Abstract
The present study was undertaken to investigate the possible protective effect of Saudi Sidr honey (SSH) on carbon tetrachloride (CCl4) induced oxidative stress and liver and kidney damage in rat. Moreover, the antioxidant activity and the phenolic and flavonoidal contents were determined. The hepatorenal protective activity of the SSH was determined by assessing biochemical, hematological, and histological parameters. Serum transaminases, ALP, GGT, creatinine, bilirubin urea, uric acid, and MDA level in liver and kidney tissues were significantly elevated, and the antioxidant status of nonprotein sulfhydryls, albumin, and total protein levels in liver and kidney were declined significantly in CCl4 alone treated animals. Pretreatment with SSH and silymarin prior to the administration of CCl4 significantly prevented the increase of the serum levels of enzyme markers and reduced oxidative stress. SSH also exhibited a significant lipid-lowering effect and caused an HDL-C enhanced level in serum. The histopathological evaluation of the liver and kidney also revealed that honey protected incidence of both liver and kidney lesions. Moreover, SSH showed a strong antioxidant activity in DPPH and β-carotene-linoleic acid assays. SSH was found to contain phenolic compounds. Additionally, the SSH supplementation restored the hepatocytes viability against 2′,7′-dichlorofluorescein (DCF) toxicity in ex vivo test.
1. Introduction
Aasal is the Arabic name for honey. It is a naturally sweet and flavorful product produced by honeybees, Apis mellifera, from the nectar of blossoms or from the exudates of trees and plants giving the nectar honey or honeydews [1]. Honey is a natural, unprocessed, and easily digested food in the diet [2]. Honey has been used in almost all cultures and traditions since ancient times as a food and medicinal product. It is considered to be used for the treatment of burns, faster wound healing [3], gastrointestinal disorders, asthma, skin, and eye diseases, besides being a natural food preservative and sweetening agent [4, 5]. Hippocrates, the father of medicine, describes the nutritional and medicinal importance of honey [6].
Numerous nutritional [7] and biological effects [8] are attributed to honey, which include antibacterial [9], antioxidant [10], antiviral [11], antiparasitic [12], anti-inflammatory [13], anticancer [14], and immunosuppressive [15] activities. Apart from various therapeutic uses, honey is used in many folkloric traditions, for instance it is given to new born babies to clean their digestive system. Honey is considered to be the first in the line to treat jaundice in traditional medicine of different countries. In the present investigation, an attempt has been made to validate its use in both liver and kidney disorders.
2. Materials and Methods
Pure honey (Saudi Sidr variety) was purchased from an exclusive honey shop in Riyadh, Saudi Arabia.
2.1. Acute Toxicity Test
The acute toxicity test was performed on the mice using the oral route. SSH was dissolved in distilled water and administered at various doses, ranging from (500–5000 mg/kg), to different groups of mice. The animals were observed continuously for 1 h and then at half-hourly intervals for 4 h on the first day for clinical signs and symptoms of toxicity and further up to 72 h followed by 14 days for any mortality.
2.2. Animals and Study Design
Wistar albino rats (180–200 g) were obtained from the Experimental Animal Care Center of the College of Pharmacy, King Saud University, Riyadh. Animals were maintained on standard chow diet and housed in polycarbonate cages in a room free from any source of chemical contamination, artificially illuminated (12 h dark/light cycle) and thermally controlled (25 ± 2°C) at the animal facility. All animals received humane care in compliance with the guidelines of the Ethics Committee of the Experimental Animal Care Society, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
After a one-week acclimatization period, animals were randomly allocated into 5 groups and treated as follows: group (1), untreated control; group (2, 3, 4, and 5) received 0.25 mL of CCl4 in liquid paraffin (1 : 1) 1.25 mL/kg body weight intraperitoneally (IP). Group 2 was administered only CCl4. Groups 3 and 4 received SSH 0.5 and 1.0 g/kg/day orally for 6 weeks. Rats in group 5 were treated with 10 mg/kg orally with silymarin for similar days. The blood was collected by cardiac puncture after 24 h following the administration of CCl4, allowed to clot and the serum was separated. After collecting the blood, the animals were sacrificed using ether anaesthesia. The liver and kidneys were dissected out and used for biochemical estimations and histological assessment.
2.2.1. Estimation of Marker Enzymes and Bilirubin
Serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) [16], alkaline phosphatase (ALP) [17], gamma-glutamyl transferase (GGT) [18], hemoglobin, and bilirubin [19] were determined using Reflotron Plus Analyzer and Roche kits (Roche Diagnostics GmbH, Mannheim, Germany).
2.2.2. Estimation of Lipid Profile
Total cholesterol [20], triglycerides [21], high-density lipoproteins (HDLC) [22], and glucose levels were estimated in serum using Roche diagnostic kits (Roche Diagnostics GmbH, Mannheim, Germany).
2.2.3. Determination of Malondialdehyde (MDA)
The method reported by Utley et al. [23] was followed. In brief, the liver and kidney tissues were removed, and each tissue was homogenized in 0.15 M KCl (at 4°C; Potter-Elvehjem type C homogenizer) to give a 10% w/v homogenate. The absorbance of the solution was then read at 532 nm. The content of malondialdehyde (nmol/g wet tissue) was then calculated, by reference to a standard curve of malondialdehyde solution.
2.2.4. Estimation of Nonprotein Sulfhydryls (NP-SH)
Hepatic nonprotein sulfhydryls were measured according to the method of Sedlak and Lindsay [24]. The liver and kidney were homogenized in ice-cold 0.02 mmol/L ethylenediaminetetraacetic acid (EDTA). The absorbance was measured within 5 min of addition of 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) at 412 nm against a reagent blank.
2.2.5. Determination of Total Protein (TP)
Total protein was estimated by the kit method, supplied by Crescent Diagnostics, Jeddah, Saudi Arabia. The absorbance of this complex at 546 nm is proportional to the protein concentration. The serum total protein was calculated using the equation:
| (1) |
2.2.6. Histopathological Evaluation
The liver and kidney tissue samples were fixed in neutral buffered formalin for 24 h. Sections of the liver tissue were histopathologically examined. These sections were stained with haematoxylin and eosin using routine procedures [25].
2.3. Ex Vivo Assay of SSH on Cultured Hepatocytes
2.3.1. Cells and Reagents
HepG2, a human hepatoma cell line was grown in RPMI medium (supplemented with 10% bovine serum, 1x penicillin-streptomycin, and 1x sodium pyruvate streptomycin (HyClone Laboratories)) at 37°C in a humified chamber with 5% CO2.
2.3.2. Hepatotoxicity and Treatment
HepG2 cells were seeded (105 cells/well in triplicate) in a 96-well flat-bottom plate (Becton-Dickinson Labware) a day before treatment and grown. 2′,7′-dichlorofluorescein (DCFH) (Sigma) commonly used to measure oxidative stress, in vitro [26], was used as a cytotoxic agent (IC50: 100 μM, personal observation), prepared in DMSO (Sigma). An aqueous suspension of pure SSH (250 mg/mL stock) was prepared in RPMI medium followed by diluting to seven doses of SSH (0.1, 0.25, 0.5, 1.0, 2.5, 5, and 10 mg/mL). The cells (in triplicate) were replenished with RPMI containing 100 μM DCF plus a dose of SSH, including untreated as well as DCF-treated controls, and further incubated for 48 hours.
2.3.3. Microscopy
A direct visual observation was made under an inverted microscope (Optica, 40x and 100x) to see any morphological changes in the cells cultured with SSH and/or DCF on day 1 and 2.
2.3.4. Cell Proliferation and Viability Test
On day 2 of treatment, hepatocyte proliferation and viability test was performed using TACS MTT Cell Proliferation and Viability Assay Kit (TACS).
2.4. Studies of the In Vitro Antioxidant Activity
2.4.1. DPPH Free Radical Scavenging Assay
The radical scavenging activity of the SSH against DPPH was evaluated as previously described [27]. The sample was redissolved in water, and various concentrations (31, 62, 125, 250, and 500 mg/mL) of the honey, 125 μL prepared DPPH (1 mM in methanol), and 375 μL solvent (methanol) were added. After 30 min incubation at 25°C, the decrease in absorbance was measured at λ = 517 nm. The radical scavenging activity was calculated from the equation:
| (2) |
2.4.2. β-Carotene-Linoleic Acid Assay
The antioxidant activity of the SSH was evaluated, using the β-carotene bleaching method as described by Mothana et al. [28]. Rutin (1 mg/mL) was used as a standard. Absorbance was read at 470 nm at 15 min intervals, using a UV-visible spectrophotometer (UV mini-1240, Shimadzu, Japan). The antioxidant activity was calculated using the equation:
| (3) |
where Abs0 and Abs0° are the absorbance values measured at zero time of incubation for sample extract and control, respectively.
Abst and Abst° are the absorbance values for sample extract and control, respectively, at t = 120 min.
2.5. Total Phenolic Content
The Folin-Ciocalteu method was used to determine the total phenolic content (TPC) of the honey according to Singleton et al. [29] and Liberato et al. [30]. Values of TPC were estimated by comparing the absorbance of each sample with a standard response curve generated using gallic acid (0, 12.5, 25, 50, 100, and 200 µg/mL). The results were expressed as mg gallic acid equivalents (GAE)/100 g of honey. All the measurements were taken in triplicate, and the mean values were calculated.
2.6. Total Flavonoid Content
The total flavonoid content was determined by using a colorimetric assay as previously described [30]. Briefly, an aliquot of 5 mL of honey solution (0.02 mg/mL) or standard solution was mixed individually with the same volumes of solution of 2% aluminum chloride (AlCl3) and allowed to stand at room temperature for 10 minutes. The absorbance was then read at 415 nm. A calibration curve was prepared with quercetin, and the results were expressed as mg quercetin equivalents (CE)/100 g of honey.
2.7. Statistical Analysis
Values are given as arithmetic means ± standard error of the mean (S.E.M.). Data was statistically analyzed by using one-way analysis of variance (ANOVA) followed by Student's t-test.
3. Results
3.1. Acute Toxicity Test
No toxicity symptoms were recorded. The LD50 value by oral route could not be determined as no lethality was observed up to 5.0 g/kg of the SSH in the animals.
3.2. In Vivo Effect of SSH on Liver and Kidney
The results indicated that animals treated with CCl4 showed a significant increase in all biochemical parameters tested. However, animals treated with Saudi Sidr honey for 6 weeks before the intoxication with CCl4 showed a significant decrease in serum GOT, GPT, ALP, GGT, creatinine, bilirubin, urea, and uric acid levels (Tables 1 and 3). CCl4-induced oxidative stress caused an elevation in lipid profile including cholesterol, triglycerides, LDL-C, and VLDL-C and reduction in the HDL-C levels in serum. The six-week pretreatment of rats with SSH in both doses, dose-dependently and significantly, reduced the cholesterol, triglycerides, LDL-C, and VLDL-C levels and significantly improved HDL-C level (Table 2). Silymarin, on the other hand, significantly prevent the CCl4-induced elevated levels of marker enzymes and lipid profile.
Table 1.
Effect of honey on CCl4-induced hepatotoxicity-related parameters in rats.
| Treatment group (n = 6) | SGOT (U/L) | SGPT (U/L) | GGT (U/L) | ALP (U/L) | Bilirubin (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 75.53 ± 2.86 | 26.93 ± 1.75 | 3.66 ± 0.37 | 282.00 ± 10.05 | 0.52 ± 0.02 |
| CCl4 only (1.25 mL/kg) | 300.00 ± 8.23∗∗∗a | 209.50 ± 8.44∗a | 15.45 ± 1.20∗∗∗a | 671.33 ± 15.15∗∗∗a | 3.45 ± 0.16∗∗∗a |
| Honey (0.5 g/kg) + CCl4 | 273.66 ± 10.28b | 182.83 ± 7.61∗b | 12.81 ± 0.61b | 614.83 ± 13.41∗b | 2.67 ± 0.14∗∗b |
| Honey (1.0 g/kg) + CCl4 | 200.00 ± 5.08∗∗∗b | 140.83 ± 6.12∗∗∗b | 10.38 ± 0.54∗∗b | 605.16 ± 12.82∗∗b | 2.01 ± 0.10∗∗∗b |
| Silymarin (10 mg/kg) + CCl4 | 133.58 ± 8.08∗∗∗b | 106.78 ± 6.33∗∗∗b | 5.08 ± 0.30∗∗∗b | 416.16 ± 11.62∗∗∗b | 1.17 ± 0.10∗∗∗b |
All values represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, followed by Dunnett's multiple comparison test.
aAs compared with normal group. bAs compared with CCl4 only group.
Table 3.
Effect of honey on CCl4-induced kidney function test in serum.
| Treatment group (n = 6) | Creatinine (mg/dL) |
Uric Acid (mg/dL) |
Urea (mmol/L) |
Sodium (mEq/L) |
Potassium (mEq/L) |
Calcium (mg/dL) |
|---|---|---|---|---|---|---|
| Normal control | 1.34 ± 0.09 | 1.28 ± 0.010 | 35.8 ± 1.90 | 54.96 ± 0.99 | 3.61 ± 0.18 | 4.42 ± 0.34 |
| CCl4 only (1.25 mL/kg) | 7.48 ± 0.31∗∗∗a | 5.68 ± 0.36∗∗∗a | 167.16 ± 4.65∗∗∗a | 101.89 ± 2.26∗∗∗a | 10.58 ± 0.48∗∗∗a | 24.64 ± 0.81∗∗∗a |
| Honey (0.5 g/kg) + CCl4 | 6.48 ± 0.31∗b | 4.25 ± 0.43∗b | 157.5 ± 5.51b | 88.67 ± 2.35∗∗b | 9.18 ± 0.27∗b | 20.42 ± 0.98∗∗b |
| Honey (1.0 g/kg) + CCl4 | 5.11 ± 0.13∗∗∗b | 2.89 ± 0.21∗∗∗b | 131.83 ± 8.15∗∗b | 78.14 ± 2.30∗∗∗b | 7.31 ± 0.23∗∗∗b | 13.76 ± 0.87*** |
| Silymarin (10 mg/kg) + CCl4 | 6.08 ± 0.27∗∗b | 4.30 ± 0.25∗b | 154.66 ± 5.85b | 93.44 ± 3.20∗b | 10.05 ± 0.40b | 25.12 ± 0.84b |
All values represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, followed by Dunnett's multiple comparison test.
aAs compared with normal group, bAs compared with CCl4 only group.
Table 2.
Effect of honey on CCl4-induced lipid profile changes in rats.
| Treatment group (n = 6) | Cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 13.79 ± 1.22 | 91.34 ± 4.57 | 52.56 ± 6.14 | 68.98 ± 6.14 | 105.33 ± 3.81 |
| CCl4 only (1.25 mL/kg) | 43.61 ± 1.58∗∗∗a | 237.63 ± 10.61∗∗∗a | 23.47 ± 1.09∗∗∗a | 218.05 ± 7.94∗∗∗a | 281.33 ± 11.01∗∗∗a |
| Honey (0.5 g/kg) + CCl4 | 34.35 ± 1.63∗∗b | 172.29 ± 8.93∗∗∗b | 31.28 ± 1.12∗∗b | 171.29 ± 8.19∗∗b | 206.66 ± 9.77∗∗∗b |
| Honey (1.0 g/kg) + CCl4 | 20.00 ± 1.17∗∗∗b | 170.50 ± 6.46∗∗∗b | 44.38 ± 1.97∗∗∗b | 100.00 ± 5.87∗∗∗b | 190.66 ± 6.97∗∗∗b |
| Silymarin (10 mg/kg) + CCl4 | 38.79 ± 0.80∗b | 198.43 ± 9.82∗b | 29.27 ± 1.55∗b | 199.98 ± 4.02∗b | 237.33 ± 9.16∗b |
All values represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, followed by Dunnett's multiple comparison test.
aAs compared with normal group, bAs compared with CCl4 only group.
The results also indicated that treatment with CCl4 resulted in a significant increase in MDA and a significant decrease in NP-SH and TP concentration in both liver and kidney tissues (Tables 4 and 5). Treatment of rats with SSH resulted in a significantly diminished level of MDA and significantly enhanced NP-SH and TP levels in both liver and kidney tissue.
Table 4.
Biochemical parameters (liver tissue) treated with honey.
| Treatment group (n = 6) | Total protein (g/L) | MDA (nmol/g) | NP-SH (nmol/g) |
|---|---|---|---|
| Normal control | 122.49 ± 4.61 | 0.97 ± 0.11 | 7.08 ± 0.34 |
| CCl4 only (1.25 mL/kg) | 41.52 ± 2.59∗∗∗a | 11.88 ± 0.77∗∗∗a | 3.81 ± 0.43∗∗∗a |
| Honey (0.5 g/kg) + CCl4 | 54.38 ± 4.29∗b | 7.96 ± 0.77∗∗b | 4.96 ± 0.47b |
| Honey (1.0 g/kg) + CCl4 | 58.44 ± 3.81∗∗b | 5.80 ± 0.58∗∗∗b | 6.13 ± 0.37∗∗b |
| Silymarin (10 mg/kg) + CCl4 | 80.83 ± 3.28∗∗∗b | 2.91 ± 0.23∗∗∗b | 6.96 ± 0.29∗∗∗b |
All values represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, followed by Dunnett's multiple comparison test.
aAs compared with normal group, bAs compared with CCl4 only group.
Table 5.
Biochemical parameters (kidney tissue) treated with honey.
| Treatment group (n = 6) | Total protein (g/L) | MDA (nmol/g) | NP-SH (nmol/g) |
|---|---|---|---|
| Normal control | 89.75 ± 4.12 | 0.69 ± 0.13 | 7.23 ± 0.46 |
| CCl4 only (1.25 mL/kg) | 31.70 ± 3.45∗∗∗a | 8.53 ± 0.91∗∗∗a | 4.08 ± 0.35∗∗∗a |
| Honey (0.5 g/kg) + CCl4 | 41.89 ± 1.62∗b | 5.77 ± 0.39∗b | 4.76 ± 0.45b |
| Honey (1.0 g/kg) + CCl4 | 46.17 ± 2.18∗∗b | 3.95 ± 0.32∗∗∗b | 5.80 ± 0.31∗∗b |
| Silymarin (10 mg/kg) + CCl4 | 64.72 ± 7.42∗∗∗b | 1.89 ± 0.28∗∗∗b | 6.29 ± 0.46∗∗b |
All values represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, followed by Dunnett's multiple comparison test.
aAs compared with normal group, bAs compared with CCl4 only group.
Upon histopathological assessment of liver, the CCl4-induced rats showed an evidence of fatty changes with necrosis in liver cells, extensive fatty and inflammatory changes along with vascular congestion, and minimal fibrosis. The rats which received SSH (0.5 and 1.0 g/kg/day) and silymarin, as oral pretreatment showed a marked improvement in liver parenchyma with remnant degenerative changes of the cytoplasm and completely intact liver hepatocytes (Figure 1).
Figure 1.
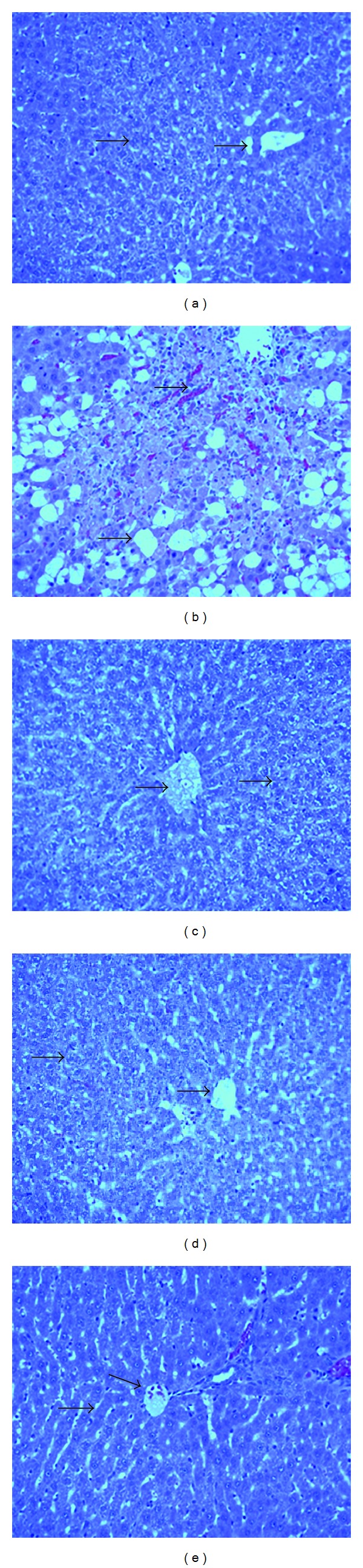
Light micrographs showing the effect of SSH on CCl4-induced hepatotoxicity in rats. (a) Normal hepatocytes. (b) CCl4-induced severe necrosis and inflammation. (c) Pretreatment of rats with SSH 0.5 g/kg. (d) Pretreatment of rats with SSH 1.0 g/kg. (e) Pretreatment of rats with silymarin 10 mg/kg.
The histological examination of kidney sections of control rat showed normal glomeruli, interstitial tubules, and blood vessels. The kidney tissue slices in rats treated with CCl4 exhibited glomerular congestion with vacuolization of the glomerular tuft and tubule with sloughing of the renal tubular lining. The kidney sections in rats pretreated with honey and silymarin showed vacuolization of glomerular tuft, evidence of tubular necrosis, and, regenerative and desquamation vacuolization (Figure 2).
Figure 2.
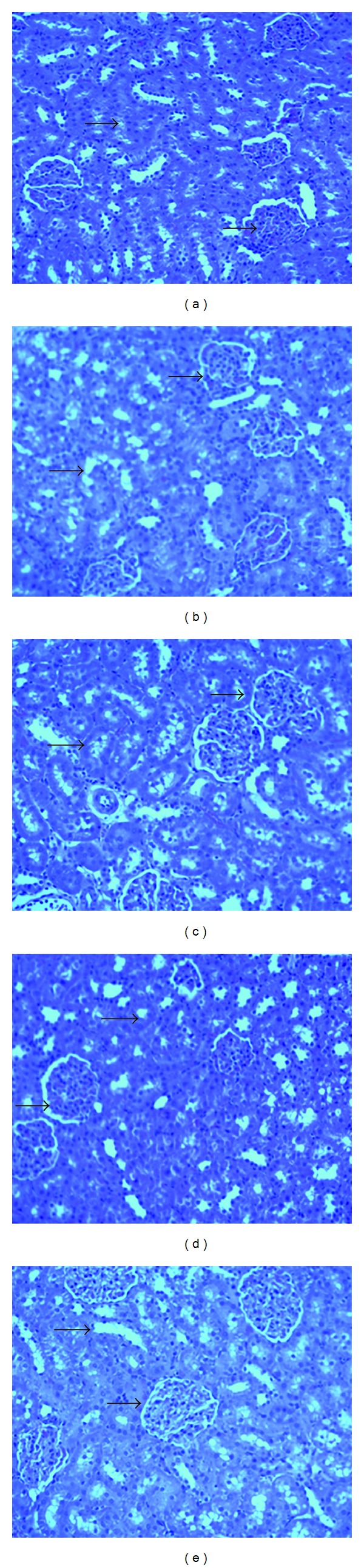
Light micrographs showing the effect of SSH on CCl4-induced nephrotoxicity in rats. (a) Normal kidney tissues. (b) CCl4-induced severe glomerular congestion and vacuolization of the glomerular tuft and tubules with sloughing of the renal tubular lining. (c) Pretreatment of rats with SSH 0.5 g/kg. (d) Pretreatment of rats with SSH 1.0 g/kg. (e) Pretreatment of rats with silymarin 10 mg/kg.
3.3. Hepatoprotective Effect of SSH on Cultured Human Liver Cells
3.3.1. Microscopy
DCF exhibited severe cytotoxic effect on the human liver cells as reflected by altered morphology compared to untreated cells. Interestingly, the DCF-treated cells supplemented with 10 mg/mL of SSH were morphologically different from the DCF-treated cells but comparable to untreated cells (data not shown).
3.3.2. Hepatocyte Protection and Viability Restoration by SSH
Our MTT test showed a protective effect of SSH (10 mg/mL) against DCF (100 μM) induced hepatotoxicity at 48 hours posttreatment (Figure 3), and in line with our microscopic observation that confirmed the ex vivo hepatoprotection by pure SSH. Under these conditions DCF-toxicated cells were recovered to about 70% with 10 mg/mL of SSH. The SSH supplementation restored the hepatocytes viability by 1.5-fold against DCF toxicity.
Figure 3.
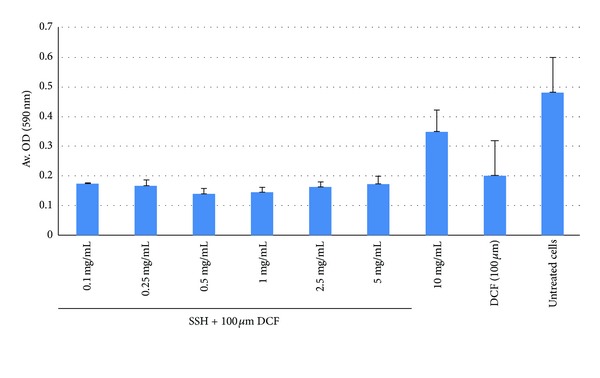
MTT test showing hepatoprotective effect of SSH (10 mg/mL) against DCF (100 μM)-induced toxicity of cultured human liver cells (HepG2).
3.4. Antioxidant Activity and Phenolic and Flavonoidal Contents of the SSH
The potential antioxidant activity of the SSH was investigated on the basis of DPPH radical scavenging activity and of inhibition of linoleic acid oxidation. As demonstrated in Table 6, SSH was able to reduce the stable free radical DPPH to the yellow-colored DPPH at low concentrations (125 and 250 mg/mL), almost near to the ascorbic acid. In addition to that, in the β-carotene/linoleic acid model system, the SSH was also able to inhibit the discoloration of β-carotene at a concentration of 125 mg/mL. The total antioxidant value was 90.8% (Table 6). The observed antioxidant activities were comparable to that of the positive control, rutin (Table 6). Moreover, the SSH showed high total phenolic and flavonoidal contents (105.1 ± 4.04 mg gallic acid equivalents/100 g and 48.3 ± 1.17 mg quercetin equivalents/100 g).
Table 6.
Free radical scavenging activity, antioxidant activity, total phenolic, and total flavonoidal contents of the honey sample.
| Plant species | Radical scavenging activity (%) | Total antioxidant activity (%) | TPC (mg GAE /100 g) | TFC (mg QE/100 g) | ||||
|---|---|---|---|---|---|---|---|---|
| 31.25 | 62.5 | 125 | 250 | 500 | 125 (mg/mL) | |||
| Honey | 28.0 | 45.0 | 72.7 | 89.3 | 91.8 | 90.5 ± 7.62 | 105.1 ± 4.04 | 48.3 ± 1.17 |
| Ascorbic acid | 18.5 | 74.1 | 88.9 | 93.0 | 94.0 | — | ||
| Rutin | 91.9 ± 5.83 | |||||||
TPC: Total phenolic content; TFC: Total flavonoidal content.
4. Discussion
The liver is an organ that not only performs physiological functions but also protect against the hazards of harmful drugs, chemicalss and xenobiotics. The liver is one of the largest and highly complex organs with multifunction, including nutrient storage, maintenance of homeostasis, secretory and excretory function, and synthesis of proteins [31]. These functions also include the metabolism of certain hormones, lipid metabolism (cholesterol, triglycerides, and high-density lipoproteins (HDL)), protein metabolism, and detoxification of xenobiotics [32]. The model used in the present study has been subjected to thorough critical appraisal and validated animal models of hepatorenal protective activity of honey in rats against CCl4 as a hepatorenal toxin to prove its claims in folklore practice against liver and kidney disorders.
Carbon tetrachloride, besides exerting its toxic effect on liver, also reportedly gets distributed at higher concentrations in the kidney than in the liver [33]. The mechanism of CCl4 renal toxicity is almost the same as that of liver, but CCl4 shows a high affinity to the kidney cortex which contains cytochrome P-450 predominantly [34, 35]. Due to CCl4 hepatorenal injury, the transport function of hepatocytes and nephrotic cells gets disturbed resulting in the leakage of plasma membrane, thereby causing an increased enzyme level in the serum [36].
In the present study CCl4 administration to rats caused a significant increase in serum GOT, GPT, GGT, ALP, urea, uric acid, bilirubin, and creatinine as well as MDA in the liver and kidney levels accompanied with a significant decrease in total protein and NP-SH in liver and kidney, which indicates the extensive disruption of the structure and function of the liver and kidney. The tendency of these marker enzymes (SGPT, SGOT, ALPG, GGT) at a near normal level in the groups of rats treated with Saudi Sidr honey and silymarin is a clear manifestation of antihepatorenal toxic effect of the honey. Our findings are in agreement with an earlier study in which feeding of honey caused liver protective effect by depleting the elevated liver marker enzymes in CCl4-treated rats [37]. On the other hand, treatment of rats with CCl4 increases the levels of total lipids, triglycerides, and cholesterol in serum [38]. The significant diminution of lipids and total cholesterol in the SSH-treated rats and an increase in HDL-C level further indicates the hepatoprotective potential of the honey.
Furthermore, the efficacy of any hepatoprotective drug is essentially dependent on its capacity of either maintaining normal physiological function or diminishing the harmful effects which were affected by hepatotoxic agents [39]. Reduction in total protein (TP) content can be deemed as a useful index of severity of hepatocellular damage [40]. The lowered levels of total proteins in the serum of CCl4-treated rats exhibited the severity of hepatopathy. Thus, this suggests that honey possesses the ability to promote protein synthesis leading to the higher concentration of protein in the liver [35].
The present study also revealed that the administration of CCl4 caused marked impairment in renal function alongside with significant oxidative stress in the kidney [41]. Serum creatinine, urea, and uric acid concentrations were significantly higher in CCl4-treated rats. Serum creatinine elevation was caused by CCl4 due to altered kidney function. Urea is the main end product of protein catabolism. It is one of the waste products of the body which is passed into blood stream to be removed by kidney [42]. Honey significantly decreased the elevated levels of serum creatinine, urea, and uric acid, which indicates that the honey possibly protects kidney tissue against oxidative damages induced by CCl4 [43] and indicates maintenance of renal function [44].
In vitro antioxidant activity of Saudi Sidr honey revealed a strong antioxidant activity in both the tests DPPH and β-carotene linoleic acid used. The observed hepatonephro protective and antioxidant activity of honey might have been due to the presence of phenolic and flavonoidal contents. In the current study, the increase in MDA level in the liver and kidney by CCl4 suggests enhanced lipid peroxidation (LPO) which is an important pathogenic event that damages biomembranes [45]. LPO is thought to be a consequence of oxidative stress which occurs when the dynamic balance between prooxidant and antioxidant mechanism is impaired [46]. Malondialdehyde is known to be a reliable marker of LPO and oxidative stress [47]. Treatment of rats with SSH significantly prevented CCl4-induced increase in MDA concentration in both liver and kidney. Hence, the observed hepato-renal protective activity of honey may be due to its antioxidant property.
Nonprotein sulfhydryls are known to be involved in several defense processes against oxidative damage; protects cells against free radicals peroxides and various poisonous substances [48]. Thus, a deficiency of GSH within the living organisms can cause tissue injury and malfunction [49]. In the current study, the liver and kidney NP-SH level in CCl4-treated group was significantly diminished when compared with the control group. These findings are in accordance with earlier reports as sulfhydryl levels were significantly depleted in different organs of rats, when exposed with CCl4 [50]. Pretreatment of rats with SSH replenished NP-SH concentration in both liver and kidney homogenate as compared with CCl4 only treated animals, suggesting the ameliorative effects of SSH on liver and kidney damage induced by CCl4, at least in part, due to its free radical scavenging activity. Furthermore, most of the parameters in the group which received CCl4 plus Sidr honey having nearer value of the control group demonstrate Sidr honey to have antiradical effect [51]. This effect is considered to be related to the phenolic compounds and in vitro antioxidant activity of Saudi Sidr honey.
On the other hand, DCFH is generally used to measure in vitro oxidative stress generated by free radicals through the principle of oxidation of DCFH to the fluorescent DCF. However, we used this agent because of its cytotoxic effect (unpublished). In this study, our ex vivo SSH protection against DCFH-induced human liver toxicity further supported the in vivo effects of SSH in CCl4-induced rat liver injury. In this way, we have confirmed our results by using two different toxins in two different systems, at least for hepatoprotection.
5. Conclusions
In conclusion, the administration of Saudi Sidr honey (SSH) prevented biochemical and histomorphological alteration induced by CCl4. This hepatorenal protective effect of SSH could be attributed to the presence of antioxidative factors for example, phenolic and flavonoidal compounds which cause significant lowering of the oxidative threat leading to normal physiological function. The findings support the use of SSH for the treatment of several liver and kidney ailments.
Conflict of Interests
The authors confirm that there is no conflict of interests.
Acknowledgments
The authors would like to express their gratitude to the Research Center, College of Pharmacy, and the Deanship of Scientific Research of King Saud University, Riyadh, for their assistance.
References
- 1.Alvarez-Suarez JM, Tulipani S, Romandini S, Bertoli E, Battino M. Contribution of honey in nutrition and human health: a review. Mediterranean Journal of Nutrition and Metabolism. 2010;3(1):15–23. [Google Scholar]
- 2.Feás X, Pires J, Iglesias A, Estevinho ML. Characterization of artisanal honey produced on the Northwest of Portugal by melissopalynological and physico-chemical data. Food and Chemical Toxicology. 2010;48(12):3462–3470. doi: 10.1016/j.fct.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Lay-flurrie K. Honey in wound care: effects, clinical application and patient benefit. British Journal of Nursing. 2008;17(11):S30–S32. doi: 10.12968/bjon.2008.17.Sup5.29649. [DOI] [PubMed] [Google Scholar]
- 4.Al ML, Daniel D, Moise A, Bobis O, Laslo L, Bogdanov S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chemistry. 2009;112(4):863–867. [Google Scholar]
- 5.Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chemistry. 2009;114(4):1438–1443. [Google Scholar]
- 6.Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews. 2008;(4):p. CD005083. doi: 10.1002/14651858.CD005083.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. Journal of the American College of Nutrition. 2008;27(6):677–689. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- 8.Jeffrey AE, Echazarreta CM. Medical uses of honey. Reviews in Biomedical. 1996;7(1):43–49. [Google Scholar]
- 9.Allen KL, Molan PC, Reid GM. A survey of the antibacterial activity of some New Zealand honeys. Journal of Pharmacy and Pharmacology. 1991;43(12):817–822. doi: 10.1111/j.2042-7158.1991.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 10.Vela L, De Lorenzo C, Pérez RA. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. Journal of the Science of Food and Agriculture. 2007;87(6):1069–1075. [Google Scholar]
- 11.Jeddar A, Kharsany A, Ramsaroop UG. The antibacterial action of honey. An in vitro study. South African Medical Journal. 1985;67(7):257–258. [PubMed] [Google Scholar]
- 12.Zeina B, Zohra BI, Al-assad S. The effects of honey on leishmania parasites: an in vitro study. Tropical Doctor. 1997;27(1):36–38. [PubMed] [Google Scholar]
- 13.Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.López-Lázaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Letters. 2007;252(1):1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Michaluart P, Masferrer JL, Carothers AM, et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Research. 1999;59(10):2347–2352. [PubMed] [Google Scholar]
- 16.Reitman SA, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 17.King EJ, Armstrong AR. Calcium, phosphorus and phosphate. In: Varley H, editor. Practical Clinical Biochemistry. New Delhi, India: CBS publishers; 1988. [Google Scholar]
- 18.Fiala S, Fiala AE, Dixon B. Gamma-glutamyl transpeptidase in transplantable, chemically induced rat hepatomas and spontaneous mouse hepatomas. Journal of the National Cancer Institute. 1972;48(5):1393–1401. [PubMed] [Google Scholar]
- 19.Stiehl A. Hyperbilirubinaemia in liver disease. Fortschritte der Medizin. 1982;100(18):842–845. [PubMed] [Google Scholar]
- 20.Demacher PNM, Hijamaus AGM. A study of the use of polyethylene glycol in estimating cholesterol. Clinical Chemistry. 1980;26:1775–1778. [PubMed] [Google Scholar]
- 21.Foster LB, Dunn RT. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clinical Chemistry. 1973;19(3):338–340. [PubMed] [Google Scholar]
- 22.Burstein M, Scholnick HR. Turbidimetric estimation of chylomicrons and very low density lipoproteins in human sera after precipitation by sodium lauryl sulfate. Biomedicine. 1973;19(1):16–19. [PubMed] [Google Scholar]
- 23.Utley HC, Bernheim F, Hochslein P. Effect of sulfhydryl reagent onperoxidation in microsome. Archives of Biochemistry Biophysics. 1967;260:521–531. [Google Scholar]
- 24.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry C. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 25.Culling CF. Handbook of Histopathological and Histochemical Techniques. 3rd edition. London, UK: Butterworth; 1974. [Google Scholar]
- 26.Rota C, Chignell CF, Mason RP. Evidence for free radical formation during the oxidation of 2’-7’-dichlorofluorescin to the fluorescent dye 2’-7’-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radical Biology and Medicine. 1999;27(7-8):873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 27.Brand-Williams WW, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Science and Technology. 1995;28(1):25–30. [Google Scholar]
- 28.Mothana RAA, Al-Said MS, Al-Rehaily AJ, Thabet TM, Awad NA, Lalk M, et al. Anti-inflammatory, antinociceptive, antipyretic and antioxidant activities and phenolic constituents from Loranthus regularis Steud. Ex Sprague. Food Chemistry. 2012;130(2):344–349. [Google Scholar]
- 29.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1998;299:152–178. [Google Scholar]
- 30.Liberato MDCTC, De Morais SM, Siqueira SMC, et al. Phenolic content and antioxidant and antiacetylcholinesterase properties of honeys from different Floral origins. Journal of Medicinal Food. 2011;14(6):658–663. doi: 10.1089/jmf.2010.0097. [DOI] [PubMed] [Google Scholar]
- 31.Williamson EM, Okpako DT, Evans FJ. The Liver and Biliary System: Selection, Preparation and Pharmacological Evaluation of Plant Material. London, UK: John Wiley and Sons; 1996. [Google Scholar]
- 32.de Medeiros BL, Costa KS, Alves J, et al. Liver protective activity of a hydroethanolic extract of Arrabidaea chica (Humb. and Bonpl.) B. Verl., (pariri) Pharmacognosy Research. 2011;3(2):79–84. doi: 10.4103/0974-8490.81954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanzgiri UY, Srivatsan V, Muralidhara S, Dallas CE, Bruckner JV. Uptake, distribution, and elimination of carbon tetrachloride in rat tissues following inhalation and ingestion exposures. Toxicology and Applied Pharmacology. 1997;143(1):120–129. doi: 10.1006/taap.1996.8079. [DOI] [PubMed] [Google Scholar]
- 34.Jaramillo-juárez F, Rodriguez-Vázquez ML, Rincón-Sánchez AR, Consolación Martinez M, Ortiz GG, Llamas J, et al. Acute renal failure induced by carbon tetrachloride in rats with hepatic cirrhosis. Annals of HepatoIogy. 2008;7(4):331–338. [PubMed] [Google Scholar]
- 35.Abraham P, Wilfred G, Cathrine SP. Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clinica Chimica Acta. 1999;289(1-2):177–179. doi: 10.1016/s0009-8981(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 36.Achliya GS, Wadodkar SG, Dorle AK. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. Journal of Ethnopharmacology. 2004;90(2-3):229–232. doi: 10.1016/j.jep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Kilicoglu B, Gencay C, Kismet K, et al. The ultrastructural research of liver in experimental obstructive jaundice and effect of honey. American Journal of Surgery. 2008;195(2):249–256. doi: 10.1016/j.amjsurg.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Seakins A, Robinson DS. The effect of the administration of carbon tetrachloride on the formation of plasma lipoproteins in the rat. The Biochemical Journal. 1963;86:401–407. doi: 10.1042/bj0860401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazeem MI, Bankole HA, Fatai AA. Protective effect of ginger in normal and carbon-tetrachloride induced hepatotoxic rats. Annals of Biological Research. 2011;2(1):1–8. [Google Scholar]
- 40.Aniya Y, Koyama T, Miyagi C, et al. Free radical scavenging and hepatoprotective actions of the medicinal herb, Crossocephalum crepidioides from the Okinawa Islands. Biological and Pharmaceutical Bulletin. 2005;28(1):19–23. doi: 10.1248/bpb.28.19. [DOI] [PubMed] [Google Scholar]
- 41.Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. African Journal of Biomedical Research. 2007;10(2):153–164. [Google Scholar]
- 42.Orth SR, Ritz E. The nephrotic syndrome. Internist. 1998;39(12):1246–1252. doi: 10.1007/s001080050297. [DOI] [PubMed] [Google Scholar]
- 43.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 44.Davison AM, Cameron JS, Gruenfeld J-P, Kerr DNS, Ritz E, Winearls G. Oxford, USA: Oxford University Press; 1998. Oxford Textbook of Clinical Nephrology; pp. 36–39. [Google Scholar]
- 45.Nayagam AAJ, Manokaran S, Sudhakar N. Hepatoprotective efficacy of Tricholepis radicans DC. against CCl4 induced liver toxicity in albino rats. Journal of Pharmacy Research. 2011;4(4):1073–1075. [Google Scholar]
- 46.Kumar RS, Asokkumar K, Murthy NV. Hepatoprotective effects and antioxidant role of scutia myrtina on paracetamol induced hepatotoxicity in rats. Journal of Complementary and Integrative Medicine. 2011;8(1):p. 8. doi: 10.2202/1553-3840.1461. [DOI] [PubMed] [Google Scholar]
- 47.Kitts DD, Yuan YV, Godin DV. Plasma and lipoprotein lipid composition and hepatic antioxidant status in spontaneously hypertensive (SHR) and normotensive (WKY) rats. Canadian Journal of Physiology and Pharmacology. 1998;76(2):202–209. [PubMed] [Google Scholar]
- 48.Sies H. Glutathione and its role in cellular functions. Free Radical Biology and Medicine. 1999;27(9-10):916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 49.Ganie SA, Haq E, Hamid A, et al. Carbon tetrachloride induced kidney and lung tissue damages and antioxidant activities of the aqueous rhizome extract of Podophyllum hexandrum . BMC Complementary and Alternative Medicine. 2011;11:p. 17. doi: 10.1186/1472-6882-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohta Y, Kongo M, Sasaki E, Nishida K, Ishiguro I. Therapeutic effect of melatonin on carbon tetrachloride-induced acute liver injury in rats. Journal of Pineal Research. 2000;28(2):119–126. doi: 10.1034/j.1600-079x.2001.280208.x. [DOI] [PubMed] [Google Scholar]
- 51.Eraslan G, Kanbur M, Silici S, Karabacak M. Beneficial effect of pine honey on trichlorfon induced some biochemical alterations in mice. Ecotoxicology and Environmental Safety. 2010;73(5):1084–1091. doi: 10.1016/j.ecoenv.2010.02.017. [DOI] [PubMed] [Google Scholar]


