Abstract
Endovascular treatment of acute and chronic iliac vein occlusions has proven to be safe and effective. Recanalization of chronic occlusions with balloon angioplasty and stenting can re-establish normal venous flow in the iliac veins and the IVC and relieve symptoms in the majority of treated patients. CDT with recanalization and stenting of underlying chronically obstructed iliofemoral segments is becoming the treatment of choice for patients with acute iliofemoral thrombosis, as anticoagulation and compression therapy alone are not satisfactory in preventing PTS. The new treatment modalities offer stimulating options for a patient group that is not adequately treated, neither by medical nor open surgical therapy. The substantial effort and additional costs of endovascular treatment appear to be justified by the encouraging mid-term results both for patients with acute and chronic occlusive iliofemoral disease. However, multi-center randomized prospective studies are required to further validate the role of these techniques.
Keywords: venous thromboembolism, deep venous thrombosis, thrombolytic therapy, catheter-directed thrombolysis, venous occlusive disease, iliac vein occlusion, venous recanalization
Introduction
The treatment for chronic and acute occlusions of the main venous trunks has changed in the 1990s with the introduction of endovascular techniques. In the past, open surgery including bypass for chronic venous occlusive disease and thrombectomy for acute thrombosis were invasive procedures, often used unselectively without proper pre- and intra-operative imaging and frequently with disappointing results. These techniques have been widely replaced by endovascular interventions such as percutaneous recanalization combined with stenting and/or catheter-directed thrombolysis (CDT).
The main venous outflow of the lower limb is the deep femoral vein.1) If deep venous thrombosis (DVT) which often origins from the veins of the soleus muscle progresses into the femoral vein, propagation to the more proximal segments is unlikely due to the substantial inflow to the common femoral vein from the long saphenous vein, the deep femoral vein and the commitant veins. Thrombosis in the iliofemoral vein segment does not characteristically commence in a more distal DVT, but usually originates in the iliofemoral segment.1) DVT usually is categorized into distal and proximal/iliofemoral DVT. Iliofemoral DVT is known to be associated with a higher risk for recurrent venous thromboembolism (VTE) and a higher risk for the development of a post-thrombotic syndrome (PTS).2) On anatomical and clinical grounds, there is considerable justification to divide these categories at the inflow of the deep femoral vein into the common femoral vein. A more proximal thrombosis obstructs the outflow from both the femoral and the deep femoral veins and is clinically equivalent to an iliac vein thrombosis. However, definition of iliofemoral DVT remains difficult as its anatomical limits are not well defined and some authors include thrombosis of the popliteal vein, femoral vein and deep femoral vein while others do not.2–4)
Anatomical conditions such as the compression of the left common iliac vein between the right common iliac artery and the fifth lumbar vertebra, also known as May-Thurner syndrome, are likely to be the source for iliofemoral DVTs.1) Chronic pulsatile compression of the vein induces focal intimal proliferation with subsequent replacement of the normal intima and media by well organized connective tissue covered with endothelium.5) The subsequent development of synechiae or spurs with partial obliteration of the vein reduces the vessel lumen and may cause outflow-obstruction (Fig. 1). Iliac vein compression is much more common in the general population than assumed earlier. While 24% of an asymptomatic population have a greater than 50% compression of the left common iliac vein on CT, 84% of patients with iliofemoral DVT have such an anatomical condition on CT venography.6, 7) Chronic obstruction in combination with coagulation disorders and other hypercoagulable risk factors appears to be a requirement for the development of an extensive thrombosis in the iliofemoral segment. We found underlying stenosis/occlusions in 36 of 44 treated limbs (82%) with acute iliofemoral thrombosis at our institution. This corresponds to the findings of Chung et al. who demonstrated anatomical abnormalities on CT venography in 37 of 44 patients with this condition.6)
Fig. 1.
Venographic picture in a patient with iliac vein compression syndrome, also known as May-Thurner syndrome. Compression by the right common iliac artery causes flattening and septation of the left common iliac vein.
Many of the independent risk factors for DVT fall within the triad of Virchow (endothelial injury, abnormal blood flow, hypercoagulability). These include prior history of DVT, postoperative state, immobilization, malignancy, pregnancy and postpartum state, hormonal therapy and acquired and inherited coagulation disorders. While the prevalence of coagulation disorders in all patients with DVT is about 40%, 78% of patients with iliofemoral DVT have one or more coagulation disorder and about 90% have at least one additional risk factor.8, 9) A combination of risk factors together with an underlying chronic obstruction appears to be a prerequisite for an extensive thrombosis in the iliofemoral segment.
Pulmonary embolism (PE) and the development of a PTS are the most serious consequences of DVT in the iliofemoral vein segments. While PE in most cases is asymptomatic and further embolization is prevented by anticoagulation, the damage to the veins and their valves frequently leads to PTS with chronic venous hypertension due to valve incompetence and/or outflow obstruction.10, 11) The socio-economic costs for long-term treatment of the PTS in young patients are significant. The substantial effort and additional costs that endovascular treatment entails appear to be justified by the encouraging mid-term results.3, 10, 12)
Imaging
The two techniques presently used for diagnosing DVT of the legs are color Doppler and venography. Both of these techniques have significant limitations when diagnosing acute thrombosis and chronic occlusions of the iliofemoral segment and the inferior vena cava (IVC). In the presence of bowel gas, color Doppler is difficult to interpret. In venography, the contrast medium is usually injected into a pedal vein, resulting in a significant dilution of the contrast when it reaches to the iliofemoral segments and the IVC (Fig. 2). More information can be collected by CT-venography. With a sufficient delay, i.e., two minutes post injection of contrast medium, visualization of the deep venous system including the iliofemoral segments and the IVC can be achieved (Fig. 3). An alternative is MR-venography, particularly when combined with a blood pool contrast agent, which remains in the circulation for several minutes and obviates the need for an exact timing and image acquisition (Fig. 4). However, CT- and MR-venography scans are made in the supine position. They cannot be performed in an upright position and therefore information on venous hemodynamics and reflux is lacking.
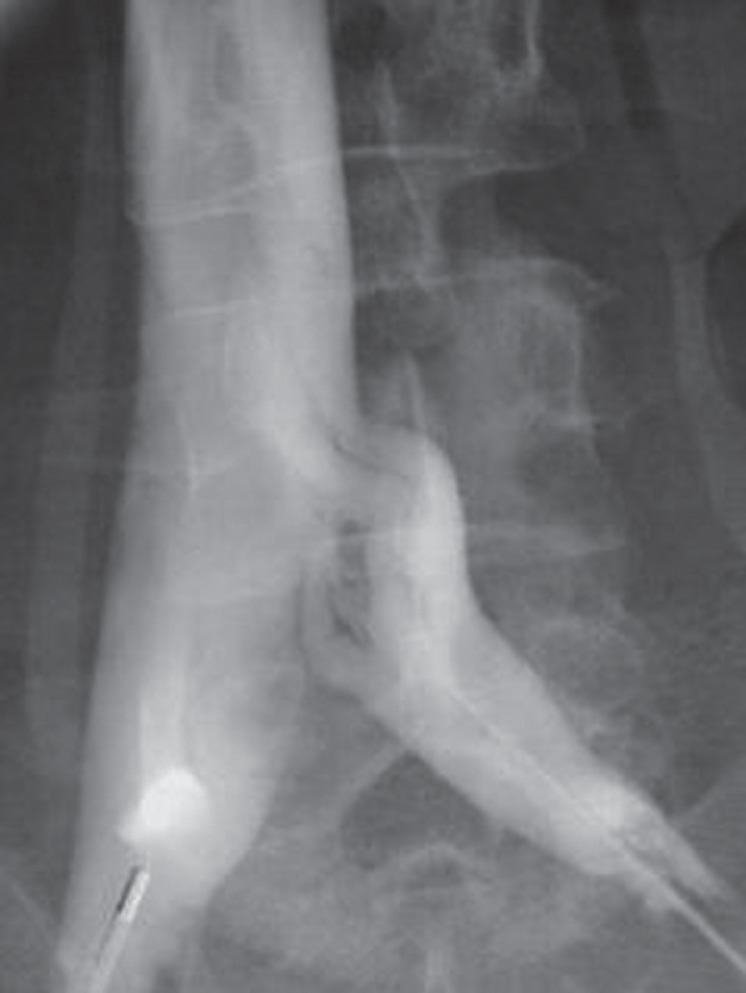
Fig. 2.
Phlebography in a 44-year-old woman with an extensive iliofemoral DVT, showing contrast-filling defects in the right femoral vein. Insufficient concentration of contrast agent in the iliac veins prevents evaluation of the proximal vein segments.
Fig. 3.
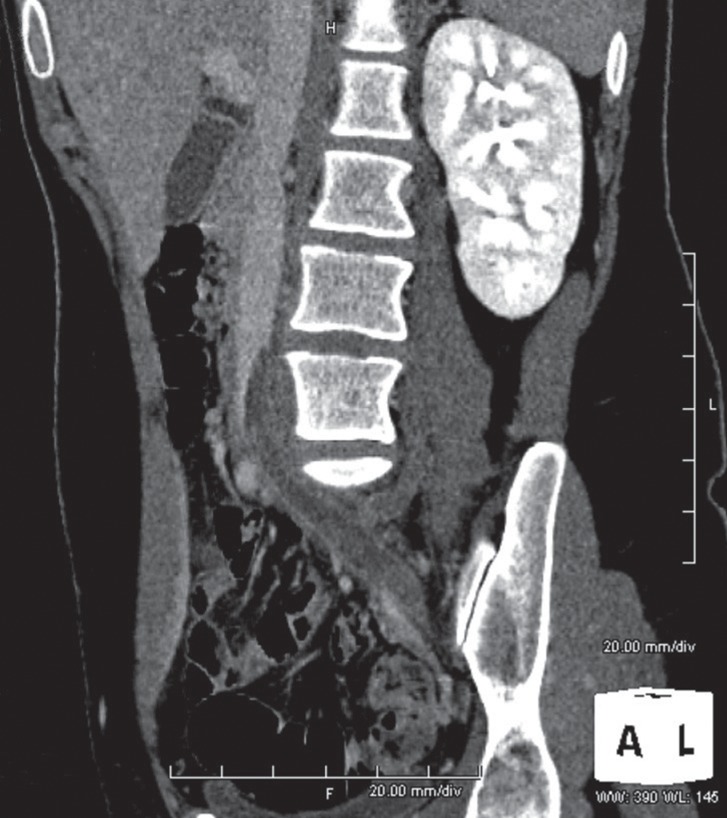
Computed tomography venography in a 23-year-old woman with acute left-sided iliofemoral DVT. Multiplanar reformation demonstrates the extension of thrombosis into the IVC.
Fig. 4.
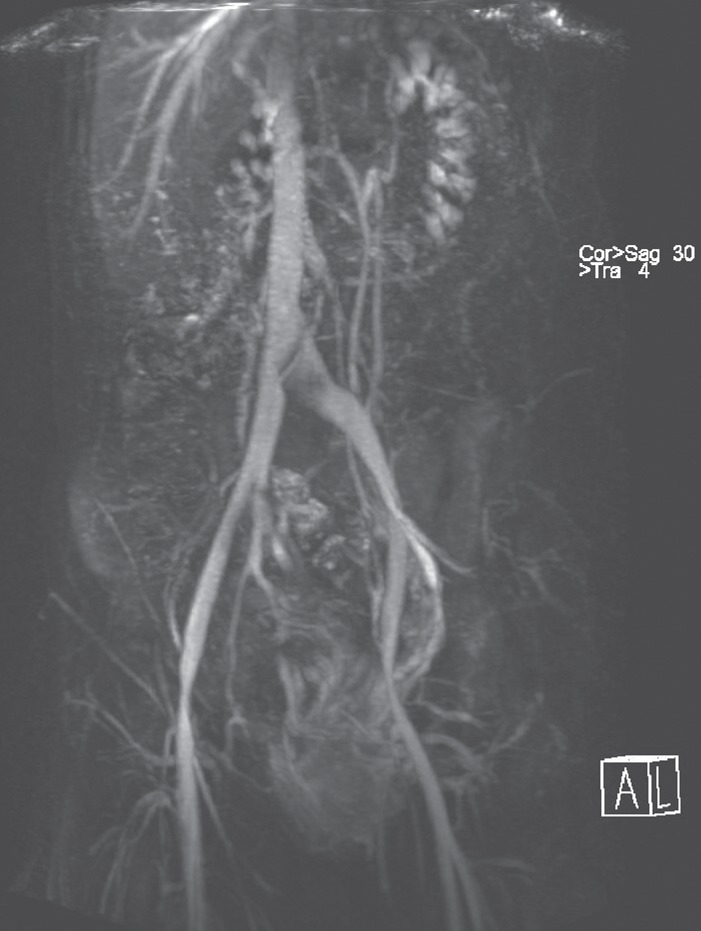
Magnetic resonance venography in a 40-year-old woman with iliac vein compression syndrome (May-Thurner syndrome) and pelvic congestion syndrome. The left common iliac vein is flattened and compressed by the right common iliac artery.
To define the degree of recanalization in chronically occluded segments and to identify the extent of thrombus in acute DVT is still difficult in spite of these new modalities. Multiple septae can be present after spontaneous recanalization of thrombosis, which is difficult to visualize on CT- and MR-venography. Furthermore, if a thrombus completely occludes the iliofemoral segment, the stagnant blood column further distally may be misinterpreted as being thrombus. Therefore, when endovascular interventions are planned, the most informative diagnostic method is venography of the segment by direct puncture with contrast injection in the immediate proximity of the iliofemoral segment. The high resolution of such venography demonstrates both the septation of chronic re-vascularisations and the extent of thrombus, along with the presence of collaterals, which are an indirect sign of obstructed outflow (Fig. 5).
Fig. 5.
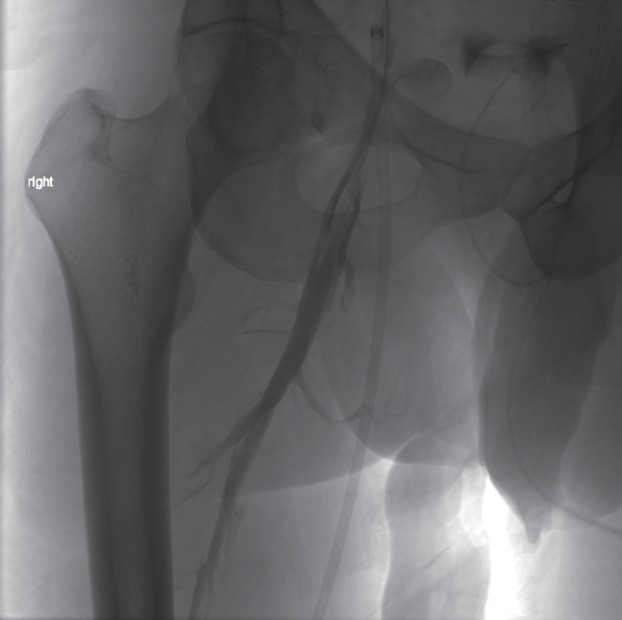
Venography in a 36-year-old man with chronic occlusion of the right iliac veins and subtotal occlusion of the left iliac veins and the IVC. The right deep femoral vein appeared to be normal on MR-angiography.
With contrast injection directly into the right deep femoral vein, the post-thrombotic changes and septation of the proximal part of the deep femoral vein are revealed. Angioplasty and stent-placement across the inguinal ligament will be needed to provide sufficient inflow after recanalization of the chronically obstructed iliac veins and IVC.
Acute Occlusions
Acute occlusions of the iliofemoral vein segments often cause pain and swelling to a moderate degree since collateral vessels may open promptly in the region of the occlusion. Patients with underlying chronic obstructions, in whom thrombosis might also affect the collateral vessels, develop more severe symptoms. Of these patients, a certain percentage develops serious clinical symptoms such as phlegmasia and may therefore require acute treatment that exceeds anticoagulation. Systemic anticoagulation by intravenous heparin infusion or subcutaneous administration of low-molecular-weight heparin followed by oral warfarin treatment is the treatment of choice for uncomplicated acute DVT of the lower extremities. PE and the development of a PTS are the most serious consequences of DVT and if the thrombus involves iliac vein segments, patients have a higher risk of recurrent VTE.2) PE is asymptomatic in most cases and recurrence or fatal embolization and progression of the DVT are prevented by traditional treatment with anticoagulation. However, anticoagulation does not eliminate the thrombus. The capability of the venous circulation to re-establish an unobstructed flow depends on the endogenous fibrinolytic activity to dissolve the thrombus at an early stage.
At a later stage, chronic venous hypertension due to valvular insufficiency or outflow obstruction frequently results in a PTS.10, 11, 13) The proximal venous obstruction causes an increased ambulatory venous pressure which leads to a progressive breakdown of distal valves and correlates well with the severity of the PTS.14) Up to 15% of patients with a history of iliofemoral DVT develop venous ulcers and claudication after five years despite adequate treatment of the acute event with systemic anticoagulation.15) Systemic thrombolytic therapy has shown better results than anticoagulation alone but is accompanied by frequent hemorrhagic complications.16–19)
Since the early 90's, catheter-directed thrombolysis (CDT) of iliofemoral DVT has become a frequently recommended strategy. It offers a significant advantage over systemic thrombolytic therapy by delivering higher local concentrations of the thrombolytic agent with lower systemic doses. This results in a more complete and faster resolution of the thrombus and reduced hemorrhagic complications. Preliminary data based on relatively small numbers of patients have been encouraging.3, 12, 20, 21) However, there is a paucity of data available on mid-term and long-term results from CDT of DVT and no multicenter randomized trial exist comparing CDT to standard anticoagulation therapy.22)
Open Surgical Thrombectomy
Loose thrombus that represents a hazard for PE can be removed by mechanical thrombectomy. Mechanical removal of adherent thrombus that is unlikely to embolize may cause damage to the intima and the valves and may promote rethrombosis. This principle applies both for open surgical and endovascular mechanical thrombectomy. Thrombolysis is a less aggressive way of removing the thrombus without additional damage of the endothelial surfaces. However, the concept of thrombus removal to prevent postthrombotic morbidity and preserve valvular function is not yet well established.23) Open surgical thrombectomy was the only available way of eliminating clot from the iliofemoral segment and restoring patency of the venous outflow in patients with acute thrombosis before effective thrombolytic therapy was introduced. Today this technique is reserved for patients with contra-indications to thrombolysis.23) Surgery is commonly performed through a groin incision under general anesthesia. The clots in the iliofemoral vein segments are removed with Fogarty-balloon catheters, while another balloon catheter may be positioned via the contralateral femoral vein in the IVC to prevent embolization during the thrombectomy. A thrombectomy of the infra-inguinal veins can be performed through the same incision as well as the ligation of the femoral vein. Plate et al. compared surgical thrombectomy combined with a temporary arteriovenous fistula in patients with acute iliofemoral DVT with conventional anticoagulation therapy in a randomized trial and showed favorable results for the surgically treated patients in early follow-up.24) However, clinical problems were not different between the two groups at late follow-up.25)
Catheter-directed Thrombolysis
In patients with unilateral iliofemoral thrombosis, the venous access site is preferably the ipsilateral popliteal vein to facilitate venography and thrombolytic therapy. It allows an easy passage through an occluded femoral vein without getting stuck in the valves. In cases when both the femoral vein and the popliteal vein are thrombosed, a jugular or contralateral femoral vein approach are alternatives. Following vein puncture under ultrasound guidance, a multi-sidehole catheter is placed in the thrombus. All manipulation and injection of contrast medium can result in an unintended loosening and embolization of thrombus. We therefore usually place a retrievable IVC-filter as a first procedure in all patients who do not have a chronically occluded IVC. Thrombolysis with r-tPA is started with an infusion rate of 1–2mg/hour, depending on the condition and amount of thrombus, the age of the patient and the estimation of the duration of the thrombolysis. Patients are monitored during the ongoing thrombolysis to detect bleeding complications at an early stage. Follow-up venography is carried out in 8–24 hour intervals to evaluate the progress of thrombolysis and to adjust the catheter position (Fig. 6). Thrombolysis should continue until the thrombus is resolved completely, a major complication has occurred, or no progress is visible over a period of 24 hours. Anticoagulation during thrombolysis can be achieved by continuous infusion of unfractioned heparin infused into the sideport of the introducer sheath. Clinical improvement is monitored by the reduction of symptoms, limb circumference measurements and pain validation.
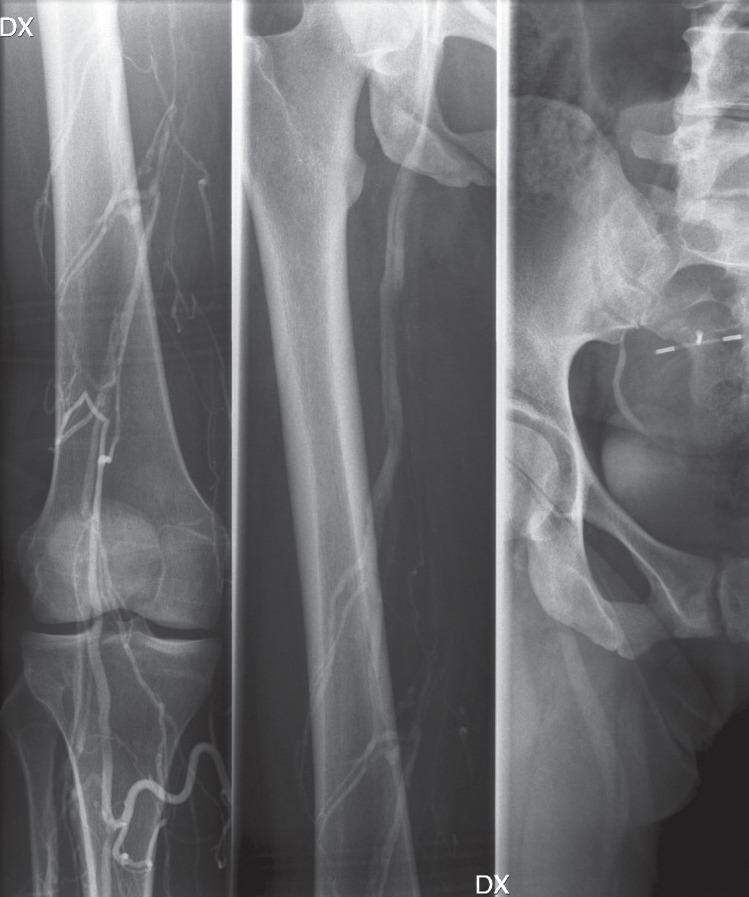
Fig. 6.
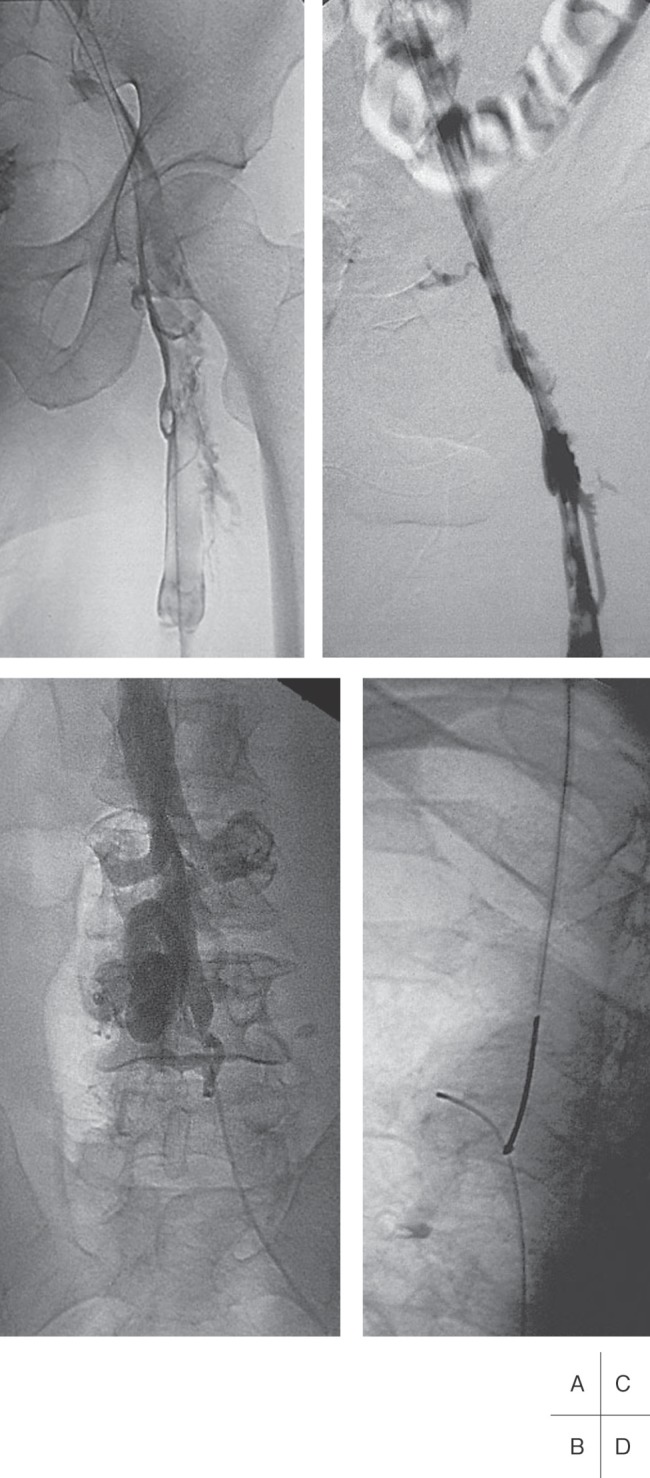
Venograms of a 48-year old man with extensive bilateral deep venous thrombosis.
A: Thrombosis of the left iliofemoral vein segment.
B: Chronic IVC occlusion to the level of the renal veins.
C: Partial recanalization of the iliac veins after 24 hours of thrombolysis and 35 mg r-tPA.
D: After completed thrombolysis, a hydrophilic guide wire was passed through the chronic IVC occlusion and grasped by a snare at the level of the right atrium.
Mechanical endovascular thrombectomy combined with CDT offers an opportunity to obtain a faster clearance of thrombus, but in our opinion, it should be restricted to patients with severe symptoms, long-segment thrombosis, a large clot burden or in cases of prolonged thrombolysis, i.e., more than 36 hours. A variety of motorized thrombectomy devices with different principal mechanisms, mainly rotational or hydrodynamic, are available for percutaneous thrombectomy. These mechanical thrombectomy devices may damage the intimal layer and the venous valves. Angioplasty balloons and various sizes of catheters used for direct aspiration are an alternative for mechanical fragmentation of thrombi.
Thrombolytic therapy should be started as early as possible. Previous studies have shown that technical success and clinical outcome deteriorated if symptom duration was longer than 4 weeks.20) The probability for complete removal of the clot burden, valve preservation and long-term clinical benefit is closely associated with symptom duration at the time of treatment. However, the patency and outcome of 6 patients in our own material with symptom duration of 15 to 21 days was similar to the patients with shorter symptom duration, although they required longer thrombolytic treatment, larger amounts of r-tPA and a higher number of stents. This might be due to the fact that patients with a long symptom duration also have thrombosed segments containing older thrombus, which may respond to thrombolytic therapy at a higher doses, thus providing sufficient recanalization and flow to maintain the patency of stented stenotic or chronically occluded iliofemoral segments.
Angioplasty and Stenting
Any visualized significant stenosis or underlying chronic occlusion should be treated with balloon angioplasty and stent placement (Fig. 7). Self-expanding stents, preferably Wallstents (Boston Scientific, Galway, Ireland) are used in combination with balloon-expandable stents if necessary. The size of the Wallstents is usually 18 to 22mm in the vena cava, 14 to 18 in the common iliac vein, 12 to 14 in the external iliac vein and 10 to 12 in the common and deep femoral vein. Whenever a stenosis cannot be resolved completely by the Wallstent, a balloon-expandable stent should be used to reinforce the Wallstent. If Wallstents are used as kissing stents in the IVC bifurcation, balloon-expandable stents can be deployed inside to prevent collapse of the Wallstents. Personal experience has shown that Wallstents exhibit a lower tendency for fracture than other self-expanding stents.
Fig. 7.
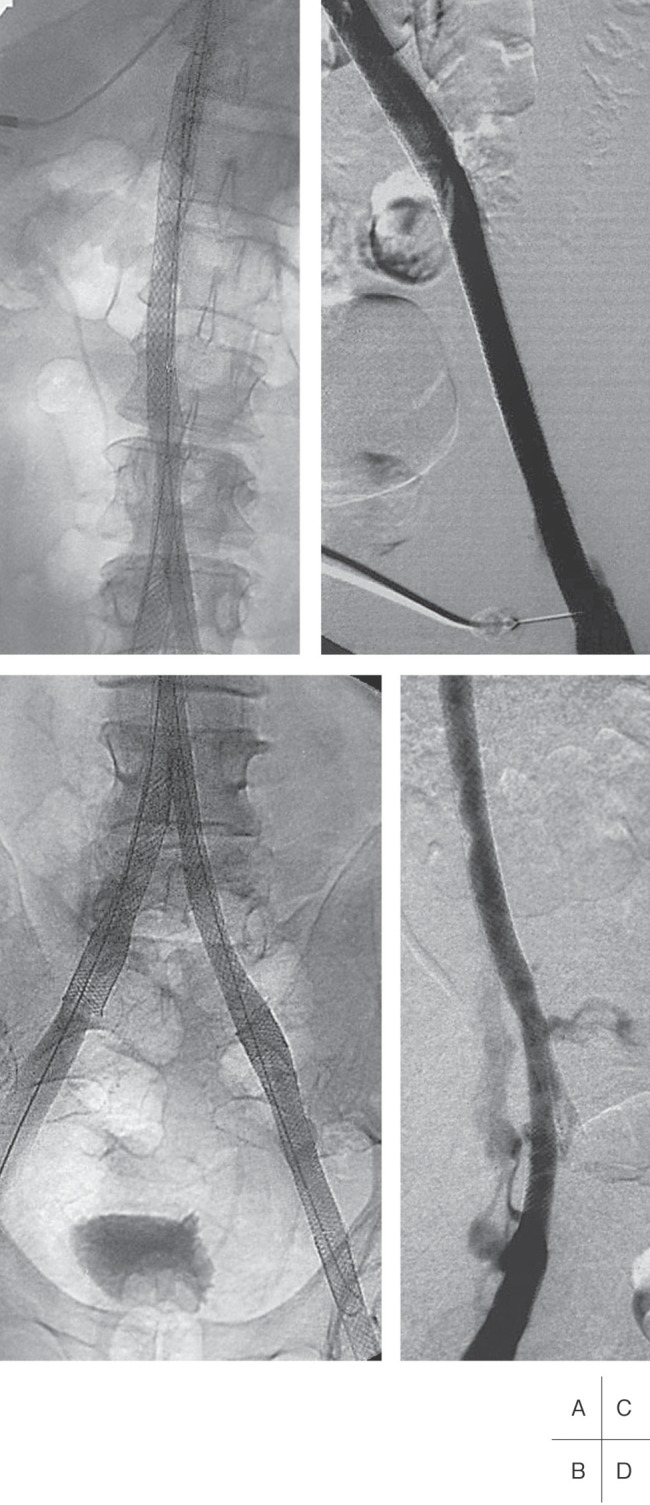
Venograms of the same patient as Fig. 6.
A and B: The chronic occlusion of the IVC has been recanalized and stented from above the renal veins to the right common iliac vein and on the left side to the common femoral vein.
C and D: The stented vein segments of the IVC, and the left and the right iliac veins, respectively, are patent at 1-year follow-up.
Stenting strategies after CDT differ widely, depending on the extension of thrombosis and personal preferences, but are mostly limited to the iliac segments.20, 26) If underlying obstructions in the iliofemoral vein segments remain insufficiently stented after thrombolysis, the chances for patency and a good clinical outcome after secondary angioplasty and stenting appear poor. In our limited experience, 3 of 37 patients needed stent placement during follow-up due to a stenosis or rethrombosis of the treated vein segment.9) The stented vein segments of all 3 patients later occluded. Therefore primary and aggressive stent placement should be used generously in the iliofemoral vein segments to prevent early rethrombosis or occlusion and to ensure patency.
In earlier reports, infra-inguinal stenting has been suggested as a contributing factor for early stent occlusion.3, 5) Our own experience does not support this finding. The patency and clinical outcome of seven patients in our material requiring infra-inguinal stenting after thrombolysis were similar or better than the others. Although the number of these patients is rather small, all of them were patent primarily and clinically improved or even became asymptomatic at follow-up. Patients who require placement of self-expanding stents across the inguinal ligament in the common femoral vein have poor inflow from the deep femoral and/or femoral vein. We recommend infra-inguinal stenting as a bailout when thrombolysis fails to provide a sufficient inflow. If the flow across a treated vein segment seems to be slow and rethrombosis is feared, especially in long stented segments with reduced inflow from the deep femoral vein, an arteriovenous fistula may be used to increase flow and prevent early stent occlusion.
Retrievable IVC-filter
Indications for the insertion of IVC filters during CDT are not well established to date. Théry and co-workers reported that emboli were caught in temporary IVC-filters in 30% of patients after initiation of systemic thrombolysis for proximal DVT.27) The accelerated thrombolysis in CDT and the mechanical stress applied to the clot might result in symptomatic or silent PE. IVC-filters protect from clinically symptomatic PE resulting in chest pain, dyspnea, and visible right heart strain, but also from silent PE. This might be of clinical importance because the angiographic severity of PE poorly correlates to the size of the perfusion defect on lung scans and some silent PE might have severe cardiorespiratory consequences.28) The IVC-filter is preferably inserted via a right jugular approach and can be removed at the end of the procedure after the occluded segments are recanalyzed and stented. Many of the complications associated with permanent IVC-filter placement can be avoided in the setting of local venous thrombolysis, as the filter is only temporarily placed in the IVC.29) Using a retrievable filter such as the Günther Tulip filter (William Cook Europe, Bjaeverskov, Denmark) which is equipped with a retrieval hook and can be inserted via either femoral or jugular venous access, we have not seen any significant complication associated with IVC-filter placement or retrieval of it in our clinical practice. Because of lack of available data from controlled studies, there remains controversy regarding the role of caval filters.30) Although there is no evidence in the literature, in our opinion, the risk for PE is high enough to justify the usage of retrievable IVC-filters. We have repeatedly seen emboli caught in the filter after stent placement (Fig. 8).
Fig. 8.
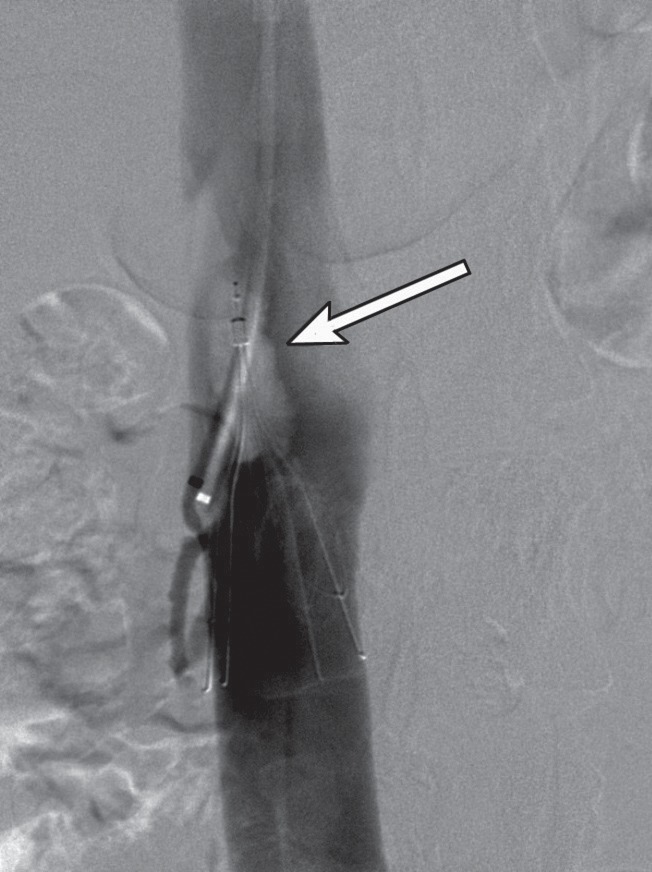
Same patient as Fig. 2. Cavogram demonstrating a trapped embolus in a temporary IVC filter (arrow) following thrombolysis and PTA for an acute thrombosis of the right external and common iliac veins.
Intermittent pneumatic compression devices such as foot impulse compression can increase the venous blood flow during thrombolysis and support CDT while patients are immobilized.31) An inflatable pad empties the plantar venous plexus and imitates the natural pumping mechanism during ambulation. In patients with proximal venous occlusive disease and phlegmasia, it should be used with care not to overload the capacity of the venous collaterals of the limb. The treatment protocol after completion of the thrombolysis includes oral anticoagulation with Warfarin with a target therapeutic range of international normalized ratio of 2-3 and graded compression stockings for at least 6 months as well as follow-up examinations after 6 and 12 months with venography or color Doppler scan. This should be repeated if necessary.
All patients with acute iliofemoral venous occlusions require a hypercoagulability work-up even though they might have other predisposing factors for the thrombosis. The likelihood of identifying underlying thrombophilic disorders is very high in patients with a proximal thrombosis. In our own material, 78% of the patients with acute iliofemoral DVT had hypercoagulable disorders compared to a prevalence of about 40% in all lower extremity DVT patients.8, 9) The hypercoagulable work-up should include APC-resistance (Factor V Leiden), antithrombin deficiency, protein C deficiency, protein S deficiency, cardiolipin antibodies, lupus anticoagulant and prothrombin gene mutation (G2002 10A).
Results of a multicenter venous registry including 287 patients treated with catheter-directed infusion of urokinase showed a 1 year patency of 79% in limbs with a 100% degree of lysis.3) In our own experience with catheter directed thrombolysis in 44 limbs of 37 patients, we obtained a patency of 89% after a median imaging follow-up of 16 months (IQR 7–26). Sixty-eight per cent of the limbs were asymptomatic and 18% were improved after a median clinical follow up of 27 months (IQR 14–57).9)
Chronic Occlusions
Venographic studies have shown that spontaneous thrombolysis and recanalization after DVT occurs in up to 95% in the more distal venous segments, while 50% of DVT in the femoral vein and only 20% of DVT in the iliofemoral vein segments reanalyze spontaneously.32, 33) Compared to distal venous occlusions which are more easily compensated by collateralization to the deep femoral vein, superficial veins or the doubled deep veins, iliofemoral occlusions have poorer chances of sufficient collateralization. About half of the patients with iliofemoral occlusions are asymptomatic in the follow-up and develop an adequate collateralization, directing the venous return of the obstructed limb to the ipsilateral and contralateral internal iliac system.1) These collateral vessels can open rapidly around the area of occlusion, providing immediate bypassing of the acute obstruction. Patients with chronic iliofemoral occlusions who developed adequate collateralization may remain asymptomatic for many years without treatment. However, they may present later with a thrombosis affecting the collateral circulation. Our experience suggests that these patients with an acute upon chronic thrombosis often present with the most severe forms of “whole leg thrombosis” with a risk for phlegmasia cerulea dolens or even venous gangrene. A combination of factors exacerbate their clinical status: the main venous trunks are chronically obstructed, collaterals are thrombosed and the thrombosis progresses distally.
Patients with iliofemoral occlusions who do not develop sufficient collateralization can present with a variety of symptoms such as varicose veins, edema, venous eczema and ulcer, as well as chronic pain and claudication. Symptoms may include bilateral limb swelling and pain, back pain and occasionally transitory impaired renal function if the renal veins or the IVC are involved. High venous pressure may also develop due to retrograde flow in collaterals without competent valves and lead to a progressive damage of distal valves with subsequent reflux if no adequate compression therapy is used to preserve the valves. Patients with chronic occlusions of the iliac veins and the IVC who do not respond to conservative treatment consisting of compression therapy and anticoagulation may benefit from endovascular techniques. Endovascular recanalization and stent-placement of chronically occluded iliac veins and the IVC can abolish outflow obstruction. However, it remains unclear whether the chronic occlusion protects against reflux in the postthrombotic limb and opening up deteriorates reflux related symptoms. In a series of 38 patients treated with recanalization of chronically occluded iliac veins, Raju et al. showed no worsening of reflux in the treated limbs measured by plethysmography.34)
Open Surgical Techniques
Open surgical techniques to treat chronic iliofemoral occlusions such as saphenopopliteal bypass, femoro-femoral and femoro-caval bypass have been used when conservative treatment failed. Results of these invasive procedures have not been satisfying. Slow flow, low pressure, collateral circulation, collapsing vessels, and thrombophilia are some of the factors responsible for a high percentage of graft occlusions. Endovascular recanalization and stenting of chronically obstructed iliofemoral segments have lately replaced open surgery as the treatment of choice.35)
Endovascular Technique
A good inflow to the stented segments through the deep femoral vein is a prerequisite for the patency of a successful recanalization. If there is no sufficient inflow at the groin due to postthrombotic changes in both the femoral vein and the deep femoral vein, the probability of successful recanalization is significantly lower and the creation of an A-V fistula might be considered. The endovascular recanalization of chronically occluded iliofemoral vein segments is preferably performed under general anaesthesia, because it can be a time-consuming and painful procedure. Access to the venous system is gained either by a jugular approach or by puncturing the common femoral vein, deep femoral vein or the proximal portion of the long saphenous vein. The aim is to approach the occlusion site as close as possible. The occlusion is then passed using a hydrophilic guide-wire supported by a rigid catheter. The looped hydrophilic guide-wire will dissect its way across the occlusion, and once it enters the patent venous segment above the occlusion, it can be snared and a “through-and-through” guide-wire condition is achieved facilitating stenting of the occluded segment.
Stenting is preferentially performed with a 10-12-French introducer sheath. Typically, Wallstents (Boston Scientific, Galway, Ireland), which have several advantages over other self-expandable stents, are used. Wallstents are easy to deploy, cover long distances, are flexible, easy to resheath and have better durability than other self-expandable stents such as Nitinol stents, when placed under the inguinal ligament. In addition, the Wallstents can be combined with balloon-expandable stents if needed, like in cases where there is an insufficient response to angioplasty or in a situation where kissing Wallstents are used in recreating the iliac confluence. The balloon-expandable stents are selectively used in this manner. They lock the Wallstents and prevent stent-collapse. Recanalization is considered complete when repeat venography shows that the collaterals, which existed prior to the intervention, are no longer opacified, meaning that the flow through the recanalyzed segment is unimpeded (Fig. 9). Pressure gradient measurements are not helpful in the venous system because of low pressure. Stents are generally placed as far distally as required to achieve maximum inflow and if necessary across the inguinal ligament and further distal to the deep femoral vein. If the inflow from the deep femoral vein, or from the femoral vein, is insufficient due to multiple septae following recanalization of chronic occlusions, it is considered unwise to stent areas further distally. In these cases, an arteriovenous fistula may be created to increase flow and prevent early stent occlusion. It can be constructed by anastomosing the long saphenous vein to the side of the superficial femoral artery and should be closed after approximately 6–12 weeks.
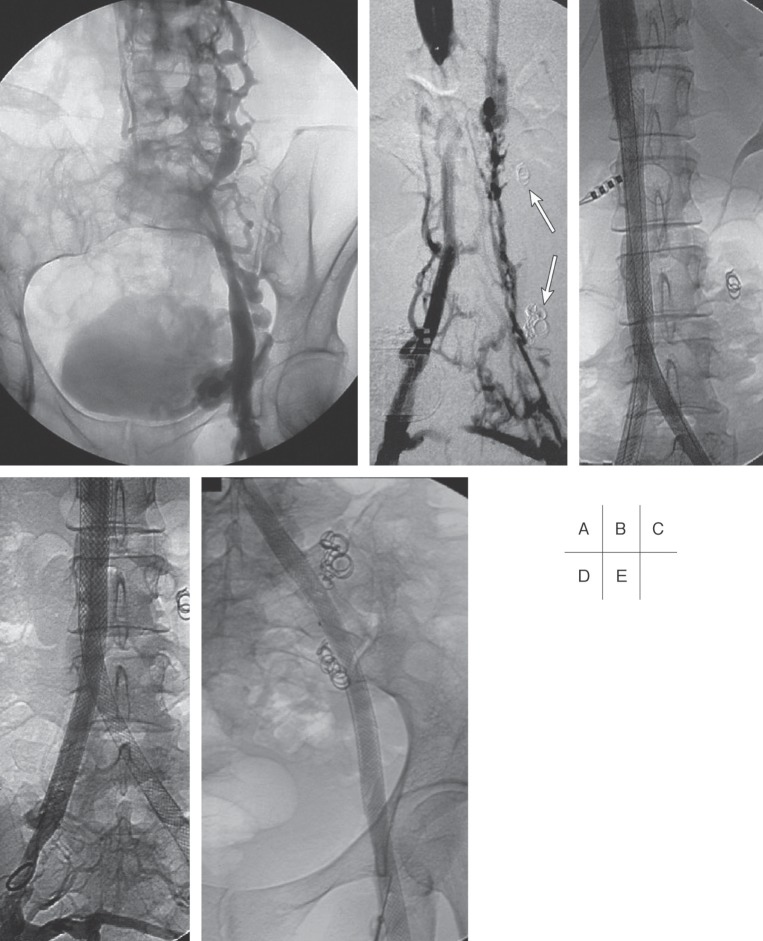
Fig. 9.
Venograms of a 35-year old woman with a chronic occlusion of the IVC following resection of a Wilms tumor of the right kidney in early childhood.
A: Collateralisation via lumbar and ovarian veins.
B: Patent IVC at the level of the left renal vein. Note: coils previously placed in the insufficient left ovarian vein (arrows).
C–E: Unimpeded flow 1 year after recanalization with stent placement from the IVC to the right common iliac vein and to the left common femoral vein.
In their series of 38 patients treated with recanalization of chronically occluded iliac veins, Raju et al. demonstrated a primary and secondary stent patency rate of 62% and 76% respectively at 24 months and 75% of the patients became free from pain after treatment.34) At our institution, 37 patients and 39 limbs were treated between 1994 and 2005 with recanalization and stenting for chronically occluded iliofemoral vein segments. Age at presentation ranged from 18 to 69 years and the male to female ratio was 1:2. Of the occlusions, 29 were left sided, 6 were right sided and 2 were bilateral. IVC occlusion was present in 4 patients. Primary patency after a median imaging follow-up of 16 months (interquartile range (IQR) 7–34) was 70% and secondary patency was 86%. After a median clinical follow up of 49 months (IQR 20–75) 68% of the treated patients were improved or even asymptomatic. These results indicate a good long-term patency.
Following the promising results of endovascular stenting, there may be a tendency to treat patients with less symptoms and “borderline obstructions” of the left common iliac vein even in the presence of untreated superficial reflux in the symptomatic limb.35) Taking the high prevalence of asymptomatic iliac vein compression into account,7) we believe that patients with non-occlusive obstructions of the left common iliac vein, without typical pathological changes in its flow profile on venography (“spurs”), should be functionally evaluated and treated for superficial venous disease before stenting of the iliac veins is considered. Aggravating factors such as superficial venous reflux, obesity, hormonal therapy and inadequate compression therapy should be corrected if possible. If symptoms persist, then intervention with recanalization and stenting of iliofemoral occlusions and significant obstructions, appears to be a safe and efficient method.
Acknowledgements
The authors want to thank Kristina Lindholm, Ph.D, for her assistance with the illustrations and the manuscript preperation.
References
- Mavor GE, Galloway JM. Iliofemoral venous thrombosis. Pathological considerations and surgical management. Br J Surg. 1969; 56: 45–59 [DOI] [PubMed] [Google Scholar]
- Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001; 110: 515–9 [DOI] [PubMed] [Google Scholar]
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999; 211: 39–49 [DOI] [PubMed] [Google Scholar]
- Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2006; 17: 417–34 [DOI] [PubMed] [Google Scholar]
- O'Sullivan GJ, Semba CP, Bittner CA, Kee ST, Razavi MK, Sze DY, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000; 11: 823–36 [DOI] [PubMed] [Google Scholar]
- Chung JW, Yoon CJ, Jung SI, Kim HC, Lee W, Kim YI, et al. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004; 15: 249–56 [DOI] [PubMed] [Google Scholar]
- Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004; 39: 937–43 [DOI] [PubMed] [Google Scholar]
- Hillarp A, Zöller B, Svensson PJ, Dahlbäck B. The 20210 A allele of the prothrombin gene is a common risk factor among Swedish outpatients with verified deep venous thrombosis. Thromb Haemost. 1997; 78: 990–2 [PubMed] [Google Scholar]
- Kölbel T, Lindh M, Holst J, Uher P, Eriksson KF, Sonesson B, et al. Extensive acute deep vein thrombosis of the iliocaval segment: midterm results of thrombolysis and stent placement. J Vasc Interv Radiol. 2007; 18: 243–50 [DOI] [PubMed] [Google Scholar]
- Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002; 24: 209–14 [DOI] [PubMed] [Google Scholar]
- Markel A, Manzo RA, Bergelin RO, Strandness DE., Jr Valvular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg. 1992; 15: 377–84 [PubMed] [Google Scholar]
- Comerota AJ, Throm RC, Mathias SD, Haughton S, Mewissen M. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000; 32: 130–7 [DOI] [PubMed] [Google Scholar]
- Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004; 239: 118–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides AN, Hussein MK, Szendro G, Christopoulos D, Vasdekis S, Clarke H. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993; 17: 414–9 [DOI] [PubMed] [Google Scholar]
- Åkesson H, Brudin L, Dahlström JA, Eklöf B, Ohlin P, Plate G. Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg. 1990; 4: 43–8 [DOI] [PubMed] [Google Scholar]
- Arnesen H, Høiseth A, Ly B. Streptokinase of heparin in the treatment of deep vein thrombosis. Follow-up results of a prospective study. Acta Med Scand. 1982; 211: 65–8 [PubMed] [Google Scholar]
- Elliot MS, Immelman EJ, Jeffery P, Benatar SR, Funston MR, Smith JA, et al. A comparative randomized trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective trial. Br J Surg. 1979; 66: 838–43 [DOI] [PubMed] [Google Scholar]
- Goldhaber SZ, Buring JE, Lipnick RJ, Hennekens CH. Pooled analyses of randomized trials of streptokinase and heparin in phlebographically documented acute deep venous thrombosis. Am J Med. 1984; 76: 393–7 [DOI] [PubMed] [Google Scholar]
- Comerota A AS. Thrombolytic therapy for acute deep vein thrombosis. Semin Vasc Surg. 1992; 5: 76–81 [Google Scholar]
- Bjarnason H, Kruse JR, Asinger DA, Nazarian GK, Dietz CA, Jr., Caldwell MD, et al. Iliofemoral deep venous thrombosis: safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997; 8: 405–18 [DOI] [PubMed] [Google Scholar]
- Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2004; 4: CD002783. [DOI] [PubMed] [Google Scholar]
- Ley EJ, Hood DB, Leke MA, Rao RK, Rowe VL, Weaver FA. Endovascular management of iliac vein occlusive disease. Ann Vasc Surg. 2004; 18: 228–33 [DOI] [PubMed] [Google Scholar]
- Comerota AJ, Paolini D. Treatment of acute iliofemoral deep venous thrombosis: a strategy of thrombus removal. Eur J Vasc Endovasc Surg. 2007; 33: 351–62 [DOI] [PubMed] [Google Scholar]
- Plate G, Akesson H, Einarsson E, Ohlin P, Eklöf B. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg. 1990; 4: 483–9 [DOI] [PubMed] [Google Scholar]
- Plate G, Eklöf B, Norgren L, Ohlin P, Dahlström JA. Venous thrombectomy for iliofemoral vein thrombosis 10-year results of a prospective randomised study. Eur J Vasc Endovasc Surg. 1997; 14: 367–74 [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, Perkins SE, Wulu JT, Ng HK. Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001; 233: 752–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Bauchart JJ, Lesenne M, Asseman P, Flajollet JG, Legghe R, et al. Predictive factors of effectiveness of streptokinase in deep venous thrombosis. Am J Cardiol. 1992; 69: 117–22 [DOI] [PubMed] [Google Scholar]
- The urokinase pulmonary embolism trial. A national cooperative study. Circulation. 1973; 47 (2 Suppl): II1–108 [PubMed] [Google Scholar]
- Arnold TE, Karabinis VD, Mehta V, Dupont EL, Matsumoto T, Kerstein MD. Potential of overuse of the inferior vena cava filter. Surg Gynecol Obstet. 1993; 177: 463–7 [PubMed] [Google Scholar]
- Lorch H, Welger D, Wagner V, Hillner B, Strecker EP, Herrmann H, et al. Current practice of temporary vena cava filter insertion: a multicenter registry. J Vasc Interv Radiol. 2000; 11: 83–8 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Hoshino S, Midorikawa H, Sato K. Intermittent pneumatic compression of the foot and calf improves the outcome of catheter-directed thrombolysis using low-dose urokinase in patients with acute proximal venous thrombosis of the leg. J Vasc Surg. 2005; 42: 940–4 [DOI] [PubMed] [Google Scholar]
- Arenander E. Varicosity and ulceration of the lower limb; a clinical follow-up study of 247 patients examined phlebographically. Acta Chir Scand. 1957; 112: 135–44 [PubMed] [Google Scholar]
- Thomas ML, McAllister V. The radiological progression of deep venous thrombus. Radiology. 1971; 99: 37–40 [DOI] [PubMed] [Google Scholar]
- Raju S, McAllister S, Neglen P. Recanalization of totally occluded iliac and adjacent venous segments. J Vasc Surg. 2002; 36: 903–11 [DOI] [PubMed] [Google Scholar]
- Neglén P, Hollis KC, Raju S. Combined saphenous ablation and iliac stent placement for complex severe chronic venous disease. J Vasc Surg. 2006; 44: 828–33 [DOI] [PubMed] [Google Scholar]