Abstract
Objectives: This study intended to confirm whether skin perfusion pressure (SPP) could predict the outcome of ischemic wound healing.
Patients and methods: Sixty-two limbs in 53 patients with conservative therapy were enrolled in this study. A SPP value of 40 mmHg was adopted as the criterion for making clinical decisions. The outcome one month after SPP measurement was classified as “improved” (diameter of ulcer decreased ≥ 20% or demarcation of gangrene became well defined) or “no change or worse” (others), and the fate of wound was classified as “healed” or “not healed”. The evaluated influential factors on the outcome at one month included age, sex, presence of arteriosclerosis obliterans, collagen disease, hypertension, diabetes mellitus, hemodialysis, wound infection, wound management, and SPP ≥ 40 mmHg.
Results: Using a criterion of SPP ≥ 40 mmHg, the outcome at one month could be predicted with a sensitivity: of 75.0%, a specificity: of 82.6%, and an accuracy: of 80.6%. The receiver operating characteristic curve indicated our criterion to be appropriate. Logistic regression analysis showed SPP ≥ 40 mmHg to be an independent factor (P < 0.0001) with the odds ratio of 14.2 (95% CI 3.6–55.8).
Conclusions: SPP, using a cutoff value of 40 mmHg, can predict the ischemic wound healing with conservative therapy.
Keywords: skin perfusion pressure, ischemic foot ulcer, wound healing, non-invasive assessment
Introduction
Among several factors influence wound healing, blood flow to the skin plays an important role. Over the past decades, skin perfusion pressure (SPP) measurement has been recognized as an accurate noninvasive test for evaluating blood flow to impaired tissue. SPP can accurately predict wound healing of ulcers and the need for amputation, and is not affected by calcified arteries. So, SPP can be measured anywhere on the limb where viable tissue is present.1–5) Measurement can also be performed in limbs that have had prior amputation.
Since 2000, we have used SPP in deciding the therapeutic strategy for foot ulcers. Although there are some reports that an SPP value of 30 mmHg is a suitable criterion for making clinical decisions on therapy, we have adopted an SPP value of 40 mmHg because of its safety margin. As a general rule, patients with SPP ≥ 40 mmHg were followed up with conservative therapy, and patients with SPP < 40 mmHg underwent revascularization if it was possible. If a patient with necrosis or gangrene were to have a minor amputation, we chose the amputation level where SPP was ≥ 40 mmHg. The objective of this study is to confirm whether SPP can predict the outcome of wound healing in patients with a foot ulcer or gangrene.
Patients and Methods
From January 2003 to December 2007, SPP was measured in 122 limbs in 109 consecutive patients with a chronic foot ulcer or gangrene who consulted a vascular surgeon at The University of Tokyo Hospital. SPP of all limbs was measured by vascular technologists at the Vascular Laboratory of The University of Tokyo Hospital. We excluded patients with acute limb ischemia, blue toe syndrome, and diabetic foot ulcers or gangrenes. Moreover, those patients with a follow-up period of less than a month were excluded.
SPP was ≥ 40 mmHg in 27 limbs. Twenty of these limbs were followed-up with conservative therapy, three underwent revascularization, and four underwent primary amputation. On the other hand, SPP was < 40 mmHg in 95 limbs. Forty two of these limbs were followed-up with conservative therapy, 38 underwent revascularization, and 15 underwent primary amputation (Fig. 1). Although we make it a rule to treat limbs with SPP < 40 mmHg by revascularization or amputation, 42 limbs with SPP < 40 mmHg were treated by conservative therapy because of the patient’s poor general condition including poor cardiac function in 24 limbs, the patient’s refusal to undergo operation in seven, mild symptoms in six, and inability to revascularize the limb for anatomical reasons in five. As a consequence, we enrolled on 62 limbs (20 limbs with SPP ≥ 40 mmHg and 42 limbs with SPP < 40 mmHg) in 53 patients who were followed-up with conservative therapy in this study.
Fig. 1.
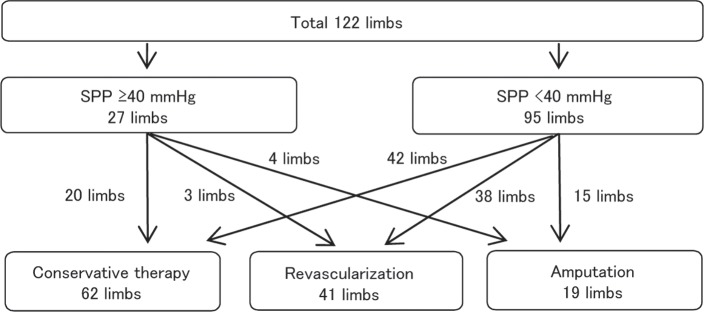
SPP was measured in 122 limbs with a foot ulcer or gangrene. Therapy, consisting of conservative therapy, revascularization, or amputation, was chosen within one month of the measurement.
We used a laser Doppler scanner LASERDOPP PV2000 (Vasamedics, St. Paul, Minn.) to measure SPP. The details of SPP measurement are described elsewhere.1, 6, 7) In this study, we adopted the SPP value measured just proximal to the ulcer or gangrene.
We examined the outcome at one month after SPP measurement to assess the tendency for wound healing and the fate of wound during the follow-up period. The outcome at one month was classified as “improved” (diameter of ulcer [square root of the product of major axis and minor axis] decreased more than 20% or demarcation of gangrene became well defined) or “no change or worse” (others) by each vascular surgeon, and the fate of wound was classified as “healed” or “not healed” (residual ulcers, worse, or therapeutic shift to revascularlization and/or amputation).
The influence of factors on the outcome was examined. The factors consisted of age, sex, presence of ASO, collagen disease, hypertension, diabetes mellitus, hemodialysis for chronic renal failure, wound infection, location of the wound, treatment of the wound (use of anti-platelet drugs, prostaglandins, and bFGF), and SPP as a parameter of local blood perfusion. Infection was defined as the presence of erythema, pain, edema, heat, exudate, foul odor, cellulitis, or wound breakdown. The location of the wound was classified as “toes” and “others” (dorsal foot, plantar sole, heel, and leg). We excluded smoking as a factor because the patients’ accounts were not reliable.
For statistical analysis, Fisher’s exact test was performed for categorical factors and Wilcoxon’s test was performed for continuous factors to determine the relationship between the outcome and risk factors. Factors with a P value less than 0.2 by univariate analysis were entered into logistic regression analysis and odds ratios were calculated. Statistical analyses were performed using JMP 7.0.1 software (SAS Institute Inc., Cary, NC).
Results
The median follow-up period was 182 days (interquartile range 65 to 584 days). There were 19 women and 34 men with a mean age of 64.8 years (21 to 84 years). The causes of foot lesions were arteriosclerosis obliterans (ASO) in 37 patients (70%), collagen disease in 11 patients (21%), and thromboangiitis obliterans (TAO) in five patients (9%) (Table 1). Forty patients (75%) had hypertension, 32 patients (60%) had diabetes, and 18 patients (34%) were receiving hemodialysis. Forty three limbs (69%) had wounds only on toes and 19 limbs (31%) had them on proximal site from toe. For conservative therapy, 40 patients with 46 affected limbs (75%) were administered anti-platelet drugs and 27 patients with 28 affected limbs (51%) were administered prostaglandins {24 patients with 25 affected limbs (45%) were administered both of them}. Furthermore, 28 patients with 31 affected limbs (53%) underwent local application of basic fibroblast growth factor (bFGF) to ulcers to promote healing (Table 2). There was no significant difference in use of each agent between two groups divided by SPP value of 40 mmHg.
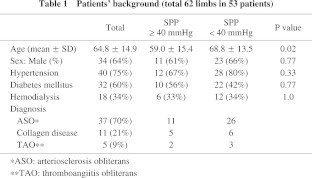
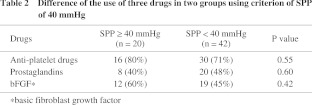
Of 20 limbs with SPP ≥ 40 mmHg, the outcome at one month of 12 limbs was “improved” and eight limbs showed “no change or worse”. Of 42 limbs with SPP < 40 mmHg, the outcome of only four limbs was “improved” and 38 limbs showed “no change or worse”. In total, the outcome of 16 limbs was “improved” and 46 limbs showed “no change or worse” (Table 3). The mean SPP value in each group was 50.0 ± 22.8 mmHg and 27.9 ± 17.0 mmHg, respectively, and the difference was significant (P < 0.0001). The expectancy of SPP ≥ 40 mmHg to predict the outcome of limbs at one month was; sensitivity: 75.0% (12/16), specificity: 82.6% (38/46), accuracy: 80.6% (50/62). The receiver operating characteristic (ROC) curve showed that SPP of 40 mmHg is the nearest to the upper left corner (Fig. 2). It indicates that the cutoff value of 40 mmHg is the best.
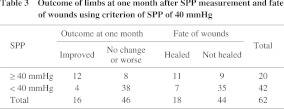
Fig. 2.
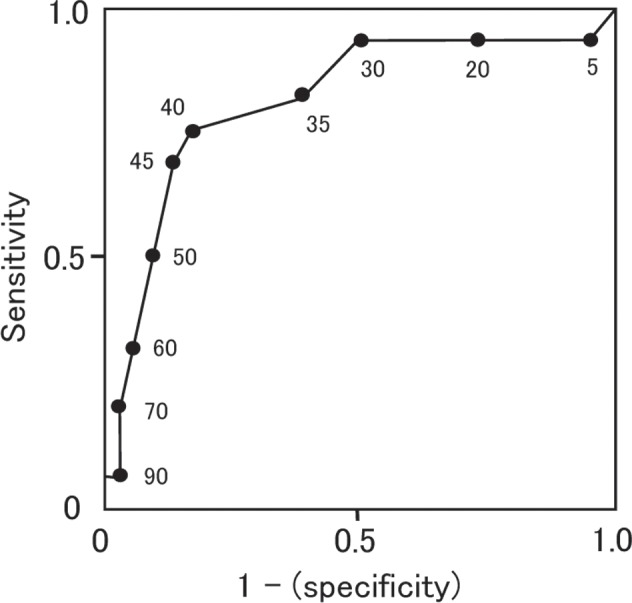
Receiver operating characteristic (ROC) curve of SPP and outcome. Values on the curve are SPP values (mmHg). As SPP 40 mmHg is the nearest point from the upper left corner, it’s the best cutoff point.
The fate of 18 limbs was “Healed”, 44 limbs was “Not healed”. Of 44 limbs with “not healed”, two limbs had revascularlization, eight limbs had secondary amputation, and 34 limbs remained unhealed. The expectancy of SPP ≥ 40 mmHg to predict the fate of wound was; sensitivity: 61.1% (11/18), specificity: 79.5% (35/44), accuracy: 74.2% (46/62) (Table 3). These results seem to be quite similar to the outcome at one month, but because 17 limbs (27%) of them were lost to follow up, they are not comparable.
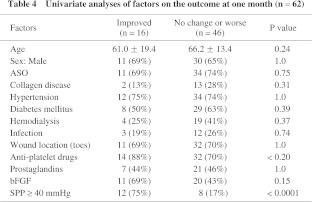
Among the factors analyzed for the influence on the outcome at one month, univariate analysis showed the P values of the factors of use of anti-platelet drugs, local administration of bFGF, and SPP ≥ 40 mmHg were < 0.2 (Table 4). After logistic regression analysis, SPP ≥ 40 mmHg was revealed to be the only independent influential factor and the odds ratio was 14.2 (95% CI 3.6–55.8).
Discussion
Our results show we can have confidence in the value of SPP as a tool for making clinical decisions in patients with ischemic foot ulcers, and an SPP value of 40 mmHg is an appropriate cutoff value. Furthermore, SPP ≥ 40 mmHg is a unique significant factor for predicting a good outcome of ischemic foot ulcers or gangrenes.
Predicting successful healing of ulcers and the need for amputation of ischemic limbs remains a major clinical challenge, particularly in diabetic patients whose systolic Doppler ankle pressure is often artifactually elevated owing to calcified arteries.8) The inability of Doppler ankle pressure to predict the healing of forefoot amputations was reported by Mehta et al.9) They concluded that a low ankle pressure did not contraindicate a transmetatarsal amputation, while a higher pressure did not guarantee success.
Unlike other noninvasive physiologic tests that measure pressure in the limbs, SPP can be measured in the limbs when skin lesions of the toe or digital amputation preclude measurement of the toe pressure, and it is not affected by calcification of arteries.4) Tsai et al. and Yamada et al. reported that there was a strong linear correlation between SPP and toe pressure,1, 10) and also, a significant correlation was found in both patients with and without diabetes.1)
Previous studies have documented that SPP is a reproducible diagnostic test and could reliably evaluate the severity of foot ischemia.11–14) Another factor that may contribute to the diagnostic accuracy of SPP is the fact that the measurement can be performed at the level of the limb or foot where the ulcer or amputation wound is located. Thus, SPP can be measured in the region of interest.4)
In previous studies, two simple functional tests, transcutaneous oxygen pressure (TcPO2) and toe blood pressure (TP) measurements, provided reliable information about the need for vascular intervention,15) but the TcPO2 test was relatively time consuming and the accuracy of TcPO2 measurements in ischemic limbs has been questioned.4) Graaff et al. concluded that the interobserver and intraobserver reproducibility of TcPO2 were moderate.15) For these reasons, we use TcPO2 measurement as a supplemental test to SPP if needed.
Our results show that SPP can predict whether an ulcer or limb gangrene will heal or improve without revascularization. Thus, the SPP measurement should be a criterion for clinical decision making in peripheral arterial disease.
Ballard et al. reported that an absolute transmetatarsal TcPO2 level of ≥ 30 mmHg appeared to be an accurate cutoff point for the selection of conservative or operative treatment for almost all diabetic foot problems, and a higher transmetatarsal TcPO2 threshold (40 mmHg) might be required to successfully manage calcaneal gangrene or some very severe non-healing ulcers.16) There are some reports that SPP of 30 mmHg is a suitable criterion for making clinical decisions on therapy,4, 5, 16–18) but we used an SPP value of 40 mmHg because of its safety margin. Recently, Yamada et al. also reported that an SPP value of 40 mmHg was a suitable cutoff value to predict wound healing.10) The ROC curve of SPP value and the outcome in our study showed that SPP 40 mmHg was the best cutoff point.
Yamada et al.10) retrospectively evaluated healing of ischemic wounds followed-up for more than three months. However, we evaluated the outcome of ischemic wounds one month after SPP measurement because SPP value changes with various factors such as infection of the ulcer or cardiac function over a longer period. Furthermore, one month is long enough to evaluate the tendency for wound healing. We also evaluated the outcome three months after SPP measurement, but 17 of 62 limbs (27%) were lost to follow up because the patients transferred to another hospital or died. The outcome of the remaining 45 limbs three months after SPP measurement was; “improved” in 23 limbs and “no change or worse” in 22 limbs. Among the 17 limbs that were lost to follow up, the outcome at one month in 16 limbs was “no change or worse”, creating a bias in the outcome of limbs three months after SPP measurement.
Previous studies did not examine factors for wound healing, but in our study, univariate and multivariate analyses showed that SPP ≥ 40 mmHg was the only independent factor that influenced the outcome of limbs. So, irrespective of a patient with an ischemic foot ulcer or gangrene has diabetes mellitus, collagen disease, wound infection, or is receiving hemodialysis, we can decide the therapy based only on the SPP value.
There were three limbs in our study whose fate of wounds were “not healed” in spite of their SPP were ≥ 40 mmHg and the outcome at one month were “improved”. One limb was in a patient with ASO and diabetes mellitus, and was also on hemodialysis. The rest two limbs of two patients were with collagen disease and TAO. On the other hand, there was only one limb whose SPP ≥ 40 mmHg but had secondary amputation. The patient had infection on ulcers, had diabetes mellitus, and was on hemodialysis. These results show that although each factors examined had no significant influence on the outcome at one month, plural factors combined can influence on the outcome later.
There were some limitations of our study. It was not a randomized controlled trial and there might have been selection bias in the outcome of limbs because 24 of 42 limbs with SPP < 40 mmHg were treated by conservative therapy mostly because of the patients’ poor condition. The heterogeneity of the pathogenesis of foot lesions might also potentially bias the results. Moreover, as each vascular surgeon was aware of the SPP value of the wound, there might have been bias in evaluating wound healing.
Conclusion
SPP can predict one month outcome of foot ulcers and gangrene treated with conservative therapy using a cutoff value of 40 mmHg.
References
- Tsai FW, Tulsyan N, Jones DN, Abdel-Al N, Castronuovo JJ, Jr, Carter SA. Skin perfusion pressure of the foot is a good substitute for toe pressure in the assessment of limb ischemia. J Vasc Surg. 2000; 32: 32–6 [DOI] [PubMed] [Google Scholar]
- Faris I, Duncan H. Skin perfusion pressure in the prediction of healing in diabetic patients with ulcers or gangrene of the foot. J Vasc Surg. 1985; 2: 536–40 [DOI] [PubMed] [Google Scholar]
- Dwars BJ, van den Broek TA, Rauwerda JA, Bakker FC. Criteria for reliable selection of the lowest level of amputation in peripheral vascular disease. J Vasc Surg. 1992; 15: 536–42 [DOI] [PubMed] [Google Scholar]
- Castronuovo JJ, Jr, Adera HM, Smiell JM, Price RM. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg 1997; 26: 629–37 [DOI] [PubMed] [Google Scholar]
- Adera HM, James K, Castronuovo JJ, Jr, Byrne M, Deshmukh R, Lohr J. Prediction of amputation wound healing with skin perfusion pressure. J Vasc Surg. 1995; 21: 823–9 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Muto A, Dardik A, Nishibe M, Nishibe T. Laser Doppler skin perfusion pressure in the diagnosis of limb ischemia in patients with diabetes mellitus and/or hemodialysis. Int Angiol. 2007; 26: 258–61 [PubMed] [Google Scholar]
- Malvezzi L, Castronuovo JJ, Jr, Swayne LC, Cone D, Trivino JZ. The correlation between three methods of skin perfusion pressure measurement: radionuclide washout, laser Doppler flow, and photoplethysmography. J Vasc Surg. 1992; 15: 823–30 [DOI] [PubMed] [Google Scholar]
- Karanfilian RG, Lynch TG, Zirul VT, Padberg FT, Jamil Z, Hobson RW., 2nd The value of laser Doppler velocimetry and transcutaneous oxygen tension determination in predicting healing of ischemic forefoot ulcerations and amputations in diabetic and nondiabetic patients. J Vasc Surg. 1986; 4: 511–6 [PubMed] [Google Scholar]
- Mehta K, Hobson RW, 2nd, Jamil Z, Hart L, O’Donnell JA. Fallibility of Doppler ankle pressure in predicting healing of transmetatarsal amputation. J Surg Res. 1980; 28: 466–70 [DOI] [PubMed] [Google Scholar]
- Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, et al. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs–comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008; 47: 318–23 [DOI] [PubMed] [Google Scholar]
- de Graaff JC, Ubbink DT, Lagarde SM, Jacobs MJ. The feasibility and reliability of capillary blood pressure measurements in the fingernail fold. Microvasc Res. 2002; 63: 270–8 [DOI] [PubMed] [Google Scholar]
- Lukkari-Rautiarinen E, Lepäntalo M, Pietilä J. Reproducibility of skin blood flow, perfusion pressure and oxygen tension measurements in advanced lower limb ischaemia. Eur J Vasc Surg. 1989; 3: 345–50 [DOI] [PubMed] [Google Scholar]
- Yvonne-Tee GB, Rasool AH, Halim AS, Rahman AR. Reproducibility of different laser Doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods. 2005; 52: 286–92 [DOI] [PubMed] [Google Scholar]
- Mayrovitz HN, Smith J, Delgado M. Variability in skin microvascular vasodilatory responses assessed by laser-Doppler imaging. Ostomy Wound Manage. 1997; 43: 66–70, 2,, 4 [PubMed] [Google Scholar]
- de Graaff JC, Ubbink DT, Legemate DA, Tijssen JG, Jacobs MJ. Evaluation of toe pressure and transcutaneous oxygen measurements in management of chronic critical leg ischemia: a diagnostic randomized clinical trial. J Vasc Surg. 2003; 38: 528–34 [DOI] [PubMed] [Google Scholar]
- Ballard JL, Eke CC, Bunt TJ, Killeen JD. A prospective evaluation of transcutaneous oxygen measurements in the management of diabetic foot problems. J Vasc Surg. 1995; 22: 485–92 [DOI] [PubMed] [Google Scholar]
- Ubbink DT, Spincemaille GH, Reneman RS, Jacobs MJ. Prediction of imminent amputation in patients with non-reconstructible leg ischemia by means of microcirculatory investigations. J Vasc Surg. 1999; 30: 114–21 [DOI] [PubMed] [Google Scholar]
- Holstein P. Level selection in leg amputation for arterial occlusive disease: a comparison of clinical evaluation and skin perfusion pressure. Acta Orthop Scand. 1982; 53: 821–31 [DOI] [PubMed] [Google Scholar]


