Abstract
Background:
High cholesterol levels are associated with increased risk of coronary heart disease and stroke. Understanding the distribution of serum cholesterol levels in each country is valuable index for use in public health planning. This study aimed to construct nomograms of total cholesterol (TC) levels and establish the cut-points specific to Iranian population.
Methods:
Data on serum TC levels of 19,630 non-institutionalized individuals aged 25–64 years from third national survey on non-communicable diseases (SuRFNCD) in 2007 were used to construct cholesterol nomograms. We proposed cutoff values for borderline and high TC levels based on rounded 75th and 90th percentiles in three age groups (25–34, 35–44 and 45–64) respectively.
Results:
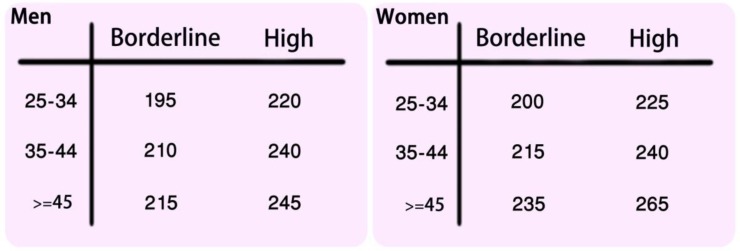
Average yearly increase of TC for males up to the age of 45 and females up to 64 were 1.15 and 1.03 mg/dl, respectively. TC levels were higher in females. In males, cutoff values for “borderline and high” TC levels were 195 and 220 mg/dl in 25–34, 210 and 240 mg/d in 35–44 and 215 and 245 mg/dl in 45–64 years old individuals. In women, these values were 200 and 225 mg/dl in 25–34,215 and 240 mg/dl in 35–44 and 235 and 265 mg/dl in 45–64 years old individuals respectively.
Conclusion:
Since TC levels are different in two sexes and change with age, we proposed different cutoffs for sex and age group. We think these cutoffs could be used in national public health planning.
Keywords: Total cholesterol, Nomograms, Population, Iran
Introduction
High cholesterol levels are associated with increased risk of coronary heart disease (CHD) and stroke (1). Worldwide, a third of CHD is caused by raised cholesterol. Since CHD is one of the most common diseases both in the developed and developing countries, high total cholesterol (TC) is a major cause of disease burden and mortality. Generally worldwide, high TC is estimated to cause 4.5% of total mortality (2.6 million deaths) and 2.0% of total disability adjusted life years (DALYS), or 29.7 million DALYS. Therefore, detecting and lowering high level of TC can be helpful in reducing CHD and total mortality (2). Understanding the distribution of serum cholesterol levels in each country is valuable index for use in public health planning.
In 1985, National Institute of Health (NIH) constructed nomorgarms of TC levels for US population. On the basis of that nomogram, TC levels were divided into three categories so that, cholesterol below the 75th percentile was defined as the desired level, between the 75th and 90th percentile was defined as moderate risk and greater than the 90th percentile was defined as high risk (3). Three years later in 1988, The Expert Panel of the US National Cholesterol Education Program (NCEP) proposed a newer classification for hypercholesterolemia which was unrelated to age. In this classification total cholesterol <200mg/dl was defined as desirable, 200–239 mg/dl as borderline and >=240 mg/dl as high (4). According to consequent treatment based classification, Adult Treatment Panel (ATP III), recommended treatment to be initiated at levels higher than 240 mg/dl and life style modification at levels higher than 200 mg/dl (5). The aforementioned cut-off values are primarily according to the western population. In fact, the association of total cholesterol values with the adverse consequences such as diabetes, coronary artery diseases (CAD), and stroke and all-cause mortality depends on age, sex and ethnicity (6–8). Americans because of their life style and particularly their diet (9, 10) have higher cholesterol levels than Iranians.
This study aimed to construct nomograms of cholesterol levels and establish the cut points for borderline and high serum TC levels specific to Iranian population.
Methods
We used data on 19630 non-institutionalized 25–64 years old Iranian individuals obtained from third national survey on non-communicable diseases in 2007 for analysis (11). The study was in line with STEPwise approach of the WHO to non-communicable disease risk factor surveillance (12). The survey was carried out with collaboration of 40 medical schools across the country. This study received ethics approval from the Center for Disease Control (CDC) of Iran. Prior to inclusion, verbal consent was taken from all individuals. In each 30 provinces of the country, a multistage random cluster sampling method, stratified by sex and age group was used to collect the data. In each province 50 clusters were taken. Each cluster comprised of 20 individuals, 10 men and 10 women, living in neighboring households. The age of target population was classified into five age groups (15–24, 25–34, 35–44, 45–54, and 55–64 years). Two men and two women were recruited from each age group in each cluster. First subject of a cluster was identified randomly based on postal addresses.
All individuals were visited at their home by trained interviewers appointed by collaborating medical schools. Data were obtained in three steps. In first step data regarding demographic and behaviors of individuals (diet, physical activity, tobacco use, history of hypertension, and history of diabetes) were gathered using a questionnaires based on WHO STEPS instrument (core and expanded) (11). In step 2 individuals underwent physical measurements (e.g. weight, height, waist circumference and blood pressure) using the same protocol for all of recruited individuals. In step 3 trained laboratory technicians took 10 ml of venous blood in sitting position after 10 to 12 hours of fasting from participant 25 years and older. Blood samples transferred in cold boxes to a referral laboratory center in at most 4 h from sampling sites. Several parameters were measured including total cholesterol level. Serum total cholesterol was measured by enzymatic method (Parsazmun, Karaj, Iran) with the coefficient of variation of 5%. In addition, in laboratory sites 10% of the blood samples were centrifuged and sera kept frozen at −20°C and then transferred to the National Reference Laboratory, a WHO collaborating center in Tehran, for double checking. Overall the measurements were found reliable.
The survey received ethics approval of the Center for Disease Control (CDC) of Iran
Statistical Analysis
Because of the sampling method, Complex Sample Survey (SVY) analysis was performed using Stata statistical software (release 11.0, StataCorp, College Station, TX) (13). The provinces were defined as strata, and in each strata clusters were defined as sampling units.
Primarily, “weights” for each sex and age groups were assigned according to the distribution of sex and age groups of the 2006 national census in each province. Then, to account for the missing data primary weights were divided by the number of measurements in the corresponding sex-age group category to correct for missing observations.
Construction of the cholesterol level nomograms
We constructed two separate nomograms of cholesterol levels for men and women. Nomograms were constructed by modeling data using fractional polynomial (14). To derive the cholesterol nomograms (reference standard curves), first the cholesterol measurements were transformed to the logarithmic scale in order to obtain approximate normal distributions. Then the variations in mean (μ) and standard deviation (SD) of the transformed cholesterol measurements were modeled with age using fractional polynomials. First, for the appropriate modeling of mean of the transformed data (μ), the powers of age were obtained using Fracpoly procedure. Then age was centered to obtain robust estimation of the model parameters. To model the mean of the logarithm of cholesterol, the powers of centered ages were used as covariates in SVY regression module, which accounts for the structure and weights of the data.
Second, the absolute residuals were computed for the above model and regressed on age using SVY module and multiplied by to estimate the age-specific standard deviation (15). Finally, the Pth percentiles of the logarithm of cholesterol levels were computed by μ + Zp × SD (eg, the Zp=1.645 for computing the 95th centile). The nomograms derived by back transformation.
Evaluating goodness of fit
The goodness of fit of the nomograms was assessed by comparing the number of cholesterol measurements in regions defined by the seven centiles (5th, 10th, 25th, 50th, 75th, 90th, and 95th) with expected number of observations for all ages using a chi-squared test.
Deriving cut points
We summarized 75th and 90th percentiles of TC in three age groups (25–34, 35–44 and 45–64). Then, 75th and 90th percentiles in each age group were rounded to derive cut-points for borderline and high levels of TC specific to Iranian population.
Results
Serum total cholesterol levels of 19,630 individuals (9,369 men and 10,261 women) aged 25–64 years from all over the Iran were used for construction of the cholesterol level nomograms.
Fractional polynomial with degree 2 was applied for modeling the mean and standard deviation of logarithmic transformation of the total cholesterol level of men with age. The powers for age were found to be in the form of −1 and 3 for mean and of 3 and 3 for standard deviation. Also, mean logarithmic transformation of the total cholesterol level of women were modeled with age using a degree 2 fractional polynomial. The age powers were obtained as 3 and 3. Furthermore, a model of the form of 2 and 3 were the powers of age when the standard deviation of the total cholesterol of women were modeled.
Blood samples of 17.4% (4162) of surveyed individuals were missing. The rate of missed cholesterol measurements were significantly more in men and younger ages and found to vary across provinces. The distribution of other non-missing available variables including body mass index (BMI), duration of exercise, frequency of consuming vegetables, fruit and oil, job related physical activity between missing and non-missing individuals in each sex and age groups were found non-significant (P>0.05; data not shown). In general, for overcoming the problem of missingness one can use imputation or weighting the data. Therefore, the following analyses were carried out using the weights computed from the national census 2006 which were divided to the number of individuals attended in each age-sex category.
Table 1 presents the smoothed 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles of the men serum total cholesterol levels for each age.
Table 1:
Cholesterol (mg/dl) levels for males (percentile: 5 10 25 50 75 90 95) according to age
| Age (yr) | P5 | P10 | P25 | P50 | P75 | P90 | P95 |
|---|---|---|---|---|---|---|---|
| 25 | 110 | 121 | 140 | 161 | 186 | 212 | 230 |
| 26 | 111 | 123 | 142 | 164 | 189 | 215 | 234 |
| 27 | 112 | 124 | 143 | 166 | 191 | 218 | 237 |
| 28 | 114 | 126 | 145 | 167 | 193 | 220 | 239 |
| 29 | 115 | 127 | 146 | 169 | 195 | 223 | 242 |
| 30 | 116 | 128 | 148 | 171 | 197 | 225 | 244 |
| 31 | 117 | 129 | 149 | 172 | 199 | 227 | 246 |
| 32 | 118 | 130 | 150 | 174 | 201 | 229 | 248 |
| 33 | 119 | 131 | 151 | 175 | 202 | 230 | 250 |
| 34 | 119 | 132 | 152 | 176 | 203 | 232 | 252 |
| 35 | 120 | 133 | 153 | 177 | 205 | 233 | 254 |
| 36 | 121 | 134 | 154 | 178 | 206 | 235 | 255 |
| 37 | 121 | 134 | 155 | 179 | 207 | 236 | 256 |
| 38 | 122 | 135 | 156 | 180 | 208 | 237 | 258 |
| 39 | 122 | 135 | 156 | 181 | 209 | 238 | 259 |
| 40 | 123 | 136 | 157 | 181 | 210 | 239 | 260 |
| 41 | 123 | 136 | 157 | 182 | 210 | 240 | 261 |
| 42 | 124 | 137 | 158 | 183 | 211 | 241 | 262 |
| 43 | 124 | 137 | 158 | 183 | 212 | 241 | 262 |
| 44 | 124 | 138 | 159 | 184 | 212 | 242 | 263 |
| 45 | 124 | 138 | 159 | 184 | 213 | 243 | 264 |
| 46 | 125 | 138 | 159 | 184 | 213 | 243 | 264 |
| 47 | 125 | 138 | 160 | 185 | 214 | 244 | 265 |
| 48 | 125 | 139 | 160 | 185 | 214 | 244 | 265 |
| 49 | 125 | 139 | 160 | 185 | 214 | 244 | 266 |
| 50 | 125 | 139 | 160 | 185 | 215 | 245 | 266 |
| 51 | 125 | 139 | 160 | 186 | 215 | 245 | 266 |
| 52 | 125 | 139 | 160 | 186 | 215 | 245 | 266 |
| 53 | 126 | 139 | 161 | 186 | 215 | 245 | 267 |
| 54 | 126 | 139 | 161 | 186 | 215 | 245 | 267 |
| 55 | 126 | 139 | 161 | 186 | 215 | 245 | 267 |
| 56 | 126 | 139 | 161 | 186 | 215 | 245 | 267 |
| 57 | 125 | 139 | 160 | 186 | 215 | 245 | 267 |
| 58 | 125 | 139 | 160 | 186 | 215 | 245 | 267 |
| 59 | 125 | 139 | 160 | 186 | 215 | 245 | 266 |
| 60 | 125 | 139 | 160 | 185 | 215 | 245 | 266 |
| 61 | 125 | 139 | 160 | 185 | 214 | 245 | 266 |
| 62 | 125 | 138 | 160 | 185 | 214 | 244 | 266 |
| 63 | 125 | 138 | 160 | 185 | 214 | 244 | 265 |
| 64 | 125 | 138 | 159 | 185 | 214 | 244 | 265 |
As Table 1 shows the cholesterol percentiles of men increased gradually by age. The median total cholesterol level of men at age of 25 was 161 mg/dl, which increased steadily to 184 mg/dl at the age of 45, and then flatten up to the age of 59 and eventually thereafter the TC level decreased around 1 mg/dl to age 64. The average yearly increase of median of TC for men up to the age of 45 was 1.15 mg/dl. The 90th percentile of the total cholesterol level also rose steadily from 212 mg/dl at the age of 25 to 240 mg/dl at the age of 41, and then this trend slowed down resulting in the 90th percentile of 245 mg/dl at the age of 50 which only decreased1 mg/dl after that.
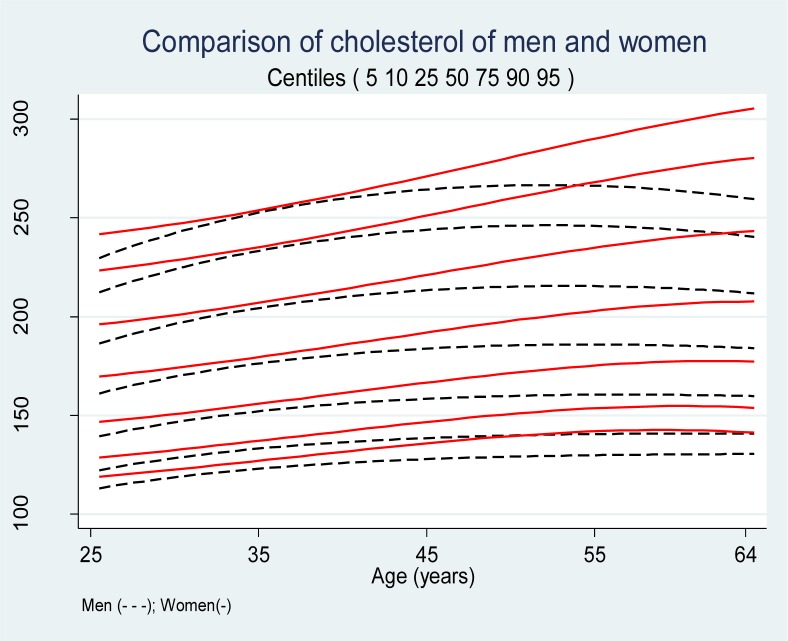
The Chi-squred test showed that the overall difference between observed and expected numbers of male cholesterol measures in eight region defined by the seven centiles (5th, 10th, 25th, 50th, 75th, 90th, and 95th) was not significant, χ2 =6.63, df=7, P=0.47; implying a good fit of the model to data. The smoothed percentiles of the female cholesterol levels are presented in Table 2. The median cholesterol level at the age of 25 was 169 mg/dl and increased steadily to 209 mg/dl at the age 64. The yearly increase of total cholesterol for females up to the age of 64 was 1.03 mg/dl. The 90th percentile of the total cholesterol was 221 mg/dl at the age of 25 and increased steadily to 277 mg/dl at the age of 64. The average yearly increase of 90th percentile was 1.44 mg/dl. The overall fit of smoothed centiles to the cholesterol levels of females was fine χ)2 =11.29, df=7, P=0.13). The comparison of serum total cholesterol levels of males and females are presented in Figure 1. All percentiles of serum cholesterol levels of females were higher than males. The spread of the percentiles was wider for females than males. The sex difference between 10th, 50th, 90th percentiles is 6, 8 and 9 mg/dl at the age of 25, respectively. This difference decreased up to the age of 33 and after that increased by age and reached to 16, 24 and 33 mg/dl for the 10th, 50th and 90th percentiles at the age of 64, respectively.
Table 2:
Cholesterol (mg/dl) levels for females (percentiles: 5 10 25 50 75 90 95) according to age
| Age (yr) | P5 | P10 | P25 | P50 | P75 | P90 | P95 |
|---|---|---|---|---|---|---|---|
| 25 | 113 | 127 | 147 | 169 | 195 | 221 | 241 |
| 26 | 113 | 128 | 148 | 170 | 196 | 222 | 242 |
| 27 | 114 | 128 | 149 | 171 | 197 | 223 | 243 |
| 28 | 115 | 129 | 149 | 172 | 198 | 225 | 245 |
| 29 | 115 | 130 | 150 | 173 | 199 | 226 | 246 |
| 30 | 116 | 131 | 151 | 174 | 200 | 228 | 248 |
| 31 | 117 | 131 | 152 | 175 | 202 | 229 | 250 |
| 32 | 117 | 132 | 153 | 176 | 203 | 231 | 251 |
| 33 | 118 | 133 | 154 | 177 | 204 | 232 | 253 |
| 34 | 119 | 134 | 155 | 179 | 206 | 234 | 255 |
| 35 | 120 | 135 | 156 | 180 | 207 | 235 | 256 |
| 36 | 120 | 136 | 157 | 181 | 209 | 237 | 258 |
| 37 | 121 | 136 | 158 | 182 | 210 | 239 | 260 |
| 38 | 122 | 137 | 159 | 183 | 211 | 240 | 262 |
| 39 | 123 | 138 | 160 | 185 | 213 | 242 | 264 |
| 40 | 124 | 139 | 161 | 186 | 214 | 244 | 266 |
| 41 | 124 | 140 | 162 | 187 | 216 | 245 | 268 |
| 42 | 125 | 141 | 164 | 189 | 217 | 247 | 270 |
| 43 | 126 | 142 | 165 | 190 | 219 | 249 | 272 |
| 44 | 127 | 143 | 166 | 191 | 221 | 251 | 274 |
| 45 | 128 | 144 | 167 | 192 | 222 | 253 | 275 |
| 46 | 128 | 145 | 168 | 194 | 223 | 254 | 277 |
| 47 | 129 | 145 | 169 | 195 | 225 | 256 | 279 |
| 48 | 130 | 146 | 170 | 196 | 226 | 258 | 281 |
| 49 | 131 | 147 | 171 | 197 | 228 | 259 | 283 |
| 50 | 131 | 148 | 172 | 199 | 229 | 261 | 285 |
| 51 | 132 | 149 | 173 | 200 | 231 | 263 | 287 |
| 52 | 133 | 149 | 174 | 201 | 232 | 264 | 289 |
| 53 | 133 | 150 | 175 | 202 | 233 | 266 | 290 |
| 54 | 134 | 151 | 175 | 203 | 235 | 267 | 292 |
| 55 | 134 | 151 | 176 | 204 | 236 | 269 | 293 |
| 56 | 135 | 152 | 177 | 205 | 237 | 270 | 295 |
| 57 | 135 | 153 | 178 | 205 | 238 | 271 | 296 |
| 58 | 136 | 153 | 178 | 206 | 239 | 272 | 298 |
| 59 | 136 | 153 | 179 | 207 | 240 | 273 | 299 |
| 60 | 136 | 154 | 179 | 208 | 240 | 274 | 300 |
| 61 | 136 | 154 | 180 | 208 | 241 | 275 | 301 |
| 62 | 137 | 154 | 180 | 208 | 242 | 276 | 302 |
| 63 | 137 | 154 | 180 | 209 | 242 | 277 | 303 |
| 64 | 137 | 154 | 180 | 209 | 242 | 277 | 303 |
Fig. 1:
Comparison of cholesterol nomograms of men and women
Table 3 summarizes the 50th, 75th and 90th percentiles of different sex and age groups.
Table 3:
The average of 50th, 75th and 90th percentiles of cholesterol of men and women according to age group
| Age (yr) groups | Men | Women | ||||
|---|---|---|---|---|---|---|
| 50th | 75th | 90th | 50th | 75th | 90th | |
| 25–34 | 169.4 | 195.7 | 223.0 | 173.7 | 200.0 | 227.0 |
| 35–44 | 180.7 | 209.0 | 238.2 | 185.4 | 213.7 | 243.0 |
| 45–64 | 185.2 | 214.4 | 244.5 | 202.3 | 234.0 | 266.8 |
These values were used to derive the cut points for borderline and high cholesterol levels specific to Iranian population (Panel 1).
Discussion
In this study we constructed cholesterol nomograms for the Iranian population specific for sex and age and proposed cut off values for borderline and high cholesterol levels.
Most studies on determination of normal cholesterol values were conducted in the United States. Cholesterol levels are considerably different between countries. Indeed, in recent years some countries tried to present normal cholesterol levels specific to their population (16, 17).
Iranian mean cholesterol level is lower than Americans (4, 18). The mean total cholesterol level of Americans was on average 200 mg/dl in 2006 (18) which is considerably higher than 186 mg/dl (derived from current data) in Iranians in 2007. In our data in males, the total serum cholesterol level of 200 mg/dl on average lay on 78th, 66th and 63th percentiles for the age groups of 25–29, 30–39 and 40–64 years, respectively. The cholesterol level of 240 mg/dl lay on percentiles 94th, 91th, and 88th for these age groups, respectively. In females, the total serum cholesterol level of 200 mg/dl on average lay on percentiles of 75th, 63th and 49th for the age groups of 25–29, 30–39 and 40–64 years. In addition, for these age groups, the total serum cholesterol level of 240 mg/dl lay on percentiles of 93th, 89th, and 72th, respectively. This data shows the difference of cholesterol levels between Iranian and Americans and warrants specific normal cholesterol levels for Iranian population.
This study showed that TC in men and women increases with age. This increase is more pronounced up to the age of 45. In men after the age of 45, age increase does not change the TC which means after the age of 45 the change of TC levels is independent of age. Oppositely, in women increase in TC levels with age continues after the age of 45 and remains dependent to age up to 64. Overall, in men TC increases with age up to middle-ages but in woman this increase continues to older ages. Several studies have shown consistent results with our study regarding the change of TC levels with age in men and women. (16, 17).
The increase in TC levels with age is partly attributed to impairment in LDL receptors in older ages. LDL receptor is responsible for endocytosis of cholesterol rich-LDL. As a consequence of peripheral impairment in uptake of LDL with age, TC concentration in blood increases. Another explanation for increase of TC with age is weight gain. Weight gain is more pronounced between 25 to 50 years of age (this is also true in our data, data not shown). This may explain why increase in TC levels in men is more obvious up to middle-ages. In women, lack of estrogen after menopause removes its stimulatory effect on production of LDL receptors which lead to the increase in TC levels in blood (19–21).
Since the cholesterol levels seems to be different in men and women and changes with age we proposed Iranian cut-points for borderline and high cholesterol levels separate for men and women and in three different age groups (25–34, 35–44 and 45–64). We proposed the age and sex related high TC values based on 75th and 90th percentiles of Iranian population. For ease, we rounded the numbers (Panel 1).
There were missing data in some clusters. Men with younger ages were more prone to be missed in obtaining blood sample (for measuring cholesterol levels). The fact that younger males were more commonly missed can be attributed to the coincidence of the blood sampling time with the work-hour of younger male individuals. However, other non-missing available variables were the same between missing and non-missing individuals in each sex and age groups. Indeed we think the missing data in each sex and age groups was in random. To compensate for missing data the primary weights derived from national census in 2006 were divided to the number of individuals attended in each age-sex category. Our study lacked relating cholesterol levels to mortality and CVD rates. Epidemiologic studies to evaluate CVD rate and mortality in conjunction with TC levels are warranted to set more accurate cut points for high cholesterol levels in the country (16). The laboratory standardization panel for cholesterol of NCEP at NIH in the US recommended the inaccuracy of measurements to be less than 5%. This was accordingly 5% in our study. Although it has been shown that when evaluating extremely large numbers of subjects, inaccuracy of measurement do not change the distribution of cholesterol levels (22).
Conclusion
Total cholesterol level of Iranians is lower than Americans. In addition, cholesterol level changes with age and is different in men and women. With taking it into account, we presented the age and sex specific cholesterol nomograms for Iranian population and proposed cholesterol borderline and high cutoff values for men and women in three age group 25–34, 35–44 and 45–64 years. Further epidemiologic studies are needed to determine the predictive abilities of these suggested cholesterol levels for CHD events and mortality.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Panel 1.
Cut points for total cholesterol levels (mg/dl) specific to Iranian population, by age
Acknowledgments
This research has been supported by Tehran University of Medical Sciences grant NO 90-01-27-12845. The authors would like to thank the non-communicable diseases risk factor surveillance Office, Center for non-communicable diseases Control & Management, Center for Disease Control (CDC), Iranian Ministry of Health and Medical Education for providing this invaluable data. Also, we are grateful to Dr. M. E. Jones at Section of Epidemiology, The Institute of Cancer Research, Sutton, UK for his help. The authors declare that there is no conflict of interest.
References
- 1.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? JAMA. 1986;256(20):2823–8. [PubMed] [Google Scholar]
- 2.World Health Organization . Preventing chronic diseases: a vital investment. World Health Organization; Genova: 2005. Available: www.who.int/chp/chronic_disease_report/en/ [Google Scholar]
- 3.Conference C. Lowering blood cholesterol to prevent heart disease. JAMA. 1985;253(17):2080–6. [PubMed] [Google Scholar]
- 4.Fulwood R, Kalsbeek W, Rifkind B, Russell-Briefel R, Muesing R, LaRosa J, et al. Total serum cholesterol levels of adults 20–74 years of age. Data from the national health survey. JAMA. 1985;253(12):1–59. [PubMed] [Google Scholar]
- 5.Expert Panel on DetectionEvaluation THBCA Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 7.Al-Mahroos F, McKeigue PM. High prevalence of diabetes in Bahrainis: associations with ethnicity and raised plasma cholesterol. Diabetes Care. 1998;21(6):936–42. doi: 10.2337/diacare.21.6.936. [DOI] [PubMed] [Google Scholar]
- 8.Bhalodkar NC, Blum S, Rana T, Bhalodkar A, Kitchappa R, Kim KS, et al. Comparison of levels of large and small high-density lipoprotein cholesterol in Asian Indian men compared with Caucasian men in the Framingham Offspring Study. Am J Cardiol. 2004;94(12):1561–3. doi: 10.1016/j.amjcard.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 9.Shekelle RB, Shryock AM, Paul O, Lepper M, Stamler J, Liu S, et al. Diet, Serum Cholesterol, and Death from Coronary Heart Disease. N Eng J Med. 1981;304(2):65–70. doi: 10.1056/NEJM198101083040201. [DOI] [PubMed] [Google Scholar]
- 10.Ernst ND, Sempos CT, Briefel RR, Clark MB. Consistency between US dietary fat intake and serum total cholesterol concentrations: the National Health and Nutrition Examination Surveys. Am J Clin Nutr. 1997;66(4):965S–73S. doi: 10.1093/ajcn/66.4.965S. [DOI] [PubMed] [Google Scholar]
- 11.Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, et al. Third national Surveillance of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007) in Iran: methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9(1):167–79. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . STEPwise approach to surveillance (STEPS) World Health Organization; Genova: 2005. Avalable from: http://www.who.int/chp/steps/en/. [Google Scholar]
- 13.StataCorp L . Stata version 11.0. College Station, TX: StataCorp LP; 2009. Available from: www.stata.com 〉 Resources & support. [Google Scholar]
- 14.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Stat. 1994;43(3):429–67. [Google Scholar]
- 15.Altman DG. Construction of age-related reference centiles using absolute residuals. Stat Med. 1993;12(10):917–24. doi: 10.1002/sim.4780121003. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Song J, Park Y, Lee H, Kim Y, Ryoo U, et al. National cholesterol treatment guidelines in Korean population--setting-up the cutpoints for high blood cholesterol. J Korean Med Sci. 1997;12(1):17–22. doi: 10.3346/jkms.1997.12.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung JC. Relationship among age, serum cholesterol level and population percentile in adults. Int J Biomed Comput. 1992;31(2):99–116. doi: 10.1016/0020-7101(92)90066-2. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, et al. 30-Year Trends in Serum Lipids Among United States Adults: Results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol. 2010;106(7):969–75. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Antonopoulos S. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(3143):3421–55. [PubMed] [Google Scholar]
- 20.Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women’s Health Across the Nation Heart women. Menopause. 2011;18(4):376–84. doi: 10.1097/gme.0b013e3181f6480e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akahoshi M, Soda M, Nakashima E, Shimaoka K, Seto S, Yano K. Effects of menopause on trends of serum cholesterol, blood pressure, and body mass index. Circulation. 1996;94(1):61–6. doi: 10.1161/01.cir.94.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Warnick GR, Kimberly MM, Waymack PP, Leary ET, Myers GL. Standardization of measurements for cholesterol, triglyceri-des, and major lipoproteins. Lab Medicine. 2008;39(8):481–90. [Google Scholar]