Turner CH. Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5-10 Hz. Bone 2004;34:261–270. [DOI] [PubMed] [Google Scholar]
- Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol 2001;204:3389–3399. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res 2002;17:1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley JR, Lanyon LE. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone 1998;23:313–318. [DOI] [PubMed] [Google Scholar]
- LaMothe JM, Hamilton NH, Zernicke RF. Strain rate influences periosteal adaptation in mature bone. Med Eng Phys 2005;27:277–284. [DOI] [PubMed] [Google Scholar]
- McKenzie JA, Silva MJ. Comparing histological, vascular and molecular responses associated with woven and lamellar bone formation induced by mechanical loading in the rat ulna. Bone 2011;48:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood MR, Owan I, Takano Y, Turner CH. Increased bone formation in rat tibiae after a single short period of dynamic loading in vivo. Am J Physiol 1996;270:E419–E423. [DOI] [PubMed] [Google Scholar]
- Uthgenannt BA, Kramer MH, Hwu JA, Wopenka B, Silva MJ. Skeletal self-repair: stress fracture healing by rapid formation and densification of woven bone. J bone Miner Res 2007;22:1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone 1998;23:275–281. [DOI] [PubMed] [Google Scholar]
- Tami AE, Nasser P, Schaffler MB, Knothe Tate ML. Noninvasive fatigue fracture model of the rat ulna. J Ortho Res 2003;21:1018–1024. [DOI] [PubMed] [Google Scholar]
- Kidd LJ, Stephens AS, Kuliwaba JS, Fazzalari NL, Wu ACK, Forwood MR. Temporal pattern of gene expression and histology of stress fracture healing. Bone 2010;46:369–378. [DOI] [PubMed] [Google Scholar]
- Hsieh Y-F, Silva MJ. In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. J Orthop Res 2002;20:764–771. [DOI] [PubMed] [Google Scholar]
- Lynch JA, Silva MJ. In vivo static creep loading of the rat forelimb reduces ulnar structural properties at time-zero and induces damage-dependent woven bone formation. Bone 2008;42:942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 2012;50:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res 2009;24:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone 2010;47:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int 1992;50:306–313. [DOI] [PubMed] [Google Scholar]
- Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res 1995;10:1544–1549. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone 2003;33:946–955. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Ausk B, Prasad J, Threet D, Bain S, Richardson T et al. Rescuing loading induced bone formation at senescence. PLos Comput Biol 2010;6:e1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PloS One 2012;7:e34980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res 2010;25:2006–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-H, Liu C, You L, Simmons CA. Boning up on Wolff's Law: mechanical regulation of the cells that make and maintain bone. J Biomech 2010;43:108–118. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Ann Rev Biomed Eng 2010;12:369–400. [DOI] [PubMed] [Google Scholar]
- Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 2007;5:464–475. [DOI] [PubMed] [Google Scholar]
- Kwon R, Meays D, Meilan A, Jones J, Miramontes R, Kardos N et al. Skeletal adaptation to intramedullary pressure-induced interstitial fluid flow is enhanced in mice subjected to targeted osteocyte ablation. PLos One 2012;7:e33336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample SJ, Collins RJ, Wilson AP, Racette MA, Behan M, Markel MD et al. Systemic effects of ulna loading in male rats during functional adaptation. J Bone Miner Res 2010;25:2016–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample SJ, Hao Z, Wilson AP, Muir P. Role of calcitonin gene-related peptide in bone repair after cyclic fatigue loading. PloS One 2011;6:e20386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone 2010;46:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pead MJ, Lanyon LE. Indomethacin modulation of load-related stimulation of new bone formation in vivo. Calcif Tissue Int 1989;45:34–40. [DOI] [PubMed] [Google Scholar]
- Turner CH, Takano Y, Owan I, Murrell GA. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Physiol 1996;270:E634–E639. [DOI] [PubMed] [Google Scholar]
- Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res 1996;11:1688–1693. [DOI] [PubMed] [Google Scholar]
- Papachristou DJ, Papachroni KK, Basdra EK, Papavassiliou AG. Signaling networks and transcription factors regulating mechanotransduction in bone. BioEssays 2009;31:794–804. [DOI] [PubMed] [Google Scholar]
- Zaman G, Sunters A, Galea GL, Javaheri B, Saxon LK, Moustafa A et al. Loading-related regulation of transcription factor EGR2/Krox-20 in bone cells is ERK1/2 protein-mediated and prostaglandin, Wnt signaling pathway-, and insulin-like growth factor-I axis-dependent. J Biol Chem 2012;287:3946–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G, Saxon LK, Sunters A, Hilton H, Underhill P, Williams D et al. Loading-related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor alpha and disuse. Bone 2010;46:628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res 2011;26:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JA, Bixby EC, Silva MJ. Differential gene expression from microarray analysis distinguishes woven and lamellar bone formation in the rat ulna following mechanical loading. PloS One 2011;6:e29328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville K, Kelly N, van der Meulen M. Skeletal mechanoresponsiveness: effects of sex hormones. In: Silva MJ (ed). Skeletal Aging and Osteoporosis: Biomechanics and Mechanobiology. Springer-Verlag Berlin Heidelberg, 2012. [Google Scholar]
- Wohl GR, Towler DA, Silva MJ. Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair. Bone 2009;44:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone 2008;42:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 2006;281:23698–23711. [DOI] [PubMed] [Google Scholar]
- Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone 2011;49:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 2008;283:5866–5875. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ et al. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem 2009;284:35939–35950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2012;50:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case N, Rubin J. Beta-catenin—a supporting role in the skeleton. J Cell Biochem 2010;110:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Newnum AB, Martin JR, Li P, Nelson MT, Moh A et al. Osteoblast/osteocyte-specific inactivation of Stat3 decreases load-driven bone formation and accumulates reactive oxygen species. Bone 2011;49:404–411. [DOI] [PubMed] [Google Scholar]
- Liedert A, Mattausch L, Röntgen V, Blakytny R, Vogele D, Pahl M et al. Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone 2011;48:945–951. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-inducible factor-1α protein negatively regulates load-induced bone formation. J Biol Chem 2011;286:44449–44456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 2012;23:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CH, Owan I, Alvey T, Hulman J, Hock JM. Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone 1998;22:463–469. [DOI] [PubMed] [Google Scholar]