Abstract
Background
Naturally occurring, thymic-derived Foxp3+CD25+CD4+ regulatory T cells (natural Tregs) are pivotal for the maintenance of self-tolerance. Natural Tregs, however, are sparse and lack alloantigen specificity and these properties pose challenges for their use in clinical transplantation.
Methods
We established mixed leukocyte reaction (MLR) with dendritic cells (DCs) as stimulators and CD4+ T cells as responders and supplemented the MLR with IL-2 and TGF-β1, and investigated whether DCs+ IL-2 +TGF-β1 differentiate the polyclonal CD4+ cells into alloantigen specific and allograft protective Tregs.
Results
We found a greater than a ten-fold increase in Foxp3+ CD25+ subpopulation (P<0.01) following stimulation of BALB/c CD4+ cells with C57BL/6 (B6) CD11c+ DCs + IL-2 + TGF-β1 in the MLR. Levels of TGF-β1 mRNA (P=0.01) and the ratios of TGF-β1 mRNA to granzyme B mRNA (P=0.0003) and Foxp3 mRNA to granzyme B mRNA (P<0.01) were higher in alloantigen induced Tregs compared to natural Tregs. Alloantigen induced Tregs suppressed MLR at a 16:1 responder to suppressor ratio whereas natural Tregs suppressed at 4:1. Suppression by alloantigen induced Tregs was alloantigen specific and was observed at the level of responder cells and at the level of stimulator cells. In a fully H-2 mismatched, non-lymphopenic, immunocompetent mouse islet transplantation model, alloantigen induced Tregs but not natural Tregs prolonged survival of islet allografts without any other immunosuppressive therapy (P=0.0003), and the protection was alloantigen specific.
Conclusions
A combination of CD11c+ DCs, IL-2 and TGF-β1 may help differentiate naïve, high abundant CD4+ T in to alloantigen specific and allograft protective Foxp3 + Tregs.
Keywords: Dendritic cells, Foxp3, Islet cell transplantation, Regulatory T cells
INTRODUCTION
Naturally-occurring, thymus-derived Foxp3+CD25+CD4+ regulatory T cells (nTregs) have emerged as key regulators of immune homeostasis and are critical for the prevention of autoimmunity and maintenance of self-tolerance (1-2). Adoptive transfer of nTregs represents a strategy not only to constrain autoimmunity but also to reduce the need for immunosuppressive therapy in organ graft recipients (3). This approach, however, is challenged by the low abundance of nTregs in-vivo, lack of alloantigen specificity and the potential for global immunosuppression following adoptive transfer of polyclonally expanded nTregs.
We investigated whether the twin hurdles – the natural low frequency of nTregs and their lack of alloantigen specificity – could be overcome by developing further the demonstration by Yamazaki et al (4) that dendritic cells (DCs) plus IL-2 induce the expansion of alloantigen specific nTregs in MLR, and our observation that BDC peptide-pulsed DCs and TGF-β1 differentiate naïve FoxP3− CD25− CD4+ BDC2.5 TCR transgenic T cells into syngeneic islet protective Foxp3+CD25+CD4+ regulatory T cells (5). In the current investigation, we examined whether the use of high abundant CD4+ T cells as the responders and splenic DCs as the stimulators in MLR and supplementation of MLR with both IL-2 (6) and TGF-β1(7) results in the generation of alloantigen specific Tregs.
We report here that C57BL/6 (B6) DCs, IL-2 and TGF-β1 differentiate naïve polyclonal BALB/c CD4+ T cells into alloantigen specific and B6 islet allograft protective Foxp3+CD25+CD4+ regulatory T cells. Alloantigen induced Tregs shared several phenotypic features with nTregs but expressed TGF-β1 mRNA at a higher level and mRNA for granzyme B and pro-inflammatory cytokines at a lower level compared to nTregs. B6 DCs, IL-2 and TGF-β1 differentiated Tregs prolonged the survival of a fully H-2 mismatched B6 islet allograft in non-lymphopenic, immunocompetent BALB/c mice in the absence of any other immunosuppressive therapy, and the protection was alloantigen specific.
RESULTS
CD11c+ DCs, IL-2 and TGF-β1 differentiation of naïve CD4+ T cells into Foxp3+CD25+CD4+ T cells
We established primary MLR using polyclonal CD4+ T cells from wild type BALB/c mice as responders and B6 CD11c+ DCs as stimulators, and supplemented MLR with IL-2 and TGF-β1. The CD4+ T cells were enriched by depletion of non-CD4 T-cells from single cell suspension of spleen of BALB/c mice. In three consecutive experiments, the mean [±SE] percentage of CD4+ T cells was 94.6 ± 1.3%, and the percentage of CD25+Foxp3+ cells was 5.4 ± 0.5%. Figure 1A illustrates the CD4+ T cell enrichment, low abundance of FoxP3+CD25+ T cells, and the paucity of CD8+ cells in the cell population used as responders in the MLRs.
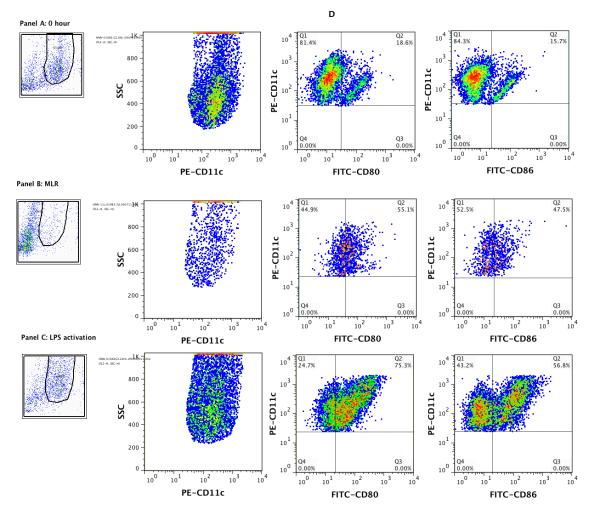
Figure 1. Flow cytometry analysis of freshly isolated BALB/c CD4+ T cells (A), B6 CD11c+ splenic DCs (B), and cells following co-culture of CD4+ T cells with B6 CD11c+ splenic DCs, IL-2 and TGF-β1 (C &D).
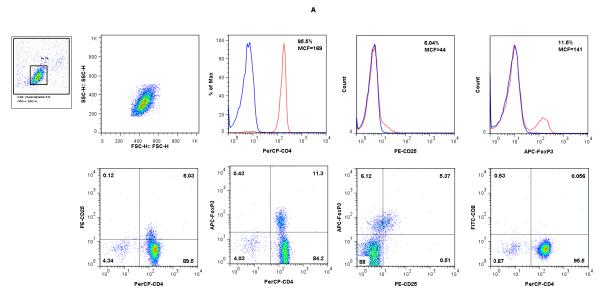
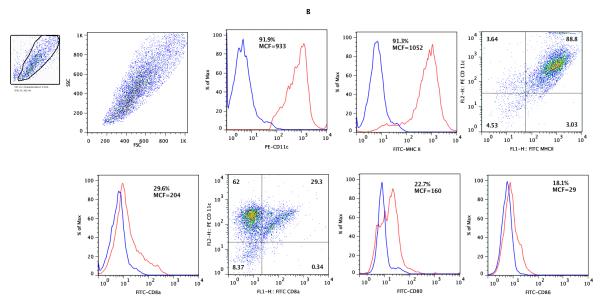
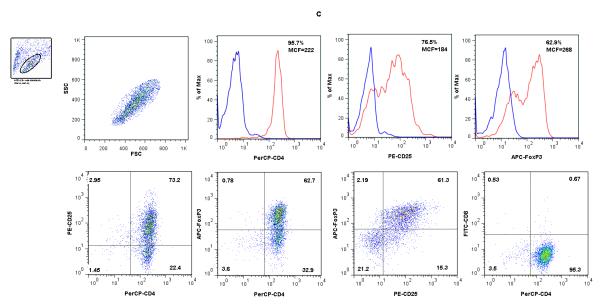
CD4+ T cells from BALB/c mice spleens (Panel A) and CD11c+ DCs from B6 mouse spleens (Panel B) were co-cultured in the presence of IL-2 and TGF-β1 and analyzed after 5 days of culture (Panels C and D). The live lymphocyte or DC population was based on size and granularity (R1) gated in the upper left corners. In each histogram, the red line shows the percentage of positive cells and MCF values for CD4, CD25, Foxp3 (panels A and C), CD11c, MHC II, CD8a, CD80, or CD86 (panel B), and the blue line shows values for respective isotype controls. (C) Following co-culture of CD4+ T cells with irradiated B6 splenic DCs in the presence of IL-2 and TGF-β1, the percentage of cells co-expressing CD25 and Foxp3 increased from 5.37% (A) to 61.3% (C). (D). Flow cytometry analysis of CD11c gated cells; CD80 or 86 expression by freshly isolated CD11c+ DCs (top panels); CD11c DCs retrieved 5 days of co-culture with CD4+ T cells, IL-2 and TGF-β1 (middle panels); CD11c+ DCs cultured overnight with 100ng/ml LPS (lower panels). Results shown are representative of two to three experiments.
The CD11c+ DCs, used as stimulators in the MLRs, were positively selected from single cell suspensions of spleens of B6 mice, using a previously described protocol (8). Flow cytometric analysis of the cells used as stimulators are shown in Figure 1B; bivariate dot plot analyses showed that 88.8% of the cells were positive for both CD11c and MHC II, 29.3% co-expressed CD11c and CD8a and a minority of freshly isolated splenic DCs expressed co-stimulatory proteins CD80 (22.7%) or CD86 (18.1%).
Following co-culture of BALB/c CD4+ T cells with irradiated B6 splenic CD11c+ DCs in the presence of IL-2 and TGF-β1, the percentages of CD4+CD25+ cells (73.5 ± 2.1%, n=3), CD4+Foxp3+ cells (64.7 ± 2.8%, n=3), and Foxp3+CD25+ cells (64.3 ± 3.0%, n=3) increased dramatically within the CD4+ T cell population. Figure 1C shows a more than 10-fold expansion of BALB/c Foxp3+CD25+ cells following stimulation with B6 CD11c+ DCs, IL-2 and TGF-β1, from 5.37% before stimulation (Fig.1A) to 61.3% following stimulation (Fig.1C).
We examined whether the phenotype of DCs following co-culture of CD4+ T cells with the CD11c+ DCs in the presence of IL-2 and TGF-β1 modified cell surface expression of CD80 and CD86. Flow cytometry analysis of CD11c gated DCs showed a remarkable increase in the percentage of DCs expressing CD80 and CD86 compared to freshly isolated DCs (Fig. 1D). In two consecutive experiments, 20.3 ± 1.7% of freshly isolated CD11C+ DCs expressed CD80 and 15.8 ± 0.1% expressed CD86 whereas 52.3 ± 2.8% of the CD11+ DCs retrieved after 5 days of co-culture with CD4+ T cells expressed CD80, and 47.2 ± 0.3% expressed CD86. Figure 1D also shows a marked increase in the expression of CD80 (77.0 ± 1.7% vs. 20.3 ± 1.7%) and CD86 (58.7 ± 1.9% vs. 15.8 ± 0.1%) following LPS-stimulation of freshly isolated DCs.
We isolated the CD4+CD25+ subset from the MLR and compared the phenotype of DC, IL-2 and TGF-β1 differentiated Tregs (alloantigen induced Tregs) to the phenotype of nTregs freshly isolated from the spleens of BALB/c mice. Figure 2 illustrates the similarities and differences between the alloantigen induced Tregs and nTregs. In three consecutive experiments, the percentage of Foxp3+ cells and the MCF intensity were numerically higher with alloantigen induced Tregs (87.4 ± 1.7%, MCF: 180 ± 66, mean [±SE], n=3) compared to nTregs (81.2 ± 3.5%, MCF: 102 ± 22, n=3) (P>0.05). The levels of expression of CD25 antigen, as assessed using MCF intensity, were significantly higher on the surface of alloantigen induced Tregs (880 ± 58) than nTregs (248 ± 17) (P=0.02). Levels of expression of CD62L antigen also differed – whereas a higher percentage of nTregs expressed CD62L+ compared to alloantigen induced Tregs (55.7 ± 2.1% vs. 36.1 ± 3.6%, P=0.02), the MCF intensity was higher among alloantigen induced Tregs (76 ± 4 vs. 27 ± 2, P=0.02).
Figure 2. Flow cytometry analyses of naturally occurring regulatory T cells (nTregs) (A) and DCs, IL-2 and TGF-β1 differentiated alloantigen specific regulatory T cells (alloTregs) (B).
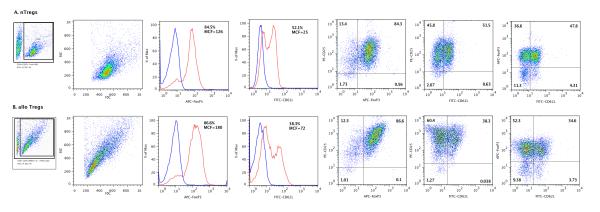
nTregs were isolated from spleens of BALB/c mice aged 6 to 10 weeks using antibody-tagged magnetic beads provided in the Mouse CD4+CD25+ Regulatory T Cell Isolation kit (Miltenyi Biotech Inc, Auburn, CA). To generate alloantigen induced Tregs, CD4+ T cells purified from BALB/c spleen cells were co-cultured with B6 splenic DCs, IL-2 and TGF-β1 for 5-6 days, followed by enrichment of the CD4+CD25+ fraction. The lymphocyte population was based on size and granularity (R1) gate. In each histogram the red line shows the percentage of positive cells and MCF values for Foxp3 and CD62L, and the blue line shows values for isotype controls. Panels on the right show two color analyses for CD25 and Foxp3, CD25 and CD62L, and for Foxp3 and CD62L. Results shown are representative of three separate experiments.
Differential mRNA expression patterns of alloantigen induced Tregs and nTregs
We quantified the absolute levels of mRNAs with the use of real-time quantitative PCR assays (Fig. 3; Ref. 9). Absolute levels of Foxp3 mRNA copies were not different between alloantigen induced Tregs and nTregs (P>0.05). In accordance with flow cytometry data, alloantigen induced Tregs expressed significantly more CD25 mRNA relative to nTregs (P=0.0003).
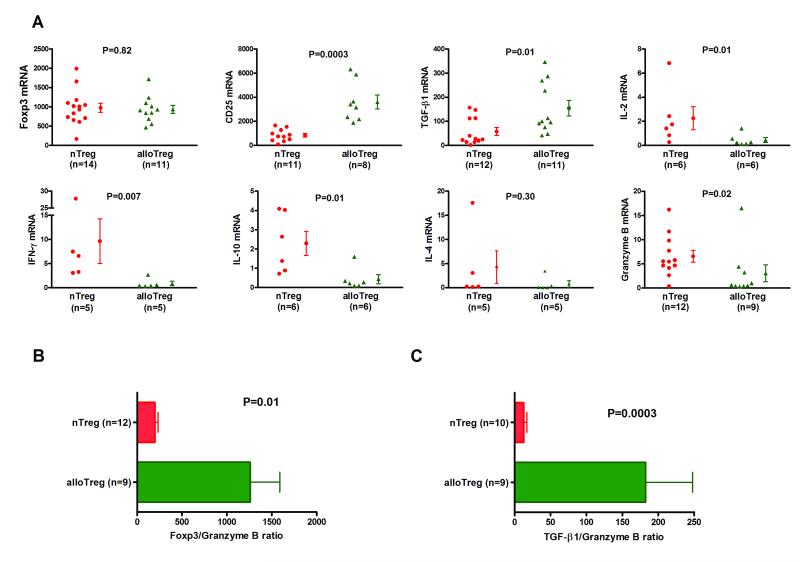
Figure 3. Differential mRNA expression patterns of nTregs and alloTregs.
Total RNA was isolated from nTregs or alloantigen induced Tregs (alloTregs), and absolute levels of mRNA for Foxp3, CD25, TGF-β1, IL-2, IFN-γ, IL-10, IL-4, or granzyme B were measured with the use of real-time quantitative PCR assays. (A) Individual and mean (±SE) mRNA levels are shown as a ratio of mRNA copies per one μg of RNA to 18S ribosomal RNA copies per one pg of RNA. Levels of Foxp3 mRNA were not different between alloTregs and nTregs (937 ± 104 vs. 976 ± 120, P>0.05) and levels of CD25 mRNA (3606 ± 588 vs. 839 ± 150, P=0.0003) and TGF-β1 mRNA (154 ± 32 vs. 58 ± 16, P=0.01) were higher in alloTregs than nTregs. Levels IL-2 mRNA (0.45 ± 0.2 vs. 2.26 ± 1.0, P=0.01), IFN-γ mRNA (0.86 ± 0.5 vs. 9.64 ± 4.6, P=0.007), IL-10 mRNA (0.44 ± 0.2 vs. 2.29 ± 0.6, P=0.01) and granzyme B mRNA 3.05 ± 1.8 vs. 6.56 ± 1.2, P=0.02) were lower in alloTregs than nTregs. (B) The ratio of Foxp3 mRNA to granzyme B mRNA (1259 ± 331 vs. 199 ± 34, P=0.01) was higher in alloTregs than nTregs. (C) The ratio of TGF-β1 to granzyme B mRNA (182 ± 66 vs. 12 ± 5, P=0.0003) was higher in alloTregs than nTregs. P values calculated using the Mann-Whitney test.
TGF-β1 mRNA was expressed at a higher level in alloantigen induced Tregs than in nTregs (P=0.01). On the other hand, levels of mRNAs encoding IL-2 (P=0.01), IFN-γ (P=0.007), IL-10 (P=0.01), and IL-4 (P=0.30) were lower in the alloantigen induced Tregs compared to nTregs.
The relative abundance of Foxp3 and granzyme B has been associated with tolerance (Foxp3>granzyme B) and rejection (Foxp3<granzyme B) in experimental transplant models (10). The levels of granzyme B mRNA were significantly lower in alloantigen induced Tregs than nTregs (Fig.3A, P=0.02) and the ratios of Foxp3 mRNA to granzyme B mRNA (1259 ± 331 vs. 199 ± 34, P=0.01, Fig.3B) and TGF-β1 to granzyme B mRNA (182 ± 66 vs. 12 ± 5, P=0.0003, Fig.3C) were higher in alloantigen induced Tregs than in nTregs.
Alloantigen induced Tregs are highly inhibitory in the suppressor assay and the suppression is alloantigen specific
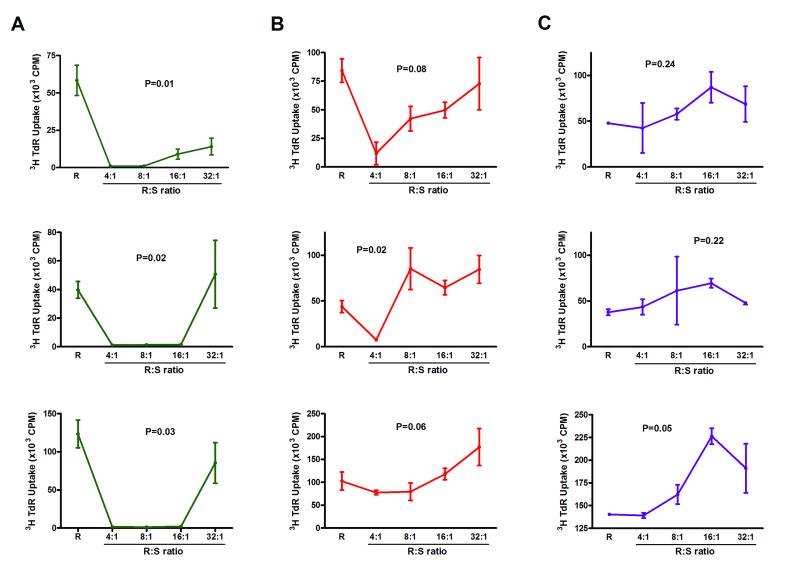
Alloantigen induced Tregs, generated using BALB/c CD4+ cell as responders and B6 DCs as stimulators in MLR supplemented with IL-2 and TGF-β1, suppressed the proliferation of BALB/c CD4+CD25− responder cells to B6 APCs up to a 16:1 responder to suppressor ratio in 3 independent experiments (93 ± 4% at 16:1 ratio) and up to a 32:1 ratio in two of three experiments (Fig.4A). nTregs were less suppressive (P=0.059), and inhibition of proliferation by nTregs was observed in two of three experiments at a 4:1 ratio (Fig.4B).
Figure 4. Allospecific inhibitory activity of alloantigen induced Tregs.
Alloantigen induced Foxp3+CD25+CD4+ Tregs were differentiated from naïve, polyclonal BALB/c CD4+ T cells using the combination of B6 DCs, IL-2, and TGF-β1. The suppressors tested were (S) were either alloantigen induced Tregs (A) or freshly isolated nTregs (B) and were mixed in various ratios with 105 CD4+CD25− cells from BALB/c [responders(R)], and stimulated with irradiated spleen cells from B6 mice (A and B). (C) AlloTregs generated from BALB/c CD4+ T cells with B6 DCs, IL-2, and TGF-β1 were mixed with 105 CD4+CD25− cells from CBA responder T cells in various ratios, and stimulated with irradiated SJL spleen cells. Proliferation was assessed by [3H]thymidine uptake. Data from each of three consecutive experiments are shown, and expressed as mean (±SE) of three replicates within each experiment. P values calculated using the Kruskal-Wallis test.
We determined whether their suppressive activity is mediated at the level of responder cells and/or at the level of stimulator cells. In three consecutive experiments, the mean (±SE) percentage of inhibition by the alloantigen induced Tregs was 96 ± 1% at a ratio of 4 responder cells to 1 suppressor cell, 80 ± 5% at 8:1 ratio, 59±5% at 16:1 ratio, and 30±3% at 32:1 ratio when BALB/c CD4+CD25− T cells were the responders and CBA splenic APCs (and not B6) were the stimulators and the percentage of inhibition was 97 ± 1% at 4 : 1 ratio, 93 ± 4% at 8:1 ratio, 57 ± 21% at 16:1 ratio, and 0 % at 32:1 ratio when CBA CD4+CD25− T cells (and not BALB/c) were the responders and B6 splenic APCs were the stimulators.
We also set up a third-party MLR with CD4+CD25− T cells from H-2k CBA mice as the responders, and H-2s SJL splenic APCs as the stimulators. In striking contrast to the suppression observed when the responder cells were BALB/c CD4+CD25− T cells and the stimulator cells were B6 splenic APCs, alloantigen induced Tregs generated using BALB/c CD4+T cells as responders and B6 DCs as stimulators failed to suppress even at a responder/suppressor ratio of 4:1 in all 3 consecutive experiments (Fig.4C).
Alloantigen induced Tregs are islet graft protective in a fully H-2 mismatched and non-lymphopenic, immunocompetent recipient mice
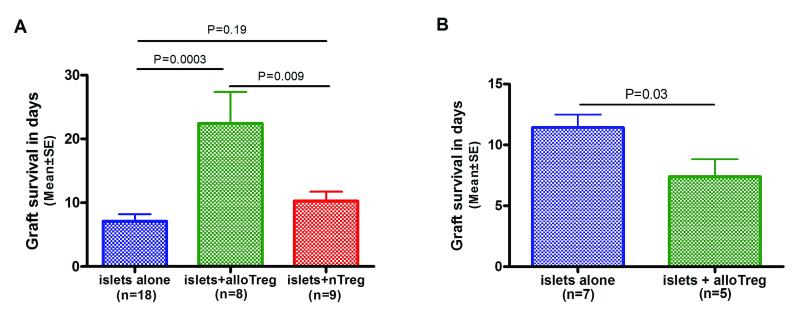
We generated Tregs in MLR using BALB/c CD4+ T cells as responders and B6 CD11c+ DCs as stimulators, and the MLR were supplemented with IL-2 and TGF-β1. In the fully H-2 mismatched donor-recipient combination of B6 islets to BALB/c recipients, co-transplantation of 5×105 BALB/c Foxp3+ CD25+CD4+ Tregs with 500 B6 islets significantly prolonged the survival time of islet allografts from 7.1 ± 1.1 days (islet alone, mean ± SE, n=18 recipients) to 22.4 ± 4.9 days (islet plus alloantigen induced Tregs, n=9 recipients, Fig.5A) (P=0.0003). In contrast, co-transplantation of 5×105 BALB/c nTregs with B6 islets did not result in a significant prolongation of islet allograft survival, and the mean survival time was 10.3 ± 1.5 days when the islet were co-transplanted with nTregs (n=8 recipients) (P=0.19 vs. islets alone).
Figure 5. Alloantigen induced Tregs prolong islet allograft survival in a fully H-2 mismatched, non-lymphopenic immunocompetent mouse islet cell transplantation model and allograft protection is alloantigen specific.
(A) A total of 500 B6 islets were transplanted under the renal capsule of diabetic BALB/c mice without Tregs, with 5×105 alloantigen induced Tregs (alloTregs), or 5×105 freshly isolated nTregs on Day 0. The alloTregs were generated in MLR using BALB/c CD4+ T cells as responders and irradiated B6 DCs as stimulators and the MLRs were supplemented with IL-2, and TGF-β1. Mean (±SE) survival times for B6 islet grafts transplanted without Tregs (blue, N=18 recipients), with alloTregs (green, N=9 recipients) or nTregs (red, N=8 recipients) are shown. Allograft rejection was defined as two consecutive blood glucose measurements exceeding 250mg/dL. (B) A total of 500 CBA islets were transplanted under the renal capsule of diabetic BALB/c mice without Tregs (blue, N=7 recipients) or along with 5×105 alloTregs (green, N=5 recipients) on Day 0. The alloTregs were generated in MLR using BALB/c CD4+ T cells as responders and irradiated B6 DCs as stimulators and the MLRs were supplemented with IL-2, and TGF-β1. P values calculated using Kaplan-Meier survival analysis and the log rank test.
Islet allograft protection by alloantigen induced Tregs is alloantigen specific
We tested whether the in-vivo islet allograft protective activity of alloantigen induced Tregs was alloantigen specific. In these experiments, 500 H-2k CBA (third-party) islets were transplanted under the renal capsule of diabetic BALB/c mice, without or with 5×105 alloantigen induced Tregs generated in MLRs by co-culturing BALB/c CD4+ T cells with B6 CD11c+ DCs, IL-2 and TGF-β1. In the allogeneic donor-recipient combination of H-2k CBA islets to streptozotocin-induced diabetic BALB/c mice, the mean (±SE) survival time of islet allografts transplanted without alloantigen induced Tregs was 11.4 ± 1 days (n=7 recipients, Fig.5B). In contrast to the significant protection observed with H-2b B6 islets (Fig.5A), co-transplantation of BALB/c alloantigen induced Tregs generated using B6 DCs failed to protect CBA islet allografts – the mean survival time of islet grafts co-transplanted with alloTregs was 7.4 ± 1 days (n=5 recipients, Fig.5B) and was even shorter compared to transplantation of islets alone (P=0.03, Fig.5B).
DISCUSSION
The central goal for our study was to generate alloantigen specific Tregs from a cell population that is more abundant naturally than nTregs. Although isolation and expansion of nTregs have been accomplished, the lack of alloantigen specificity of polyclonally expanded nTregs and the potential for global immunosuppression were also the rationale for our investigation. Our data, summarized here, suggest that the use of high abundant CD4+ T cells as the responders and donor DCs as the stimulators in MLR supplemented with IL-2 and TGF-β1 is efficacious in inducing Tregs with alloantigen specificity. The fine specificity was evident more in-vivo than in-vitro since B6 DC induced BALB/c Tregs protected B6 but not CBA islet allografts in BALB/c recipients whereas the induced Tregs inhibited not only BALB/c + B6 MLR but also BALB/c + CBA MLR, albeit to lesser extent.
Most studies investigating the efficacy of Tregs in preclinical models have used immunodeficient recipients or have combined Treg therapy with T-cell depletion strategies or other immunosuppressive agents (11-13). In order to better reproduce the clinical situation and minimize exogenous immunosuppressive therapy, we selected a fully H-2 mismatched donor-recipient combination and used non-lymphopenic, immunocompetent mice as allograft recipient and examined the efficacy of alloantigen induced Tregs without any additional interventions. In these stringent experimental conditions, the donor DC, IL-2 and TGF-β1-induced Tregs protected the allograft, albeit to modest extent, in the absence of any other immunosuppressive therapy. The islet cell transplantation model we used however could be critiqued for not being directly applicable to the clinic since human islets are currently transplanted via the intra-portal route rather than placed under the renal capsule. However, the ideal site for islet cell transplantation has not been resolved and extra-hepatic strategies are being explored to as achieve better success with islet cell grafts (14).
Issues such as whether the alloantigen induced Tregs would protect solid organ allografts such as cardiac allografts or whether the Tregs are graft protective when administered intravenously remains to be defined. Our preliminary findings that intravenous infusion (one million cells on the day of transplantation and repeated on post transplant day 7 and 14) of B6 CD11c+ DCs, IL-2 and TGF-β1 induced Tregs increased the survival time of B6 islets grafts in the untreated BALB/c from 7.1 ± 1.1 days (n=18) to 17.5 ± 2.5 days (n=2) are in accord with the earlier observations that intravenous infusion of DC− differentiated antigen specific Tregs restores normoglycemia in diabetic NOD mice (15) and protects syngeneic islet grafts in diabetic NOD recipients (5).
Our comparison of the inhibitory activity of alloantigen induced Tregs with that of nTregs suggest that the allospecific Tregs may be more potent than nTregs. We used freshly isolated nTregs rather polyclonally activated Tregs and the activated nTregs may have greater regulatory activity. We hypothesize that alloantigen specificity rather than the activation status of Tregs contributed to the greater regulatory activity since Tregs generated with B6 DCs were protective of B6 islet allografts but not of CBA islet allografts (Figure 5A vs. 5B). Additional support for the idea that antigen specificity rather than activation status is responsible is provided by the elegant demonstration that CD4+CD25+CD62L+ T cells sorted from the spleen and lymph nodes of CD4+ TCR− transgenic mice and expanded in vitro using IL-2, anti-CD3/anti-CD28 were more potent on a cell-for-cell basis compared to similarly expanded CD4+CD25+CD62L+ T cells from the wild-type mice (16).
TGF-β1 plays a central role in Foxp3 expression and may help prevent the conversion of Tregs to T effectors (17, 18). mRNA encoding TGF-β1 was over-expressed in alloantigen induced Tregs compared with nTregs and those associated with acute rejection of allografts such as granzyme B, IL-2, and IFN-γ were all under-expressed, suggesting that the molecular program of alloantigen induced Tregs may be conducive to allograft protection. The overexpression of Foxp3 compared to granzyme B, observed in the alloantigen induced Tregs, may also be significant since a higher expression of Foxp3 to granzyme B ratio reported to favor tolerance and a lower one, to allograft rejection (10). Further studies are needed not only to better resolve mechanisms underlying the suppressive activity of alloantigen induced Tregs but also to increase their therapeutic efficacy since we failed to accomplish permanent survival of the allograft in our stringent transplant model. In a preliminary analysis to understand whether the islets are rejected despite the presence of alloantigen induced Tregs, we investigated Foxp3 mRNA expression in the islet bearing kidney removed several days after rejection and found no detectable mRNA for Foxp3. At this stage, it is not clear how long the Tregs co-transplanted with the islet graft remain at the graft site and whether the Tregs migrate to the draining lymph node and/or recruit endogenous Foxp3+ cells. In an earlier study, it was observed that intravenous infusion of DC-expanded, islet antigen–specific CD4+CD25+ Tregs results in a substantial increase in Foxp3+ cells in the draining pancreatic lymph nodes and that these Foxp3+ cells were derived from the recipient mice and not from the injected Tregs (15). Longitudinal studies to characterize the stability of co-transplanted Tregs and the use of congenic strains to distinguish the origin of Foxp3+ cells are clearly needed.
Our system for generating alloantigen induced Tregs involved direct antigen recognition, and areas worthy of exploration include whether education through the indirect antigen recognition pathway would enhance their in-vivo regulatory activity. Joffre et al. (19) found that Tregs with direct alloantigen specificity are only able to suppress acute rejection, whereas Tregs with both direct and indirect specificities induce long-term allograft survival and immune tolerance. Others have shown using transgenic T-cell receptors that Tregs with dual direct and indirect specificities were more potent than Tregs only capable of direct alloantigen recognition (20).
In summary, we have demonstrated the differentiation of naïve CD4+ T cells into alloantigen specific and allograft protective Foxp3+CD25+CD4+ Treg cells with the use of donor-derived DCs, IL-2 and TGF-β1. DC-differentiated Tregs exhibited exquisite donor alloantigen specificity in their regulatory activity, and prolonged the survival of fully H-2 mismatched islet allografts in immunocompetent diabetic mice.
MATERIALS AND METHODS
A more detailed version of Methods is provided as supplemental digital content (SDC, Materials and Methods).
Mice
Male H-2d BALB/c, H-2b C57BL/6 (B6), H-2k CBA and H-2s SJL mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mice were treated according to the guidelines and protocols approved by the Institutional Animal Care and Use Committee of Joan and Sanford I. Weill Medical College.
Cell isolation and MLR
CD11c+ DCs (by positive selection using anti-CD11c beads, [8]) and CD4+T cells (by negative selection) were isolated from mouse spleen. nTregs were isolated from spleen using antibody-tagged magnetic beads provided in the Mouse CD4+CD25+ Regulatory T Cell Isolation kit (Miltenyi Biotech Inc, Auburn, CA).
We established MLR using CD4+ T cells as responders and splenic CD11c+ DCs as stimulators. A total 4×104 per well of CD4+T cells were co-cultured with irradiated DCs at a 4:1 T: DC ratio in 96-well plates, and the MLR were supplemented with 2ng/ml TGF-β1 (R&D Systems, Minneapolis, MN), and 100 units/ml IL-2 (Chiron, Emeryville, CA). After 5-6 days of culture, CD25+CD4+ T cells were enriched using the Miltenyi Mouse CD4+CD25+ Regulatory T Cell Isolation kit.
Flow Cytometry Analysis
Antibodies used for cell staining and the protocols for flow cytometry analyses are provided as supplemental digital content (SDC, Materials and Methods). Flow cytometry data were acquired on a FACSCalibur (BD Biosciences) using CellQuest Pro v5.11 and analyzed using FlowJo 8.0 software (TreeStar, Ashland, OR). The quadrants were set based on isotype control antibody staining, and the number in each quadrant represents the percentage of cells. Statistical analysis was performed using the Mann-Whitney test.
Quantification of mRNA levels by real-time quantitative PCR assay
Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA), reverse transcribed to cDNA and absolute levels of mRNA were measured using an ABI Prism 7500 sequence detection system (Applied Biosystems) (9, 21). Data were analyzed using the Mann-Whitney test. Additional details of PCR assay conditions including the sequence and location of the probes and primers are provided as supplemental digital content (SDC, Table 1).
Suppressor assays
Freshly isolated CD4+CD25− T cells (responders) from spleens of mice were cultured along with irradiated (17Gy) splenocytes as antigen-presenting cells (APCs) at a ratio of one responder T cell to 4 APCs. Alloantigen induced Tregs or nTregs were added at graded concentrations. [3H]Thymidine (2μCi per well) was added for the last 18 hours of a 120-hour culture period, and [3H]thymidine uptake was measured at the end of the culture period. Appropriate responder and stimulator combinations were established to assess allospecificity of alloantigen induced Tregs. Data from suppressor assays were analyzed using the Kruskal-Wallis one-way analysis of variance. Dunn’s test for multiple comparisons was used to correct for type I error.
Islet cell transplantation
Recipient mice were rendered diabetic by a single intraperitoneal injection of streptozotocin (180-185mg/kg, Sigma, St. Louis, MO). Islets were isolated as described (22) and about 500 islets were co-transplanted under the renal capsule of diabetic mice without or with 5×105 alloantigen induced Tregs or nTregs. Islet allograft function was assessed by serial blood glucose measurements following transplantation. Islet graft rejection was defined as blood glucose levels exceeding 13.9mmol/L (250mg/dL) on two successive readings. Islet graft survival was analyzed using the Kaplan-Meier method and the log rank test.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. S. Yamazaki for her expert advice on dendritic cell isolation and for generously providing the recombinant human interleukin-2 used in this study and Dr. X. R. Luo for sharing her expertise on Treg cell generation.
This investigation was supported in part by grants UL1RR024996 and TLRR024997 of the Clinical and Translational Science Center at Weill Cornell Medical College. H. Yang is a recipient of the Clinical and Translational Science Center Pilot Award, E. Cheng is a recipient of the KL2 Post-doctoral Research Training Award, T. Muthukumar is a recipient of the NIH Career Development (K08-DK087824) Award, and M. Suthanthiran is a recipient of the NIAID MERIT (2R37 AI51652) Award.,
We thank Dr. Ralph M Steinman for the thoughtful review of the manuscript and dedicate this work to him in recognition of years of scientific collaboration and inspiring friendship.
ABBREVIATIONS
- alloTregs
Alloantigen induced regulatory T cells
- DCs
Dendritic cells
- MCF
Mean channel fluorescence
- MLR
Mixed leukocyte reaction
- nTregs
Naturally-occurring, thymus-derived regulatory T cells
Footnotes
Parts of the information contained in this article were presented as an abstract entitled “Donor Dendritic Cells and TGF-beta1 Plus IL-2 Differentiate CD4+ T Cells to Alloantigen Specific and Allograft Protective T Regulatory Cells” by Yang H, Song P, Chang C, Cheng E, Weir R, Lagman M, Sharma VK, Ding R, Thomas D, Suthanthiran M at the American Transplant Congress, Toronto, May, 2008.
Author Contributions: Hua Yang, and Elaine Y. Cheng participated in research design, the performance of the research, data analysis, and the preparation of the manuscript; no conflicts of interest.
Vijay K. Sharma participated in the performance of the research, data analysis, and in the preparation of the manuscript; no conflicts of interest.
Christina Chang participated in the performance of the research and data analysis; no conflicts of interest.
Ruchuang Ding, Mila Lagman, and Ping Song participated in the performance of the research; no conflicts of interest.
Thangamani Muthukumar participated in data analysis; no conflicts of interest Manikkam Suthanthiran participated in research design, data analysis, and in the preparation of the manuscript; no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki S, Patel M, Harper A, et al. Effective expansion of alloantigen-specific Foxp3+CD25+CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc Natl Acad Sci U S A. 2006;103:2758. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo X, Tarbell K, Yang H, et al. Dendritic cells with TGF-beta1 differentiate naïve CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:2821. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki S, Iyoda T, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 10.Zheng XX, Sánchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19:503. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 11.Raimondi G, Sumpter TL, Matta BM, et al. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010;184:624. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia G, He J, Zhang Z, Leventhal JR. Targeting acute allograft rejection by immunotherapy with ex vivo-expanded natural CD4+CD25+ regulatory T cells. Transplantation. 2006;82:1749. doi: 10.1097/01.tp.0000250731.44913.ee. [DOI] [PubMed] [Google Scholar]
- 13.Xia G, He J, Leventhal JR. Ex-vivo expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant. 2008;8:298. doi: 10.1111/j.1600-6143.2007.02088.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibly RF, Graham JG, Luo X, Lowe WL, Jr, Hering BJ, Shea LD. Advancing islet transplantation: from engraftment to the immune response. Diabetologia. 2011;54:2494. doi: 10.1007/s00125-011-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+CD25+CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan TV, Tang Q, Liu FC, Hoang V, Bi M, Bluestone JA, Kang SM. Requirements for prolongation of allograft survival with regulatory T cell infusion in lymphosufficient hosts. J Surg Res. 2011;169:e69. doi: 10.1016/j.jss.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4+Foxp3+ T cells. Curr Opin Immunol. 2009;21:281. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsang J, Tanriver Y, Jiang S, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Ding R, Sharma V, et al. Hyperexpression of Foxp3 and IDO during acute rejection of islet allografts. Transplantation. 2007;83:1643. doi: 10.1097/01.tp.0000263991.74052.46. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Thomas D, Boffa D, et al. Enforced c-REL deficiency prolongs survival of islet allografts. Transplantation. 2002;74:291. doi: 10.1097/00007890-200208150-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.