Abstract
Background: Acute pulmonary embolism (APE) has high mortality. Some APEs with circulatory collapse or cardiopulmonary arrest have been treated by percutaneous cardiopulmonary support (PCPS) in Japan. But there have been no reports with a large number of series of APE treated with the use of PCPS.
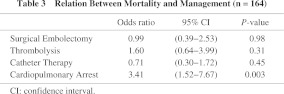
Methods and Results: We collected all the reported cases with acute thrombotic pulmonary embolism treated with PCPS before surgical embolectomy or those without surgical embolectomy in Japan, and assessed the effectiveness of PCPS. PCPS was combined with surgical embolectomy in 35% (68 of 193), thrombolytic therapy in 62% (120/193), and catheter therapy in 24% (46/193). The survival rate treated with PCPS was 73% (80% in surgical embolectomy, 71% in thrombolytic therapy, and 76% in catheter therapy). Logistic regression analysis showed that the mortality rate was elevated in cases with cardiopulmonary arrest (odds ratio [OR], 3.41; 95% confidence interval [CI], 1.52–7.67; p-value, 0.003) but not by surgical embolectomy (OR, 0.99; 95% CI, 0.39–2.53; p-value, 0.98), catheter therapy (OR, 0.71; 95% CI, 0.30–1.72; p-value, 0.45), and thrombolysis (OR, 1.60; 95% CI, 0.64–3.99; p-value, 0.31) as regards to the concomitant therapies with PCPS.
Conclusion: PCPS might improve the survival rate in APE patients with circulatory collapse or cardiopulmonary arrest, but there was no differences in outcome among cases treated by surgical embolectomy, catheter therapy, and thrombolysis as the concomitant therapies.
Keywords: cardiopulmonary arrest, catheter therapy, extracorporeal membrane oxygenation, surgical embolectomy, thrombolysis
Mortality in acute pulmonary embolism (APE) is high with a quarter of APE with circulatory collapse dying within one hour,1) and with half of those with cardiopulmonary arrest (CPA) dying within 30 days (most of the deaths occurred on the day of diagnosis or 1 day after diagnosis was made).2) Intra-venous administration of thrombolytic therapy was introduced for the patients with CPA who did not respond to usual cardiopulmonary resuscitation.3, 4) Percutaneous cardiopulmonary support (PCPS; = extracorporeal membrane oxygenation) may be another alternative of choice to treat fulminant APE.5, 6) However there have been no reports with a large number of series of APE treated with the use of PCPS.
Our aims are to examine the effectiveness of the treatment by PCPS for the patients of APE and to compare the efficacies of PCPS among those patients treated concurrently with surgical embolectomy, catheter therapy (aspiration and/or fragmentation), and thrombolysis.
Methods
Search Strategy
Electronic search strategies were used to identify relevant studies. The following electronic databases were searched: PubMed (1966 to February 2006) and Ichushi web (Japana Centra Revuo Medicina) (1983 to February 2006) using the following search terms: [percutaneous cardiopulmonary support or extracorporeal membrane] and [pulmonary embolism]. We augmented our search by reviewing the reference lists of retrieved articles. Studies published in both English or Japanese were used for our analysis.
Study Selection and Data Extraction
All cases with APE, who showed circulatory collapse or cardiopulmonary arrest and who were treated with PCPS, were included. The exclusion criteria was chronic pulmonary embolism, non-thrombotic pulmonary embolism (fat emboli, tumor emboli, amniotic fluid emboli, and air emboli) and cases in which PCPS was started during or after surgical embolectomy.
Statistical Analysis
Statistical analysis was conducted using SPSS 13.0 (SPSS Inc, Chicago, IL, USA). Categorical data were analyzed by chi-square statistics and multiple logistic analysis. The results of the logistic regression model are presented as estimated odds ratios with corresponding 95% confidence intervals.
Results
There have been no controlled clinical trials. All reports were case reports or case series, and most of them were written in Japanese (see Appendix). We identified total of 251 cases including our own seven cases (Table 1 and 2). Gender was described in 187 cases (132 females and 55 males), and the severity of APE in 165 cases (80 circulatory collapses and 85 cardiopulmonary arrests). Cardiopulmonary arrest occurred in the general hospital wards (25 cases), in intensive care units (9 cases), in examination rooms or roentgen suite (7 cases), in emergency clinics (4 cases), during transportation (4 cases), in the operation room (2 cases), in another hospital (1 cases), and in a patient’s house (1 case). The site of locations were not described in 32 cases.
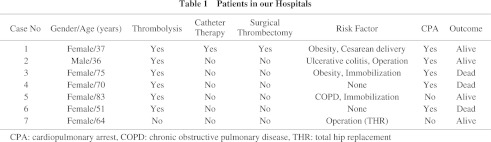
There were 126 cases in which definite diagnostic method for APE was described. Pulmonary angiography was used in 68, enhanced CT in 31, and perfusion lung scan in 16 cases. Two methods mentioned above were combined to apply in 11 patients. Embolism in the right atrium, the right ventricle, or pulmonary artery was found by trans-thoracic echocardiography in 14 and by trans-esophageal echocardiography in 5 patients. Pulmonary embolism was confirmed during surgical embolectomy in two and by autopsy in one case. Diagnosis was confirmed in 58% (67/115) of patients before and in 42% (48/115) after equipping with PCPS.
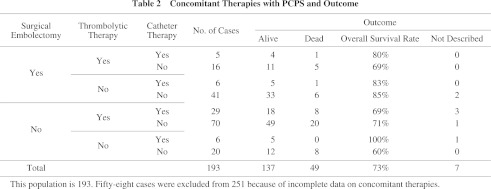
In 193 cases (Table 2), PCPS was combined with surgical embolectomy in 35% (68/193), with thrombolytic therapy in 62% (120/193), and with catheter therapy in 24% (46/193). Ten percent (20/193) of cases with PCPS were not treated by these three therapies, and only heparin was administered. The survival rates calculated in surgical embolectomy, thrombolytic therapy, and catheter therapy were 80% (53/66), 71% (82/116), and 76% (32/42) respectively (Table 2). The survival rates of those patients who were treated with only one of these three modalities, surgical embolectomy, thrombolitic therapy and catheter therapy, were 85% (33/39), 71% (49/69) and 100% (5/5) respectively. In cases with circulatory collapse and with cardiopulmonary arrest, the survival rates were 86% and 65% respectively (p = 0.001). While in cases where cardiopulmonary arrest occurred in intensive care units, general wards, and examination rooms or roentgen suite, their survival rates were 78%, 74% and 57% respectively (p = 0.62). Logistic regression analysis showed that only cardiopulmonary arrest was related to the mortality (Table 3).
As an underlay risk factor, surgery was a risk for APE in 74 cases (15 gastrointestinal, 13 orthopedic [7 total hip replacement], 9 gynecological, 9 cesarean section, 4 neurosurgical, 3 aortic aneurysm, 3 laparoscopic, 2 coronary artery bypass grafting, and 12 other surgeries). In the remaining 4 cases, the surgical sites were not described.
PCPS was used for 24 hours as the median (25 percentile, 20 hours; and 75 percentile, 48 hours), when the cases with surgical embolectomy were excluded.
Discussion
PCPS may improve the survival rate in APE patients with circulatory collapse or cardiopulmonary arrest and there are no differences in the outcome among cases treated by surgical embolectomy, catheter therapy, and thrombolysis as the concomitant therapies.
Indication of PCPS
PCPS has been used in cases whose general condition was improved by temporary support of circulation and/or gas exchange.7) The indications of PCPS are: In the surgical field; patient with cardiac or respiratory dysfunction. In the non-surgical field; patient with severe left-side heart failure and pulmonary embolism. In emergencies; patient with cardiopulmonary arrest, cardiac failure, and respiratory failure. PCPS is also used to support percutaneous coronary intervention (PCI).
In the most recent paper, Maggio et al. described that PCPS should be considered in the algorithm for the management of massive pulmonary embolism in unstable patients.8) PCPS had been implemented in the strategy for the diagnosis and the treatment of APE in Japanese Guideline 2004.9, 10)
It is advisable to equip PCPS before cardiopulmonary arrest because survival rate is high in patients with circulatory collapse compared with that in patients with cardiopulmonary arrest (86% vs., 65%) based on the findings of the present investigation. Cardiopulmonary arrest in patients equipping with PCPS occurred mostly in hospitals. Diagnosis was confirmed in 42% after equipping with PCPS. Therefore, it will be reasonable to state that the indication of PCPS is for those patients suspected of APE with circulatory collapse or whose cardiopulmonary arrest occurs in hospitals.
Timing of introducing PCPS
The time interval has a strong predictive value. In cases in whom the time interval between arrest and initiation of PCPS was over 30 min, the survival rate was less than 10%. There was no survivors of unwitnessed arrest undergoing PCPS except hypothermic condition. Therefore, unwitnessed arrest might be a contraindication for PCPS.11)
Some patients developed cerebral sequelae or multi-organ failure even when hemodynamics was stabilized by PCPS therapy. The duration from the onset of cardiopulmonary arrest to installing of PCPS is one of the prognostic factors.12) When PCPS is used, discretion in selecting the cases is required, taking into consideration of the occurrence of these disorders.
Concomitant therapies with PCPS
In APE patients, most PCPSs have been used before surgical embolectomy.13) Recently, Endo et al. reported that PCPS was an excellent modality in the cases treated with thrombolysis as a concomitant therapy.14) The improvement in hemodynamics is appears to be obtainable when the treatment by surgical embolectomy or catheter therapy is applied in comparison with treatment only by thrombolysis, however, our results indicate that the outcome is similar among three therapies (surgical embolectomy, catheter therapy, and thrombolysis). Moreover, logistic regression analysis, including a factor of severity of APE at the time of equipping with PCPS, did not show the superiority of three managements in mortality (Table 3). Therefore, when PCPS is available, fulminant APE can be treated without special therapeutic modality such as surgical embolectomy or catheter therapy.
Duration of PCPS use
The half of the cases were able to weaned from PCPS within 24 hours even when the cases treated with surgical embolectomy were excluded. Endo et al.14) also reported that the mean weaning time from PCPS was 15 hours 38 min (range from 1 hour 47 min to 33 hours 55 min). This may indicate that the hemodynamics become so stable by this duration that PCPS be able to terminate. However, if hemodynamic is compromised and unless sufficient flow to maintain systemic circulation is obtained by PCPS, surgical embolectomy should be considered as an additional approach.
Survival rates
Prognosis of APE with circulatory collapse is extremely poor,2) therefore the improvement of prognosis is a major concern of APE therapy. It is agreed that PCPS may improve the prognosis in some fulminant APE as well as it may use in cases with unstable hemodynamics or immediately after cardiopulmonary arrest.
The outcome of early experience of PCPS for the treatment of APE was poor. In a study reported in 197915) and in patients between 1975 and 1993,16) all APE treated with PCPS (three cases in Ref. 15 and two in Ref. 16) had died. It appears that the survival rate has been improving recently and it is 74% by our calculation. In adult cases between 1985 and 1996, the survival rate was 30% (8/27) of all causes including myocardial failure, emergency cardiopulmonary resuscitation, and bridge to transplant.17) In APE between 1989 and 2003, survival of PCPS was 72% (13/18) and survival to discharge was 61% (11/18).18) PCPS Study Group in Japan showed that survival rate was 42% (14/33) in APE patients.19)
Complication resulting from PCPS use
There were a few descriptions on complications of PCPS in the present-gathered reports and these reported complications are as follows. In adults patients treated with PCPS, bleeding was found in 74% but clinically significant only in 16%. Renal failure and neurological complications (related to cerebral hypoxia or intracerebral hemorrhage) were 56% and 19% respectively.17) In cases with APE, mechanical and physiological complications during PCPS are hemodynamic instability requiring vasopressors (57%), cannulation site bleeding (38%), cardiac arrhythmias (38%), intracerebral hemorrhage/infarction (24%), culture-proven new infection (24%), surgical site bleeding (24%), creatine > 3.0 mg/dL (24%), hyperbilirubinemia (19%), pneumothorax (14%), and oxygenator failure (14%).8)
The complications associated with the use of PCPS in Japan occurred in 32–40% between 1997 and 2002, including procedural problems (bleeding at the puncture site, retroperitoneal hematoma, vascular injury, and ischemia in lower extremities; 65–77% of all complications), bleeding (in brain, lung, and digestive tract; 16–25%), and thromboembolism (in brain, abdominal organs, and extremities; 7–13%).19)
Intracranial hemorrhage was related to female gender and as well as to thrombocytopenia of less than 50,000 cells/mm3. The duration of time of the use of PCPS was longer in cases with impaired of renal function.20)
Diagnosis of APE treated with PCPS
The patients with APE and treated by PCPS were diagnosed using pulmonary angiography and CT21) in a similar way in milder APE, although trans-thoracic or trans-esophageal echocardiography which detected thrombus echo in the right atrium, the right ventricle or the proximal pulmonary artery were occasionally applied in certain circumstances.22) PCPS was often used to obtain hemodynamic stability before the definitive diagnosis was established. When APE was suspected by the characteristic situation at the time of onset, presence of risk of venous thromboembolism, right ventricular overload in echo-examination and so on, PCPS was installed before the confirmation of the diagnosis of APE.
Effectiveness of PCPS
Surprisingly, some patients with cardiac collapse or CPA could be rescued treated by only PCPS without thrombolytic therapy, catheter therapy, or surgical embolectomy (60% survive, shown in Table 2). There have been similar reports from other countries.8, 23, 24) Kolvekar et al. emphasized that tightly controlled systemic heparinization in conjunction with natural thrombolytic mechanismus allows the resolution of the pulmonary embolism while oxygenation and circulation are maintained by PCPS.23) PCPS allows rapid correction of physiologic derangements in massive APE. Mean while, diagnostic approachs and therapeutic interventions including PAG, implantation of inferior vena cava filter, or even pulmonary embolectomy will be able to proceed during this period.17)
Random-allocation studies are ideal to assess the effectiveness of PCPS. However, these studies are difficult to perform in the emergency/intensive care unit because patients who need PCPS are too ill. APE is a potentially life-threatening disease, and the survival rate is as low as 35% in APE patients requiring cardiopulmonary resuscitation.25-27) The survival rate seems to be low compared with the present data in cases with the use of PCPS (86% in patients with circulatory collapse, and 65% in those with cardiopulmonary arrest). The present results and previous reports suggest that the use of PCPS in the management of patients with APE appears to be effective and safe in experienced centers, however, there have been no high-evidence level reports on PCPS for the management of APE, routine use of PCPS cannot be recommended through our present study.
Limitations of PCPS
Long cardiopulmonary resuscitation relates to low survival rate. Therefore, PCPS may be limited in use when cardiopulmonary arrest occurs outside hospitals. Careful decision is required to the use of PCPS in the following situations such as cardiac arrest and suspicion of brain damage in a patient who regain spontaneous circulation. On the other hand, if the flow of PCPS is inadequate or insufficient to maintaining systemic circulation, emergent surgical embolectomy should be considered as a life saving procedure.
Conclusion
PCPS may improve the survival rate in APE patients with circulatory collapse or cardiopulmonary arrest, however, our results shows there are no differences in outcome among patients treated by surgical embolectomy, catheter therapy, and thrombolysis as the concomitant therapies.
Appendix
We analyzed the cases in the following studies.
Kato Y, Morita H, Negoro N, Koike Y, Fukumoto H, Nishimoto T, et al. Thrombolytic therapy for acute massive pulmonary embolism with the aid of a percutaneous cardiopulmonary support system. J Jpn Ass Acute Med. 1992; 4: 148–52.
Ookawa Y, Kamata K, Tanaka A, Maekawa K, Watanabe N. A case report of massive pulmonary embolism with cardiac arrest at ICU−the effect of emergency percutaneous cardiopulmonary support system. Nippon Kyobu Geka Gakkai Zasshi. 1993; 41: 1035–9.
Kurose M, Okamoto K, Sato T, Ogata K, Yasumoto M, Terasaki H, et al. Extracorporeal life support for patients undergoing prolonged external cardiac massage. Resuscitation. 1993; 25: 35–40.
Sakai M, Ohteki H, Doi K, Masumoto A, Akatsuka H, Hayashida K, et al. Clinical application and indications for percutaneous cardiopulmonary bypass support in cardiopulmonary failure of various etiologies. J Jpn Ass Acute Med. 1996; 7: 345–52.
Amagasa S, Takaoka S, Kudo M, Hoshi H, Nunokawa H, Horikawa H. Cardiac arrest following induction of anesthesia in a patient with acute massive pulmonary thromboembolism. J Anesth. 1997; 11: 228–30.
Murata S, Adachi H, Ino T, Yamaguchi A, Kamio H, Okada M. An emergent surgical case of acute massive pulmonary embolism supported by antithrombotic percutaneous cardiopulmonary support system. Nippon Kyobu Geka Gakkai Zasshi. 1997; 45: 1159–64.
Oda H, Sendai M, Maruyama M, Sakai Y, Yamakawa O, Furusawa A, et al. An effective case of percutaneous cardiopulmonary support that was used in order mainly to prevent brain hypoxia due to pulmonary infarction associated with Lupus anticoagulant that had recurrent cerebral infarction. Respir Circ. 1998; 46: 803–7.
Sudo K, Ide H, Fujiki T, Tonari K, Nasu Y, Ikeda K. Pulmonary embolectomy for acute massive pulmonary embolism under percutaneous cardiopulmonary support. J Cardiovasc Surg. 1999; 40: 165–7.
Akasu K, Kashikie H, Yokose S, Chihara S, Kosuga T, Tayama E, et al. Treatment for massive pulmonary thromboembolism utilizing percutaneous cardiopulmonary support. J Kurume Med Ass. 1999; 62: 237–43.
Miyamoto T, Kato Y, Yasuda T, Matsuura Y, Mori I. A case of massive and recurrent pulmonary embolism with antiphospholipid antibody syndrome resuscitated by the percutaneous cardiopulmonary support. Ther Res. 1999; 20: 2739–47.
Hashimoto S, Sugawara Y, Tsuruya Y, Yazu T, Kubo N, Ohmura N, et al. Management and outcome of acute pulmonary thromboembolism: effect of thrombolytic therapy. Heart (Jpn). 2000; 32: 773–80.
Misawa Y, Fuse K, Yamaguchi T, Saito T, Konishi H. Mechanical circulatory assist for pulmonary embolism. Perfusion. 2000; 15: 527–9.
Araki Y, Tajima K, Iwase J, Abe T, Kato W, Tanaka K, et al. Surgical salvage of acute pulmonary thromboembolism supported by a percutaneous cardiopulmonary bypass system. Jpn J Cardiovasc Surg. 2000; 29: 122–5.
Kawahito K, Murata S, Adachi H, Ino T, Fuse K. Resuscitation and circulatory support using extracorporeal membrane oxygenation for fulminant pulmonary embolism. Artif Organs. 2000; 24: 427–30.
Niino T, Shiono M, Yagi S, Yamamoto T, Okumura H, Inoue T, et al. A case report of pulmonary thrombo-embolism. J Nihon Univ Med Ass. 2001; 60: 204–7.
Nakamura H, Teramoto K, Takamatsu S, Baba H, Saeki I, Goseki N, et al. A case cured of thromboembolism after laparoscopic cholecystectomy with percutaneous cardiopulmonary support system. Jpn J Gastroenterol Surg. 2001; 34: 1747–50.
Sasaki S, Ishitani T, Nanzaki S, Morimoto Y, Kemmotsu O, Gando S, et al. Percutaneous cardiopulmonary support in the last decade. Circ Cont. 2001; 22: 125–31.
Shimizu M, Morimura N, Uchida K, Suzuki J, Sugiyama M. Examination of the example of medical treatment by percutaneous cardiopulmonary support to severe, acute pulmonary embolism. J Jpn Biomed Forum. 2001; 11: 47–50.
Tayama E, Takaseya T, Hiratsuka R, Akasu K, Teshima H, Hayashida N, et al. Percutaneous cardiopulmonary support for treatment of massive pulmonary embolism. J Artif Organs. 2002; 5: 228–32.
Imanaka K, Kyo S, Tanabe H, Asano H, Kato M, Ohuchi H, et al. Allograft pulmonary artery root replacement for refractory isolated pulmonic valve endocarditis. Heart Vessels. 2002; 16: 198–200.
Tayama E, Ouchida M, Teshima H, Takaseya T, Hiratsuka R, Akasu K, et al. Treatment of acute massive/submassive pulmonary embolism. Circ J. 2002; 66: 479–83.
Watanabe M, Tanno K, Miyata K, Imai A, Ohmi Y, Koide A, et al. A successful resuscitation case of postoperative pulmonary thromboembolism treated with percutaneous cardiopulmonary support. Hakodate Med J. 2003; 27: 27–30.
Nomoto K, Murayama T, Nishimura M, Otsuka Y, Hirasaki Y, Inada S, et al. A case of irreverently applied PCPS in an undiagnosed leukemia patient who developed cardiopulmonary collapse. J Jpn Soc Intensive Care Med. 2003; 10: 347–51.
Nakayama S, Miyabe M, Tabata K, Toyooka H. Anesthetic management of massive endobronchial hemorrhage after pulmonary embolectomy. Masui. 2003; 52: 863–5.
Kimotsuki H, Kuroiwa M, Arai M, Takenaka T, Okamoto H, Hoka S. A case of delayed diagnosis of pulmonary thromboembolism in a patient with mitral stenosis undergoing cesarean section. MasuiAnesth. 2003; 52: 1100–3.
Samejima C, Motomura A, Hoshino M, Sakuramoto C, Hoka S. A case of pulmonary thromboembolism just before spinal anesthesia, resuscitated with percutaneous cardiopulmonary support. Circ Cont. 2003; 24: 386–9.
Hasai M, Inamori N, Hitomi K, Tanigami H, Hirata T, Mori T. Intensive care management of acute pulmonary thromboembolism. Masui. 2003; 52: 14–9.
Koshiko S, Yamazaki K, Akasaka N, Azuma N, Goh K, Hirata S, et al. Acute pulmonary embolism performed embolectomy under percutaneous cardiopulmonary support successfully after lung cancer operation. Kyobu Geka. 2003; 56: 769–72.
Kemmoku K, Tahara S, Sugimoto I, Ichinose A. A case of pulmonary embolism after eye socket reconstruction with a free radial forearm flap. J Jpn Soc Reconstructive Microsurg. 2003; 16: 25–9.
Yuasa Y, Hirai S, Arioka I, Matsumoto H, Dohi T. A case of acute massive pulmonary embolism successfully saved with the aid of a percutaneous cardiopulmonary support system. J Jpn Surg Ass. 2003; 64: 1876–9.
Okada Y, Terada T, Inagawa H, Ishida J, Kojima N. Pulmonary thromboembolism in psychiatric hospital: significance of prophylaxis in patients with physical restrains. Jpn J Clin Psychiatry. 2003; 32: 1539–44.
Kadoi Y, Kunimoto F, Saito S, Goto F. A case of acute massive pulmonary thromboembolism one week after the fracture of the supine. Jpn J Reanimatology. 2003; 22: 36–8.
Hiraoka G, Izumiyama O, Hasagawa T, Tanno K, Koide A, Takahashi M, et al. Two cases with acute pulmonary thromboembolism treated with percutaneous cardiopulmonary support. J Med Ass South Hokkaido. 2004; 39: 167.
Miyazu O, Yamauchi M, Watanabe Y, Uno M, Seki A, Sakakibara M, et al. Successfully resuscitated case of acute pulmonary embolism after hip replacement arthroplasty operation. St. Marianna Med J. 2004; 32: 181–7.
Onuma K, Takahira N, Uchiyama K, Boku T, Itoman M, Uchino M, et al. Fatal pulmonary thromboembolism with acetabular fracture: a recovered case report. Kossetsu. 2004; 26: 54–8.
Jojima H, Naito M, Takamori Y, Mori T, Serikawa T, Murayama T, et al. Pulmonary embolism after pelvic osteotomy: a case report. Orthoped Surg. 2004; 55: 423–6.
Yamanouchi S, Arai M, Koyama A, Azuma K, Maki M, Shin’ya F. Report of three cases of acute pulmonary embolism after gastrointestinal surgery. J Jpn Soc Abd Emerg Med. 2004; 24: 99–103.
Shinoura S, Morimatsu H, Katayama H, Kunitomi A, Sadamori H, Yagi T, et al. A case of acute pulmonary embolism after rectal cancer operation. J Okayama Med Assoc. 2004; 116: 141–7.
Sekimoto K, Kadoi Y, Hinohara H, Kunimoto F, Saito S, Goto F. Acute massive pulmonary thromboembolism in a patient recovering from subarachnoidal hemorrhage. J Clin Anesth (Jpn). 2004; 28: 1187–90.
Tazawa F, Hinohara H, Isa Y, Obata H, Kunimoto F, Goto F. Treatment of pulmonary thromboembolism for postoperative patients and patients with active bleeding. Jpn J Intensive Care Med. 2004; 28: 615–9.
Yamamoto T, Sato N, Tanaka K, Takano T, Nakazawa K, Tajima H, et al. Catheter-based therapy is an optimal strategy for acute massive and submassive pulmonary thromboembolism. Jpn J Phlebol. 2005; 16: 79–85.
Kohitu Y, Iwahashi T, Matsumoto A, Tanaka N, Amano K, Ishimaru A, et al. A case of acute pulmonary thromboembolism with the thrombus trapped in patent foramen ovale. J Tokyo Med Univ. 2005; 63: 252–62.
Ando M. Surgical indications, procedures, and results for acute pulmonary thromboembolism. Surg Frontier. 2005; 12: 42–7.
Following studies have Japanese title but no English title.
Shigeta O, Fukuda I, Maeda H, Oohashi N, Ueda H, Meguro K. Jpn J Thoracic Cardiovasc Surg. 1988; 36: 819–20.
Takai K, Ogawa H, Mochizuki M, Kondo T, Kakugawa K, Shinoda M, et al. Jpn Soc Extra-Corpoteal Tech. 1992; 18: 89–91.
Ookawa Y, Kamata K, Tanaka A, Maekawa K, Watanabe N, Sato A, et al. Jpn J Intensive Care Med. 1993; 17 (Suppl): 219.
Masahisa Y, Kosako Y, Shiraishi R. J Jpn Ass Acute Med. 1993; 4: 497.
Mouri A, Nishimura T, Okamoto T, Aoli A, Tashiro C. J Jpn Coll Surg. 1995; 20: 271.
Dote K, Nomura K, Ninomiya M, Nakano Y, Sasaki S, Mannda H, et al. Ther Res. 1995; 16: 1305–6.
Numaguchi K, Hayashi K, Kondo J, Tsuboi E, Sone T, Sasa H, et al. Ther Res. 1996; 17: 1549–51.
Higashioka H, Shinozaki M, Tomobuchi Y, Morinaga T, Bessyo T, Ono T, et al. J Wakayama Med Soc. 1996; 47: 539.
Ootaki Y, Ozawa S, Asada M, Mukaihara N, Hinoue T, Ooho H, et al. Jpn J Phlebol. 1996; 7: 215.
Kokubo J, Ide H, Mathison M, Fukawa M, Nonaka K, Tonari K, et al. Jpn J Intensive Care Med. 1997; 21: 994.
Kamata H, Fukuda M, Ikeda D, Kameyama N, Mtsumoto A, Fujita T, et al. J Jpn Intensive Care Med. 1997; 4 (Suppl): S184.
Tezuka M, Nakamura S, Takagi T, Anzai H, Kosakai K, Sato K. Jpn J Interventional Cardiol. 1998; 13 (Suppl 1): 191.
Okayama H, Sumimoto T, Kawata H, Kawasaki S, Tahagaki Y. Jpn J Geriat. 1998; 35: 501.
Nakagiri K, Okada M, Yoshida M, Ataka K, Yamashita C. Jpn Circ J. 1998; 62 (Suppl II): 729.
Komura M, Nitta J, Okuda H, Hagiwara N, Konagai H, Sato Y, et al. Jpn Circ J. 1998; 62 (Suppl II): 664.
Tsubouchi H, Umeda H, Yamada K, Ishiki Y, Takai J, Ishihara H, et al. Jpn Circ J. 1998; 61 (Suppl III): 819.
Kadono Y, Murashima R, Tsobota K, Tokishige J, Kin A, Iga T, et al. Kantou J Orthoped Traumatology. 1998; 29 Suppl: 90.
Hirohashi T, Watanabe Y, Koyama N, Masuhara D. KANTO J Jpn Assoc Acute Med. 1998; 19: 516–7.
Komiya A, Shibui K, Sawano M, Hamabe Y, Sonozaki H. KANTO J Jpn Assoc Acute Med. 1998; 19: 522–3.
Iwasaki M, Irie A, Nakajima T, Maeda K, Nomura K, Kato H, et al. Jpn Soc Extra-Corpoteal Tech. 1999; 26: 140.
Souma K, Hyashi M, Aoyama N. J Jpn Soc Intern Med. 1999; 88: 2342–7.
Kusama N, Yamazaki J, Aimi Y, Yasuda K, Ito A, Miura M, et al. Jpn J Anesth. 1999; 48: 916.
Narimatsu A, Morooka T, Ogata T, Tsuji T, Oomori K, Utsunomiya T, et al. Jpn Circ J. 1999; 63 (Suppl III): 947.
Usui S, Sagara K, Aizawa T, Fu T, Watanabe R, Kato K. Jpn Circ J. 1999; 63 (Suppl II): 703.
Kubota Y, Noriyasu Y, Sakurada H, Yanase O, Nomura S, Teshima T, et al. Ther Res. 1999; 20: 1025–7.
Obara Y, Arima K, Nagao K, Furuya S, Takahashi H, Ootsuki J, et al. KANTO J Jpn Assoc Acute Med. 2000; 21: 36–7.
Ookubo S, Yoneda M, Koto H, Maeda K, Nomura K, Nakajima T. Jpn J Thracic Cardiovasc Surg. 2000; 48 (Suppl): 155.
Mitani T, Nakajima Y, Watanabe K. J Tottori Med Ass. 2000; 2: 89.
Sugito Y, Tange M, Nogawa N, Ui G, Tsuchio Y, Kumakura H, et al. Jpn Circ J. 2000; 64 (Suppl III): 867.
Irie H, Kurioka N, Hiranuma O, Yoshizumi M, Araki S, Nakahara S, et al. Jpn Circ J. 2000; 64 (Suppl II): 800.
Harada A, Machii M, Sasaki T, Hukuda N, Iwahara S, Kobayashi A. Vascular Surg. 2000; 19: 94.
Uesugi M, Sone T, Tsuboi H, Kondo J, Mukawa H, Kasogabe Y, et al. Ther Res. 2000; 21: 1107–9.
Tomizuka T, Tsuzuki A, Nagao K, Ootsuki J, Kushi H, Yazaki M, et al. J Nihon Univ Med Ass. 2001; 60: 118.
Naya M, Inaba Y, Hamaguchi S, Ozaki T, Takagi C, Okada H, et al. Jpn Circ J. 2001; 65 (Suppl III): 700.
Ootsuki A, Tamagawa R, Hosoda S, Abe M, Nagai S, Saito N. Med J Matsue City Hospital. 2001; 5: 71–3.
Abe H, Yanagishita Y, Kisara M, Sichimatu Y. J Jpn Intensive Care Med. 2001; 8 (Suppl); 227.
Cyujyo K, Nasu K, Morino Y, Endo R, Kawaguchi S, Ogura S, et al. J Jpn Intensive Care Med. 2001; 8 (Suppl): 198.
Tabata T, Kusuhara T, Hamagami K, Matsuda M, Kamigouchi T, Takeuchi Y, et al. Jpn Circ J. 2001; 65 (Suppl III): 782.
Endo A, Inoue S, Sekii H, Takayama K. J Obst Gyne Res. 2001; 53: 332.
Kimura H, Kotani A, Siomi K, Matsuura H, Marusawa K, Oku K, et al. J Med Ultrasonics. 2001; 28: J136–7.
Sakano H, Kato Y, Matsuda T, Hashimoto S. J Jpn Soc Respir Care. 2001; 11: 176.
Sone T. J Ther Engineering. 2001; 13: 169–76.
Imai H, Machii M, Suzuki K, Fujita H, Inoue F, Hikida T, et al. J Jpn Soc Emer Med. 2002; 5: 225.
Serikawa T, Kakuta O, Mori T, Goto H, Tanaka K, Shibano R, et al. Jpn J Clin Exp Med. 2002; 79: 327–30.
Hamada M, et al. Circ J. 2002; 66 (Suppl II): 995.
Takahashi N, Imai H, Masuda Y, Asai Y, Abe T. J Jpn Coll Surg. 2002; 27: 388.
Takahashi S, Abe M, Koseki T, Murai T, Takeuchi M, Yahagi T, et al. J Jpn Intensive Care Med. 2002; 9 (Suppl): 213.
Ooue K, Miyamoto S, Minamimura H, Ishikawa T, Nishi H, et al. Jpn J Thorac Cardiovasc Surg. 2002; 50 (Suppl): 298.
Oono O, Naruse M, Nakajima Y, Hayashi Y, Furukawa T, Torii T, et al. Circ J. 2002; 66 (Suppl III): 1055.
Kawase Y, Ishii Y, Yajima T, Bessyo R, Imura H, Yamashita H, et al. Jpn J Thoracic Cardiovasc Surg. 2002; 50 (Suppl): 80.
Sugimura K, Sakuma M, Takahashi T, Demachi J, Nawata J, Namata H, et al. Ther Res. 2002; 23: 589–90.
Numata T, Ogiwara H, Sasaki K, Hanafusa Y, Kitamura S, Ando M. Ther Res. 2002; 23: 648–9.
Kubota R, Shimizu K. Ther Res. 2002; 24: 595–7.
Satou T, Ishikawa S, Ishikawa M, Kikuie T, Haga Y, Shimada M. J Jpn Soc Intensive Care Med. 2003; 10 (Suppl); 165.
Toujyou K, Fujii M, Takeda A, Kinoshita H, Furuhira S, Nishikawa A, et al. Jpn Soc Extra-Corpoteal Tech. 2003; 30: 326–9.
Mishima T, Kawahito H, Murata S, Adachi H, Ino T. Jpn J Vasc Surg. 2003; 12: 290.
Akaeda S, Takahashi T, Takeuchi M, Fushimi E, Sekiguchi N, Tabata M, et al. Akita J Rural Med. 2003; 49: 27.
Nishimura K, Okayama H, Hiasa T, Sumimoto T. Jpn J Geriat. 2003; 40: 527.
Tabata T, Hasegawa H, Sakamoto E, Igami T, Mori T, Tokumaru S, et al. J Jpn Ass Acute Med. 2003; 14: 604.
Kouno S, Ito A, Imai T, Ashida K, Abe Y, Komatsu R, et al. Circ J. 2003; 67 (Suppl III): 985.
Nanba S, Sato K, Bando H, Ooya M. Circ J. 2003; 67 (Suppl II): 857.
Obana M, Yaga S, Nakayama T, Saito T, Kawashima H, Kamino D, et al. J Jpn Surg Ass. 2003; 64: 2063.
Ichihara T, Eda T, Sasaki M, Ueda Y. Proceedings of the 39th Annual Meeting of Cyubu Surg Soc. 2003; 39: 58.
Azuma A, Yamashita M, Iwamoto S, Suehiro S, Yamamoto ZY, Kobayashin A, et al. Jpn J Thoracic Cardiovasc Surg. 2003; 51 (Suppl): 159.
Ogino Y, Niinuma H, Oohira A, Makita S, Moriai Y, Suzuki T, et al. Ther Res. 2003; 24: 579–81.
Uda M, Dote K, Shimizu I, Nakanishi K, Yano M, Hiraga N, et al. J Jpn Soc Clin Anesth. 2003; 23: S294.
Nakamura Y, Kanaoka T, Ishiguro S, Oogi S. Ther Res. 2003; 24: 533–5.
Sugihara T, Ban K, Nakajima T, Kumagai A, Tanabe A. Ther Res. 24: 536–539, 2003.
Imamoto S, Minami T, Matsuyama A, Shimazu Y, Hashimoto K, Mochizuki M. Circ J. 2004; 68 II: 770.
Sakano Y, Misawa Y, Fuse K, Nomura Y, Ootani K, Katsuki T. Circ J. 2004; 68 (Suppl III): 895.
Sato K, Kubo M, Tada T, Kobayashi H, Yano M, Tukioki M, et al. Med J Onomichi Municipal Hospital. 2004; 20: 85–9.
Kanaoka Y, Kojima T. Jpn J Vasc Surg. 2004; 13: 632.
Syoubukawa Y, Kinoshita H, Miyajima M, Tanaka T, Hirose Y, Iinuma Y, et al. Niigata Med J. 2004; 118: 225.
Ishikawa M, Kato M, Okiwara M, Imanaka K, Nishimura N, Asano H, et al. Jpn J Surg. 2004; 105 (Suppl): 689.
Hayashida K, Takahashi R, Nakajima T, Kawauchi Y, Yaku H. Jpn J Surg. 2004; 105 (Suppl): 601.
Nanzaki S, Hayakawa T, Morishita Y, Takahashi I. J Jpn Intensive Care Med. 2004; 11 (Suppl): 236.
Furuichi T, Arima K, Kawamata H, Yamaoka K, Takahashi K, Kosuge K, et al. J Jpn Intensive Care Med. 2004; 11 (Suppl): 199.
Nakamura T, Konobe T, Hata Y, Murao Y, Seki T, Okuchi K, et al. J Jpn Coll Angiol. 2004; 44: 508.
Tamura D, Kurogane K, Kajiura K, Takai E, Kaname C, Sawada T, et al. Circ J. 2004; 68 (Suppl III): 936.
Hirabayashi T, Oota S, Ishikura K, Kitamura T, Yamada N, Okinaka T, et al. Circ J. 2004; 68 (Suppl III): 917.
Yoshitani K, Nakamura T, Ono D, Chiba T, Fujita K. Circ J. 2004; 68 (Suppl II): 756.
Imakama I, Toda R, Shimozono T, Oohashi I, Yuda T. Jpn J Thoracic Cardiovasc Surg. 2004; 52 (Suppl 1): 147.
Ishida K, Kawae J, Mabuchi H, Maeda K, Murakami T. Shiga Med J. 2004; 26: 143.
Yoshikawa R, Shide J, Araya T, Kawai H, Matsumoto H, Watanabe T, et al. Ther Res. 2004; 25: 1214–6.
Yamashita T, Ookita Y. Med Clinics Jpn. 2004; 30: 353–5.
Fujita S. Kyukyu, Shuchu Chiryo. 2004; 16: 401–6.
Koma M, Meda M, Matsuda H. Jpn J Surg. 2005; 106 (Suppl): 465.
Endo T, Saito T, Saka K, Kawaura N, Mitsuhashi T, Murayama M, et al. J Cardiol. 2005; 46 (Suppl): 513.
Yamaguchi D, Yahagi N, Ito H, Nakajima T, Komatsu S, Oobayashi T, et al. J Jpn Intensive Care Med. 2005; 12 (Suppl): 223.
Komura Y, Kushiyama Y, Shirai N, Iwabuchi N, Yasumoto H, Nosaka H, et al. J Jpn Intensive Care Med. 2005; 12 (Suppl): 208.
Yamada R, Watanabe N, Motomiya K, Yoshimura Y, Saito A, Oyama Y, et al. Circ J. 2005; 69 (Suppl II): 814.
Tsumaru S, Nakayama K, Yamauchi M, Kitano T. Circ J. 2005; 69 (Suppl III): 914.
Kaneshiro T, Arai A, Matsubara S, Miyazaki S, Hattori E, Koumoto T, et al. Circ J. 2005; 69 (Suppl III): 893.
Kojima N, Ishida J, Inagawa H, Okada Y, Terada Y. J Jpn Soc Emer Med. 2005; 8: 135.
Komatsu T. Kyukyu, Shuchu Chiryo. 2005; 17: 889–96.
Sato A, Nakano S, Nakatani H, Gomi A, Nakamura Y, Sugimoto K. Jpn J Thoracic Cardiovasc Surg Kanto-Kosinnetsu. 2005; 8.
Inafuku S, Morishima Y, Nagano T, Kaneshiro M, Mabuni K, Niigaki K, et al. Okinawa Med J. 2005; 44: 97.
References
- Ota M, Nakamura M, Yamada N, Yazu T, Ishikura K, Hiraoka N, et al. Prognostic significance of early diagnosis in acute pulmonary thromboembolism with circulatory failure.Heart Vessels. 2002; 17: 7–11 [DOI] [PubMed] [Google Scholar]
- Sakuma M, Nakamura M, Nakanishi N, Miyahara Y, Tanabe N, Yamada N, et al. Inferior vena cava filter is a new additional therapeutic option to reduce mortality from acute pulmonary embolism.Circ J. 2004; 68: 816–21 [DOI] [PubMed] [Google Scholar]
- Böttiger BW, Bode C, Kern S, Gries A, Gust R, Glätzer R, et al. Efficacy and safety of thrombolytic therapy after initially unsuccessful cardiopulmonary resuscitation: a prospective clinical trial. Lancet. 2001; 357: 1583–5 [DOI] [PubMed] [Google Scholar]
- Spöhr F, Arntz HR, Bluhmki E, Bode C, Carli P, Chamberlain D, et al. International multicentre trial protocol to assess the efficacy and safety of tenecteplase during cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest: the Thrombolysis in Cardiac Arrest (TROICA) Study. Eur J Clin Invest. 2005; 35: 315–23 [DOI] [PubMed] [Google Scholar]
- Ohteki H, Norita H, Sakai M, Narita Y. Emergency pulmonary embolectomy with percutaneous cardiopulmonary bypass. Ann Thorac Surg. 1997; 63: 1584–6 [DOI] [PubMed] [Google Scholar]
- Kawahito K, Murata S, Adachi H, Ino T, Fuse K. Resuscitation and circulatory support using extracorporeal membrane oxygenation for fulminant pulmonary embolism. Artif Organs. 2000; 24: 427–30 [DOI] [PubMed] [Google Scholar]
- Conrad SA, Rycus PT, Dalton H. Extracorporeal Life Support Registry Report 2004. ASAIO J. 2005; 51: 4–10 [DOI] [PubMed] [Google Scholar]
- Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007; 62: 570–6 [DOI] [PubMed] [Google Scholar]
- Japanese Circulation Society Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2004). Circ J. 2004; 68Suppl IV: 1079–134 (in Japanese). [DOI] [PubMed] [Google Scholar]
- Sakuma M, Nakamura M, Nakanishi N, Miyahara Y, Tanabe N, Yamada N, et al. Diagnostic and therapeutic strategy for acute pulmonary thromboembolism. Intern Med. 2006; 45: 749–58 [DOI] [PubMed] [Google Scholar]
- Kurusz M, Zwischenberger JB. Percutaneous cardiopulmonary bypass for cardiac emergencies. Perfusion. 2002; 17: 269–77 [DOI] [PubMed] [Google Scholar]
- Chen YS, Chao A, Yu HY, Ko WJ, Wu IH, Chen RJ, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003; 41: 197–203 [DOI] [PubMed] [Google Scholar]
- Mattox KL, Feldtman RW, Beall AC, Jr, DeBakey ME. Pulmonary embolectomy for acute massive pulmonary embolism. Ann Surg. 1982; 195: 726–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Saka K, Kawaura N, Mitsuhashi T, Murayama M, Saito T, et al. Clinical Experience with percutaneous cardiopulmonary support for massive acute pulmonary embolism. Ther Res. 2006; 27: 1147–9 (In Japanese). [Google Scholar]
- Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prespective study. JAMA. 1979; 242: 2193–6 [DOI] [PubMed] [Google Scholar]
- Phillips SJ. Resuscitation for cardiogenic shock with extracorporeal membrane oxygenation systems. Semin Thorac Cardiovasc Surg. 1994; 6: 131–5 [PubMed] [Google Scholar]
- Kolla S, Lee WA, Hirschl RB, Bartlett RH. Extracorporeal life support for cardiovascular support in adults. ASAIO J. 1996; 42: M809–19 [DOI] [PubMed] [Google Scholar]
- Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004; 240: 595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T. Registry. In: Matsuda H. ed. Cutting edge of percutaneous cardiopulmonary support. Tokyo: Shujunsha, 2004: 141–8 [Google Scholar]
- Kasirajan V, Smedira NG, McCarthy JF, Casselman F, Boparai N, McCarthy PM. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 1999; 15: 508–14 [DOI] [PubMed] [Google Scholar]
- Marty AT, Hilton FL, Spear RK, Greyson B. Postcesarean pulmonary embolism, sustained cardiopulmonary resuscitation, embolectomy, and near-death experience. Obstet Gynecol. 2005; 106: 1153–5 [DOI] [PubMed] [Google Scholar]
- Tsai SK, Wang MJ, Ko WJ, Wang SJ. Emergent bedside transesophageal echocardiography in the resuscitation of sudden cardiac arrest after tricuspid inflow obstruction and pulmonary embolism. Anesth Analg. 1999; 89: 1406–8 [DOI] [PubMed] [Google Scholar]
- Kolvekar SK, Peek GJ, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenator for pulmonary embolism. Ann Thorac Surg. 1997; 64: 883–4 [PubMed] [Google Scholar]
- Bauer C, Vichova Z, Ffrench P, Hercule C, Jegaden O, Bastien O, et al. Extracorporeal membrane oxygenation with danaparoid sodium after massive pulmonary embolism. Anesth Analg. 2008; 106: 1101–3 [DOI] [PubMed] [Google Scholar]
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation. 2005; 112: e28–32 [DOI] [PubMed] [Google Scholar]
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999; 353: 1386–9 [DOI] [PubMed] [Google Scholar]
- Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997; 30: 1165–71 [DOI] [PubMed] [Google Scholar]


