Abstract
Objective: To analyze relationships between plaque-morphology classified by intravascular ultrasound (IVUS) and risk factors in patients with peripheral arterial disease (PAD).
Methods: We performed IVUS in 203 patients with PAD. Multiple regression and logistic analysis were used to assess relationships between plaque-morphology (degree of calcification, presence of a lipid core, intimal flap and thrombus) and risk factors including diabetes mellitus, hypertension, dyslipidemia, estimated glomerular filtration rate (eGFR), HbA1c and the homeostasis model assessment-insulin resistance ratio (HOMA-IR).
Results: IVUS data led to 22% of lesions being classified as soft, 18% as fibrous, 32% as calcified, and 28% as mixed. Calcification was present in the superficial and deep layers in 65% and 35% of cases, respectively, and a lipid core, intimal flap and thrombus were found in 31%, 5.4% and 3.0%, respectively. The calcified angle correlated with HbA1c and eGFR (p < 0.05). Associations were found between deep calcification and HOMA-IR (odds ratio: 4.4, p < 0.05) and a lipid core and hypercholesterolemia (odds ratio: 3.2, p < 0.05). The odds ratio for intimal flap was 15.6 times with hypercholesterolemia (p < 0.05) and 16.9 times with a high HOMA-IR (p < 0.01).
Conclusion: Plaque calcification and morphology are associated with chronic kidney disease, insulin resistance and dyslipidemia in PAD patients.
Keywords: peripheral arterial disease, plaque-morphology, intravascular ultrasound, chronic kidney disease, calcification
Introduction
Peripheral arterial disease (PAD) is an atherosclerotic disease of the peripheral arteries that has multiple atherosclerosis risk factors and a high incidence of coexisting atherosclerotic vascular disease.1) Atherosclerotic stenotic lesions consist of various components, and the morphology and character of the plaques may vary depending on the associated risk factors.2) Diabetes mellitus (DM) and hypertension are reported to be strongly associated with calcification in coronary arteries and DM increases the progress of calcification.3) Intravascular ultrasound (IVUS) provides cross sectional images of atheromatous plaques, intimal thickening, media and adventitia in real time, such that the plaque cross-section and intimal proliferation within the stent can be determined.4–6) There are also reports that use of IVUS in endovascular treatment for PAD improves late patency,6, 7) but there are no IVUS studies of the relationship between lesion characteristics in PAD and risk factors. In this study, we evaluated the lesion morphology and characteristics of PAD using IVUS and investigated the relationship of the lesion morphology and characteristics with risk factors for arteriosclerosis to elucidate the cause of peripheral arteriosclerosis.
Patients and Methods
Patients
The study included PAD patients who were admitted to our hospital and received endovascular treatment between July, 1996 and April, 2004 because of iliac artery stenosis of ≥ 70% defined by angiography. Prior to starting the study, the patients received a full explanation of the methods of treatment and examination, and submitted written informed consent.
Intravascular ultrasound
The cross section of the lesion with a minimal luminal diameter was observed using IVUS (Ultracross catheter, 30 MHz, Boston Scientific, Natick, MA) just before the start of endovascular treatment. Atherosclerotic plaques were classified into 4 types (soft, fibrous, calcified, and mixed) based on echogenicity, following the classification for coronary arteries described by Hodgson et al.8) In this classification, soft plaque has no calcification and ≥ 80% of the plaque has a homogeneous echo pattern with lower echogenicity than that of the adventitia; fibrous plaque has no calcification, and ≥ 80% of the plaque has a homogeneous echo pattern with equivalent or higher echogenicity compared to that of the adventitia and no acoustic shadow; calcified plaque has a high echogenicity associated with an acoustic shadow that occupies ≥ 90° of the circumference of the blood vessel; and mixed plaque has calcified lesions of < 90° of the circumference of the blood vessel or shows a mixture of fibrous and soft echo patterns. Each plaque was also categorized as a concentric or eccentric type according to its location. The severity of calcification was determined based on the angle at which hyperechoic calcification was found, and calcification was classified into a superficial or deep type according to the location of calcification in the plaque.9) The presence or absence of a lipid core in the plaque, thrombi attached to the plaque,10) and intimal flap around the plaque were also evaluated.
Smoking history, hypertension, DM, cerebral infarction, and ischemic heart disease were studied as risk factors for arteriosclerosis. Cerebral infarction was considered positive if the patient had a history of this condition or if lesions due to cerebral infarction were found in a brain computed tomography scan (CT). Ischemic heart disease was considered to be present if the patient had a history of this disease or showed a positive sign in stress/rest myocardial perfusion scintigraphy. Blood was collected during fasting to determine total cholesterol (TC), HDL cholesterol (HDL-C), triglyceride (TG), lipoprotein (a) (Lp (a)), remnant-like particles-cholesterol (RLP-C), glycosylated hemoglobin A1c (HbA1c), homocysteine, fasting glucose (FG), and immunoreactive insulin (IRI). The glomerular filtration rate was estimated using the Modification of Diet in Renal Disease (MDRD) Study equation for creatinine, as modified by the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × (Scr) −1.094 × (Age) −0287 (× 0.739 if female). Renal dysfunction was diagnosed based on eGFR ≤ 60 ml/min, and lipid abnormalities were diagnosed based on TC > 219 mg/dl, HDL-C < 40 mg/dl, TG > 150 mg/dl, RLP-C > 7.5 mg/dl, and Lp (a) > 40 mg/dl. Insulin resistance was calculated from FG and IRI using the insulin resistance formula: HOMA-IR (homeostasis model assessment-insulin resistance) = FG × IRI/405. Insulin resistance was defined as positive when HOMA-IR was > 2.0.
Statistical analysis
Data are expressed as mean values ± standard deviation. The two groups were compared using a t-test and proportions were compared using Chi-square analysis and Yates’ correction. Relationships between atherosclerotic lesions and risk factors were studied using multiple regression analysis and multiple logistic analysis with SPSS v.15.0 (SPSS Inc, Chicago, IL). A p-value of < 0.05 was considered to indicate a significant difference.
Results
Patient characteristics
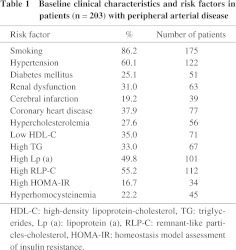
The study population comprised 203 patients with PAD (mean age: 71.1 ± 8.3 years old, 185 males and 18 females) of iliac arteries (98 patients with common iliac artery stenosis and 105 patients with external iliac artery stenosis). Patients with chronic total occlusion were excluded from the study. All subjects had symptoms of intermittent claudication. The patient characteristics including complications and risk factors are shown in Table 1. Patients receiving hemodialysis were also excluded from the study.
Intravascular ultrasound
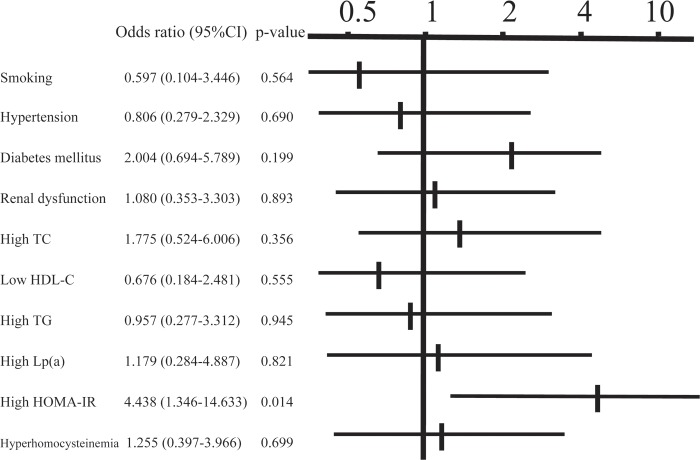
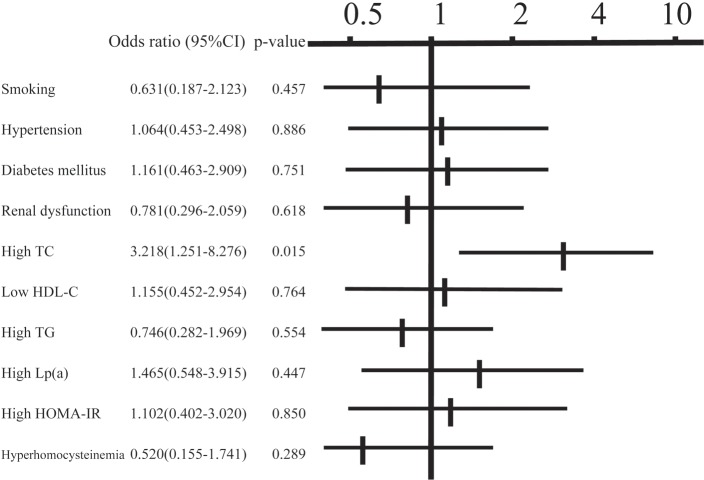
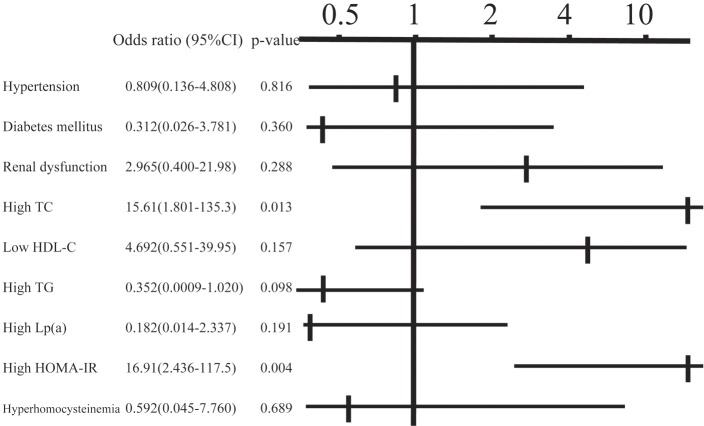
In the classification of plaques, 45 patients (22%) had soft plaques, 37 (18%) had fibrous plaques, 65 (32%) had calcified plaques, and 56 (28%) had mixed plaques. Concentric and eccentric plaques were found in 79 (39%) and 124 (61%) patients, respectively. The angle of calcification was 0°, 1–90°, 91–180° and ≥ 181° in 87 (43%), 55 (27%), 22 (11%) and 39 (19%) patients, respectively, and calcification of the plaque was classified as superficial and deep in 132 (65%) and 71 (35%) patients, respectively. Examination of the relationship between the calcified angle and risk factors using multiple regression analysis (Table 2) revealed a positive correlation with HbA1c and a negative correlation with eGFR (p < 0.05). For deep calcification, the odds ratio was 4.4 times higher in cases of positive insulin resistance (p < 0.05) (Fig. 1). A lipid core was found in 63 patients (31%), an intimal flap was present in 11 patients (5.4%), and mural thrombi were noted in 6 patients (3.0%). The odds ratio for the presence of a lipid core was 3.2 times higher in cases with hypercholesterolemia (p < 0.05) (Fig. 2). An intimal flap was found on the surface of the plaque or at the end of plaque ulcer, which may be associated with plaque rupture. The odds ratio for an intimal flap around the plaque was 15.6 times higher in cases with hypercholesterolemia (p < 0.05) and 16.9 times higher in those with insulin resistance (p < 0.01) (Fig. 3). No significant relationship between mural thrombi and risk factors was found.
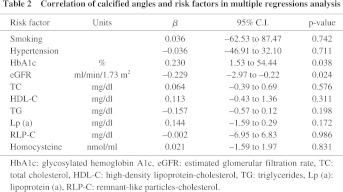
Fig. 1.
Relationship between deep calcification and risk factors in multiple logistic analysis. TC: total cholesterol, HDL-C: high-density lipoprotein-cholesterol, TG: triglycerides, Lp (a): lipoprotein (a), HOMA-IR: homeostasis model assessment of insulin resistance.
Fig. 2.
Relationship between the presence of a lipid core and risk factors in multiple logistic analysis. TC: total cholesterol, HDL-C: high-density lipoprotein-cholesterol, TG: triglycerides, Lp (a): lipoprotein (a), HOMA-IR: homeostasis model assessment of insulin resistance.
Fig. 3.
Relationship between the presence of an intimal flap and risk factors in multiple logistic analysis. TC: total cholesterol, HDL-C: high-density lipoprotein-cholesterol, TG: triglycerides, Lp (a): lipoprotein (a), HOMA-IR: homeostasis model assessment of insulin resistance.
Discussion
There are many different risk factors for atherosclerosis, include smoking, lipid abnormalities and C-reactive protein in proximal arteries and DM in distal arteries.10) Calcified lesions are classified into two types by location, including superficial and internal calcification of plaque and media calcification, which is also known as Mönckeberg-type atherosclerosis.11) Our study of the relationship between plaque morphology and risk factors using IVUS revealed a positive association of the calcified angle of iliac arteries with HbA1c and a negative correlation with eGFR, which indicates that DM and chronic kidney disease are strongly related to plaque calcification. Furthermore, the significant relationship found between deep calcification and insulin resistance suggests that hyperinsulinemia might be related to calcification inside the plaque.
Patients with a high coronary calcification score on a CT scan may have a poor prognosis. This score is higher in patients with DM, and such patients have a poorer prognosis than those without DM.12) Plain foot radiography of femoral artery calcification in patients with DM showed that 41.5% had arterial media calcification and 29.3% had intimal calcification, and patients with intimal calcification tended to have a longer history of DM, a high mortality, and a probable indication for leg amputation.13) A hyperglycemic and hyperinsulinemic status also induces transformation from vascular smooth muscle cells to osteoprogenitor cells, and hyperinsulinemia may be especially likely to induce calcification by β-glycerophosphate.14, 15) On the other hand, the incidence and progress of calcification is greater in chronic kidney disease, which has a strong relationship with media and a possible relationship with calcification of the plaque surface and lipid deposits such as cholesterol.11) The incidence of coronary artery calcification may also increase when eGFR decreases,16) which corresponds to our results in iliac arteries.
The relationship between soft plaque and hypercholesterolemia is evident from the results of the current study, in terms of the character of the plaque. We also found that an intimal flap in the intravascular lumen as a result of plaque rupture may be associated with hypercholesterolemia. Soft plaque is more likely to break and remain as an ulcer or intimal flap, compared with other types of plaque. Therefore, hypercholesterolemia may be involved in acute thrombotic occlusion including formation and rupture of soft plaque. A histological examination of a patient who died suddenly of acute coronary syndrome showed that rupture of coronary artery plaque may be involved in hypercholesterolemia, but no significant relationships with low HDL cholesterolemia, smoking, hypertension, and HbA1c were found.17) Only a low incidence of thrombus attached to plaque was observed in the current study, and this suggests that intimal flap and thrombi around plaque may be involved in atheroma rupture, which may correspond to acute coronary syndrome.
Acceleration of soft plaque formation and plaque rupture by hypercholesterolemia were observed in peripheral arteries. Moreover, we found that hyperglycemia and hyperinsulinemia were associated with the calcification of atherosclerotic plaque and arterial medial layer. Because these findings are well recognized in coronary arteries,14, 15, 17) we propose that distinct risk factors cause the atherosclerosis in a similar fashion between coronary and peripheral arteries. However, occluded lesions were excluded in this study, and thrombus formation may not result in acute total occlusion even if plaque rupture occurs because of the greater diameter of the iliac artery compared to the coronary artery.
The incidence of superficial calcification was higher in our study,13) which may be because deep calcification could not be evaluated by IVUS because an acoustic shadow due to superficial calcification covered the deep lesions. This may be viewed as a limitation of the study. Integrated Backscatter IVUS and Virtual Histology IVUS have recently been developed for detailed analysis of plaque character and can be used to analyze features of atheroma in more detail.18, 19) However, these methods give some differences compared to histopathological findings and further development is required. All ultrasonography approaches are limited in that they cannot show areas behind severely calcified lesions. New technology for analysis of the character of plaque will allow clarification of the cause of arteriosclerosis and will be useful for device selection in endovascular treatment.
Conclusion
In this study, we observed atherosclerotic lesions of PAD using IVUS and studied the relationship with risk factors. The results showed that calcification of artery walls is associated with DM, insulin resistance, and chronic kidney disease and that hypercholesterolemia is associated with soft plaque formation and residual intimal flap.
References
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007; 45 (Suppl. S): S5–67 [DOI] [PubMed] [Google Scholar]
- Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001; 104: 365–72 [DOI] [PubMed] [Google Scholar]
- Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007; 115: 2722–30 [DOI] [PubMed] [Google Scholar]
- van Lankeren W, Gussenhoven EJ, Qureshi A, van der Lugt A. Intravascular ultrasound and histology in in vitro assessment of iliac artery angioplasty. Cardiovasc Intervent Radiol. 1999; 22: 50–5 [DOI] [PubMed] [Google Scholar]
- Schwarzenberg H, Müller-Hülsbeck S, Glüer CC, Steffens JC, Heller M. Evaluation of maximum neointima proliferation and plaque morphology in iliac self-expanding nitinol stents with intravascular sonography. Am J Roentgenol. 1998; 171: 1627–30 [DOI] [PubMed] [Google Scholar]
- Kumakura H, Kanai H, Ichikawa S, Ogino T, Koyano T, Mitsui K. Evaluation of the Palmaz stent in iliac artery stenosis using intravascular ultrasound. Jpn J Cardiovasc Surg. 2004; 33: 319–24 (in Japanese with English abstract). [Google Scholar]
- Nishimura RA, Welch TJ, Stanson AW, Sheedy PF, 2nd, Holmes DR., Jr Intravascular US of the distal aorta and iliac vessels: initial feasibility studies. Radiology. 1990; 176: 523–5 [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Reddy KG, Suneja R, Nair RN, Lesnefsky EJ, Sheehan HM. Intracoronary ultrasound imaging: correlation of plaque morphology with angiography, clinical syndrome and procedural results in patients undergoing coronary angioplasty. J Am Coll Cardiol. 1993; 21: 35–44 [DOI] [PubMed] [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995; 91: 1959–65 [DOI] [PubMed] [Google Scholar]
- Aboyans V, Lacroix P, Criqui MH. Large and small vessels atherosclerosis: similarities and differences. Prog Cardiovasc Dis. 2007; 50: 112–25 [DOI] [PubMed] [Google Scholar]
- David Smith C, Gavin Bilmen J, Iqbal S, Robey S, Pereira M. Medial artery calcification as an indicator of diabetic peripheral vascular disease. Foot Ankle Int. 2008; 29: 185–90 [DOI] [PubMed] [Google Scholar]
- Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004; 43: 1663–9 [DOI] [PubMed] [Google Scholar]
- Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996; 16: 978–83 [DOI] [PubMed] [Google Scholar]
- Olesen P, Nguyen K, Wogensen L, Ledet T, Rasmussen LM. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am J Physiol Heart Circ Physiol. 2007; 292: H1058–64 [DOI] [PubMed] [Google Scholar]
- Fadini GP, Pauletto P, Avogaro A, Rattazzi M. The good and the bad in the link between insulin resistance and vascular calcification. Atherosclerosis. 2007; 193: 241–4 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Oka M, Maesato K, Ikee R, Mano T, Hidekazu M, et al. Coronary artery calcification, ADMA, and insulin resistance in CKD patients. Clin J Am Soc Nephrol. 2008; 3: 1289–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997; 336: 1276–82 [DOI] [PubMed] [Google Scholar]
- Diethrich EB, Pauliina Margolis M, Reid DB, Burke A, Ramaiah V, Rodriguez-Lopez JA, et al. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther. 2007; 14: 687–8 [DOI] [PubMed] [Google Scholar]
- Okubo M, Kawasaki M, Ishihara Y, Takeyama U, Yasuda S, Kubota T, et al. Tissue characterization of coronary plaques: comparison of integrated backscatter intravascular ultrasound with virtual histology intravascular ultrasound. Circ J. 2008; 72: 1631–9 [DOI] [PubMed] [Google Scholar]