Abstract
Kawasaki disease is a disease of unknown etiology that most frequently affects infants and children under 5 years of age. Inflammation occurs in medium-sized muscular arteries throughout the body including the coronary artery, being classified as a systemic vasculitis syndrome. Histopathological investigations of Kawasaki disease have mainly focused on the coronary artery because it is directly associated with the cause of death. However, to identify the cause and pathology of Kawasaki disease, it is necessary to investigate lesions of whole organs. Thus, we attempted to review lesions in organs other than the heart and hypotheses of pathogenesis recently attracting attention.
Keywords: Kawasaki disease, pathology, systemic vasculitis syndrome, granulomatous angitis, infant
Introduction
Kawasaki disease was first reported as an acute febrile mucocutaneous lymph-node syndrome by Tomisaku Kawasaki in 1967.1, 2) The prognosis of the disease was initially considered favorable, but the presence of fatal cases was clarified by a nationwide survey. On autopsy, systemic vasculitis was observed and a vasculitis-based aneurysm was formed in the coronary artery, clarifying that thrombotic occlusion of the lumen occurring in the aneurysm caused fatal ischemic heart disease.3)
The diagnosis of Kawasaki disease is based on clinical signs and symptoms, which are classified as principal clinical and other clinical and laboratory findings.4) The principal symptoms include: 1) fever persisting for 5 days or more; 2) bilateral conjunctival congestion; 3) changes in the lips and oral cavity: reddening of the lips, strawberry tongue, and diffuse infection of the oral and pharyngeal mucosa; 4) polymorphous exanthema; 5) changes in the peripheral extremities: initial stage = reddening of the palms and soles, and indurative edema, convalescent stage = membranous desquamation from the fingertips; and 6) acute nonpurulent cervical lymphadenopathy. At least five of the above six items must be satisfied for a diagnosis of Kawasaki disease. However, patients showing four of the principal symptoms can be diagnosed with Kawasaki disease when a coronary aneurysm or dilation is detected by two-dimensional echocardiography or coronary angiography.
The involvement of an infective factor in the development of Kawasaki disease has been assumed, but the cause is still unclear. A nationwide epidemiological survey of Kawasaki disease has been continued from 1970, and the total number of registered patients exceeds 240,000. The peak onset age is one year, and 70% of pediatric patients develop the disease at 3 years of age or younger. More than 10,000 patients have been reported annually in recent years, and the number of patients is still increasing.5) After Furusho et al.6) reported the efficacy of immunoglobulin, the incidence of coronary arterial complication decreased and the mortality was markedly reduced. However, 15% of pediatric patients do not sufficiently respond to immunoglobulin. The development of new therapeutic methods that provide alternatives to immunoglobulin is another task.7–10)
The histopathological investigation of Kawasaki disease is still focused on the coronary artery, as in the past, because coronary arterial lesions are directly associated with fatality and their details have to be elucidated. Regarding organs other than the heart, many histopathological investigations were reported in the 1980s, but rarely reported thereafter as the number of autopsies of Kawasaki disease patients decreased. Moreover, many of these were abstracts of academic meetings or written in Japanese. To investigate the pathology and cause of Kawasaki disease, it is necessary to understand lesions of not only the heart but also the whole body. Thus, this report firstly aims at reviewing the histopathology of organs other than the heart, focusing on vascular lesions, in English. The histopathology of various organs we investigated is presented for your understanding.
Outline of Systemic Vascular Lesions
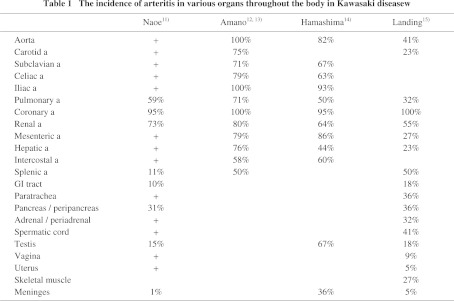
Whole body examination for Kawasaki disease as a systemic vasculitis was outlined by Naoe et al.,11) Amano et al.,12, 13) Hamashima et al.,14) and Landing et al.15) All reported that coronary arteritis occurred at the highest incidence but vasculitis developed at various sites throughout the body (Table 1). Naoe et al.11) reported that vascular lesions of Kawasaki disease started in the tunica interna and externa of medium-sized muscular arteries, such as the coronary artery, whereas Amano et al.12, 13) and Hamashima et al.14) reported that vasculitis started in arterioles, venules and capillaries, and inflammation disseminated to large arteries including the coronary artery. The following points are consistent among Japanese researchers: Firstly, the histological characteristic of vasculitis in Kawasaki disease is proliferative granulomatous inflammation consisting of markedly accumulating monocytes/macrophages, but fibrinoid necrosis rarely occurs. Secondly, vasculitis in Kawasaki disease starts simultaneously with the onset, rapidly reaches an inflammatory peak, and then slowly remits and heals with cicatrization, showing a monophasic course. However, Landing et al.15) observed late lesions corresponding to scars of vasculitis in about 1/3 of arteries in patients who died in the acute phase within 2 weeks after onset, and acute inflammatory findings (acute lesions) in about half of the arteries even at 3 months after the onset, showing that vasculitis in the acute and cicatrical phases is mixed in Kawasaki disease. However, in our study, the course of Kawasaki disease vasculitis was synchronous throughout the body.16) The mixed presence of acute- and cicatrical phase vasculitis is a histological feature of polyarteritis nodosa (PAN), suggesting that cases of PAN in childhood were included in the survey reported by Landing et al.
Lung
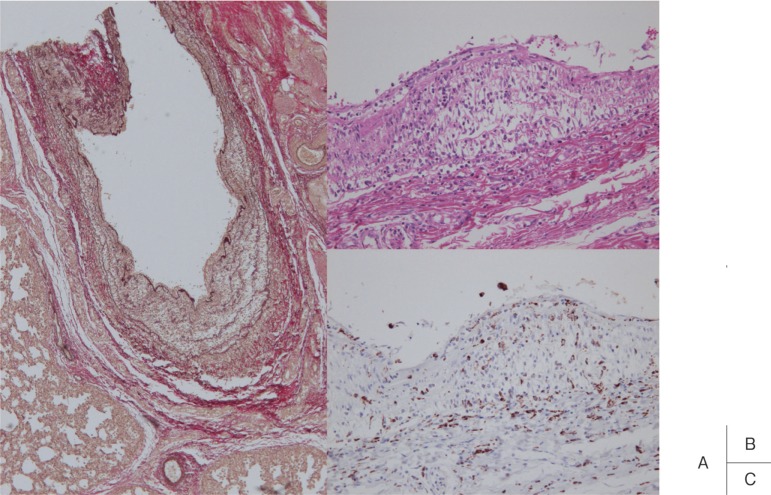
Shibuya et al. histopathologically investigated the pulmonary artery.17) Panangiitis occurred in the pulmonary artery in 20 of 34 fatal cases (59%) within 60 days after onset, and all these were localized to the elastic pulmonary artery at sites up to the 4th branching. The earliest change in the pulmonary artery was edematous dissociation of the tunica media observed in a patient who died on the 13th illness day. The condition progressed to severe panarteritis on the 25–30th illness day (Fig. 1). After day 30, inflammation started to remit and scars were formed in patients who died at 3 months. No aneurysm or arterial dilatation was noted in the pulmonary artery, which may have been due to the low blood pressure.
Fig. 1.
Arteritis observed in the elastic type pulmonary artery. The tunica media was edematously thickened, and a small number of CD68-positive monocytes/macrophages infiltrated the vascular wall. Pulmonary arterial inflammation was milder than that of the coronary artery (A: elastic van Gieson stain, B: H&E, C: CD68).
In the acute phase of Kawasaki disease, interstitial lung shadows appear in some cases. On autopsy, interstitial changes were observed in 4 of 13 patients (31%) who died on the 29–57th illness day. Histologically, the changes corresponded to diffuse alveolar damage (DAD) (Fig. 2), but there was no apparent correlation between pulmonary arteritis and the interstitial changes.18)
Fig. 2.
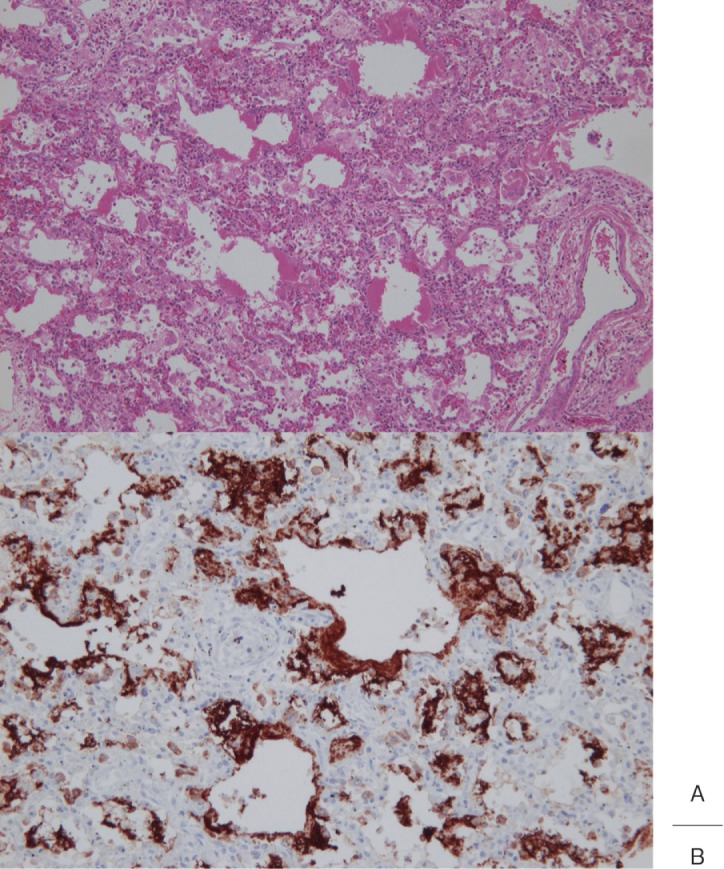
A: Interstitial pneumonia observed in a patient who died 29 days after the onset of Kawasaki disease. Hyaline membranes appeared over a wide area (H&E).
B: The hyaline membranes positively reacted with anti-surfactant apoprotein antibody.
Kidney
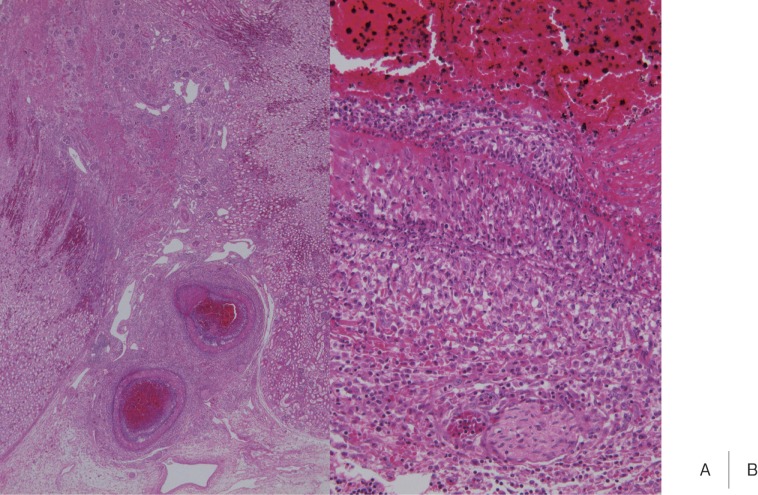
The incidence of panarteritis in the kidney varies among reports.19–21) Asaji et al. 19) observed panangiitis or its scars in arteries in the kidney on autopsy in 35 of 48 Kawasaki disease patients (73%) who died 6 days-11 years after the onset. Arteritis developed as edematous dissociation of the tunica media in a patient who died on the 13th illness day, and granulomatous and proliferative inflammation were noted in patients who died on days 17–28 (Fig. 3). Inflammation remitted after day 30. Compared to coronary arteritis, arteritis in the kidney developed several days later and inflammation was milder. Panangiitis was localized in the interlobar arteries and rarely occurred in the arcuate and interlobular arteries. The occurrence of renal aneurysm is recognized, and the development of renal hypertension due to renal arterial stenosis has been reported,22) but descriptions concerning the renal artery in autopsy cases are lacking.
Fig. 3.
Proliferative and granulomatous arteritis of the interlobar artery in the renal hilar region. The vascular lumen was obstructed with a thrombus.(H&E, A: × 2, B: × 20)
Regarding glomerular changes, the presence of segmental or global glomerulosclerosis was described in many reports.19–21) However, these are considered to be physiological changes occurring with childhood development, called infantile glomerulosclerosis, not specific to Kawasaki disease. Focal segmental mesangial proliferation was also reported as another glomerular change noted in Kawasaki disease.20, 23) We observed many monocytes/macrophages in the glomerular capillary, showing a feature of intracapillary proliferative glomerulonephritis, in only one case (Fig. 4), but no immune complex deposition in glomeruli was noted. The observation of tubular changes in 8% of cases was reported.19)
Fig. 4.
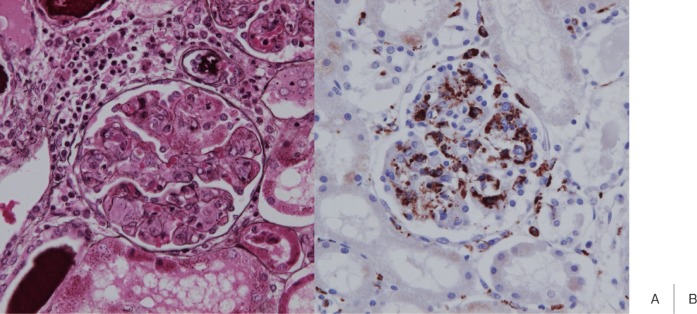
The renal glomerular lesion in a sole case in which we observed an intracapillary proliferative change. Many CD68-positive monocytes/macrophages were present in capillary blood vessels. Generally, no renal glomerular lesion develops in Kawasaki disease (A: PAM stain, B: CD68).
Liver
Liver dysfunction occurs at a high incidence in the acute phase of Kawasaki disease. Tanaka et al.24) performed liver biopsy in 19 patients at 7–36 days after onset, and observed the fatty and edematous degeneration of hepatocytes and severe inflammatory cell infiltration in the portal area in most patients (Fig. 5). Portal area vascular inflammation was unclear, and hepatic changes were assumed to be toxic rather than circulatory impairment. Ohshio et al.25) also reported that inflammatory cell infiltration in the portal area was observed in the acute phase at a high frequency, and cholangitis and pericholangitis were more noticeable than vasculitis of the portal area.
Fig. 5.
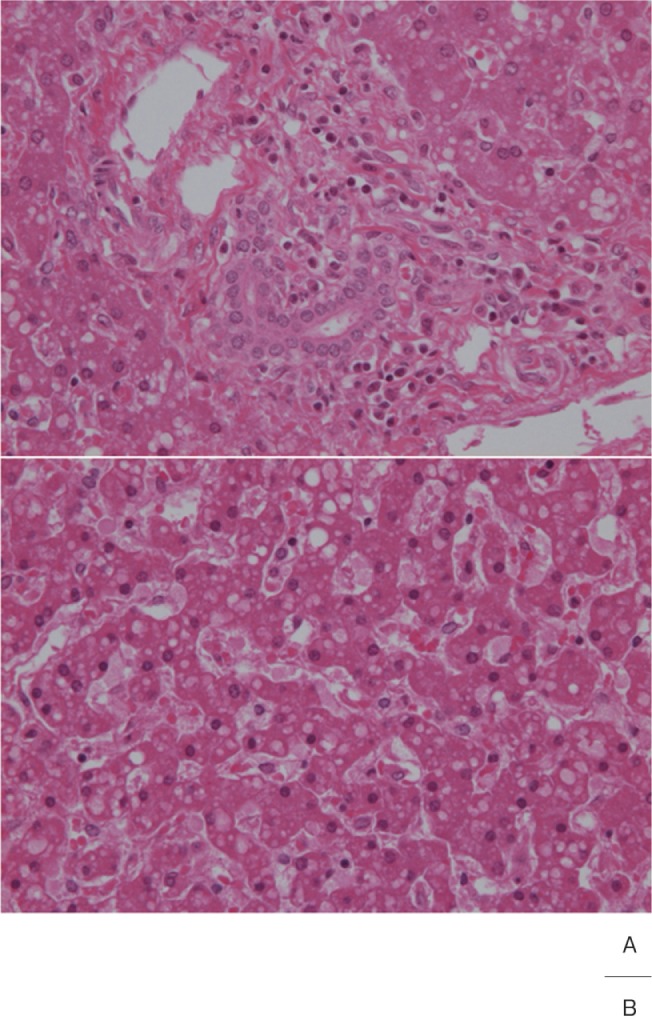
A: Mild lymphocyte and plasma cell infiltration was noted in the portal area of the liver.
B: Droplet fatty degeneration in hepatocytes.
Gallbladder
Suddleson et al.26) observed swelling of the gallbladder in 16 of 117 patients (14%) with acute Kawasaki disease on ultrasonographic examination. The gallbladder inflammation remitted as Kawasaki disease resolved, showing that surgical excision of the swollen gallbladder in Kawasaki disease is unnecessary. Masuda et al.27) histopathologically investigated gallbladders that were surgically excised based on a diagnosis of cholecystitis in 4 patients with acute Kawasaki disease, and observed characteristic non-specific acalculous cholecystitis. Regarding vascular changes, perivascular cell infiltration was observed, but panangiitis was noted only in one of the 4 cases, and the panangiitis occurred in a artery with a 400-μm diameter in the subserosal layer.
Spleen and Pancreas
Pancreatic vascular lesions developed in 14 of 45 patients (31%), and the lesions were located at sites up to the pancreatic interlobular arteries. Vasculitis started on the 10th illness day, reached an inflammatory peak at about day 28 (Fig. 6), and then healed while fibrous intimal thickening remained. In the spleen, arteritis was noted in arteries in the hilar and trabecular regions, and the histological findings were similar to those in the pancreas.28) Yoshioka et al.29) reported that inflammatory cell infiltration in the pancreatic duct and its surrounding and vasculitis were characteristic, and the inflammatory findings of the pancreatic duct were marked in acute cases by day 27.
Fig. 6.
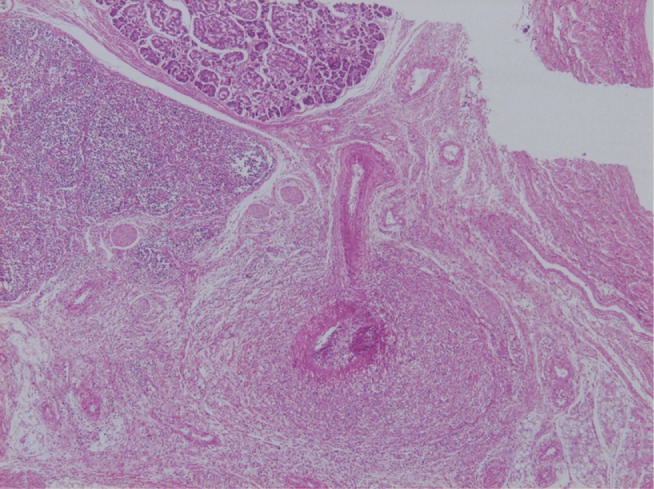
Inflammation of the medium-sized muscular artery in the pancreatic interstitium.
Gastrointestinal Tract
Kurashige et al.30) histopathologically investigated gastrointestinal lesions in 31 autopsy cases of Kawasaki disease. Vasculitis was noted in 3 patients, but all were localized in arteries in the subserosa, not between mucosal and muscular layer, showing a difference from PAN with regard to the distribution of lesions. On the other hand, ulcers graded as Ul-III were present in 3 cases (Fig. 7). In addition, the reactive hyperplasia of lymphoid follicles was often noted in mucosa at the end of the ileum.
Fig. 7.
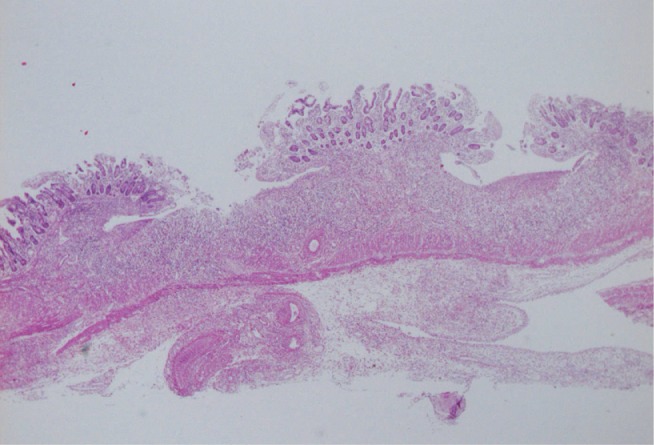
Multiple ulcers in the small intestine
Lymph Node
Cervical lymph node swelling is one of the principal clinical findings described in the diagnostic guidelines for Kawasaki disease, and it is present in 70% of acute cases. Histopathologically, lymph node swelling in Kawasaki disease was a nonspecific reactive change in many cases but characterized by focal necrosis which started from the marginal sinus.3, 31–33) Necrotized regions were mixed with karyorrhexis, and enlarged endothelial cells, luminal obstruction and fibrin thrombi were noted in micro blood vessels continuous with the necrotized lesions (Fig. 8). Some researchers consider that the cause of necrosis is microinfarction, while others consider changes with inflammation.32, 33) We suggest that necrosis is due to infarction, and extracapsular dissemination of inflammation is the point of differentiation from histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto's disease).
Fig. 8.
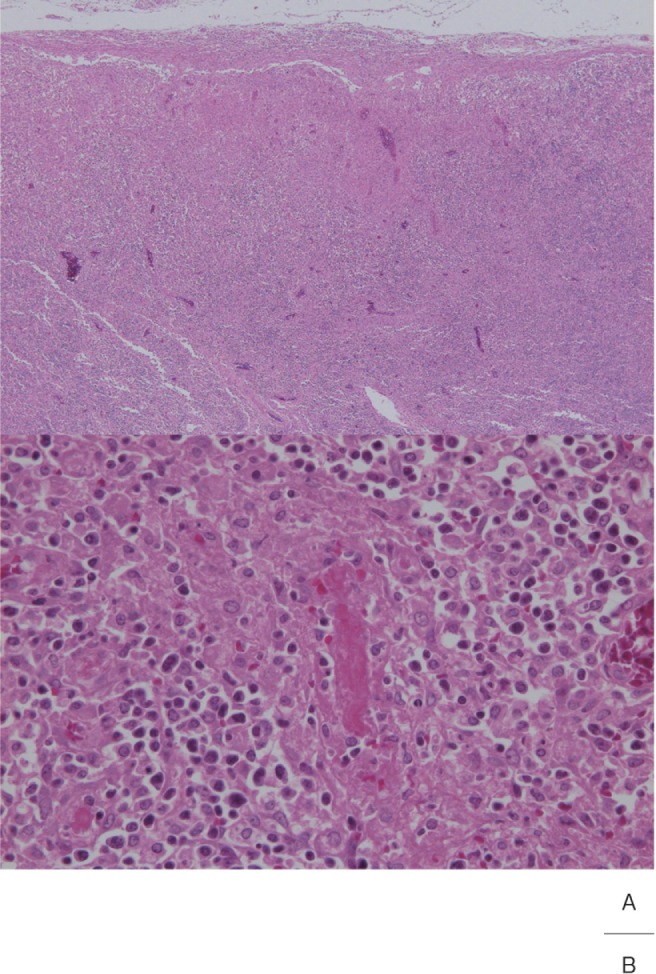
A: Small necrosis directly below the lymph node capsule
B: Fibrin thrombus in a small blood vessel
Skin
Changes in the skin are also a principal clinical finding, as well as lymph node swelling, described in the diagnostic guidelines, and many pathological investigations have been reported. Many points are consistent among these reports, summarized as follows: Skin lesions are characterized by markedly inflammatory edema accompanied by vasodilatation in the dermal papillary layer and fibrin exudation.34–36) These changes are marked in scars of BCG vaccination, and granulomatous inflammation was noted in some cases.37) Endothelial cells enlarged and were surrounded by infiltrating monocytes/macrophages and CD4-positive T cells, but very few neutrophils and B cells were present. No panangiitis was noted. It has been reported that IL-1α and TNF-α were strongly positive in the acute phase but negative in the recovery phase on immunohistological investigation.38)
Other Organs
In the intercostal arteries which directly branch from the aorta, similarly to the coronary artery, lesions develop at the proximal site of the arteries.39) The histology of vasculitis is also similar to that of the coronary artery, and aneurysms may be formed.
Regarding conjunctival changes, one of the main signs of Kawasaki disease, Burns et al.40) reported that these were nonspecific, such as vasodilatation and mild infiltration by lymphocytes and plasma cells.
In the salivary glands, severe inflammatory cell infiltration by lymphocytes, plasma cells, and neutrophils occurs around the acinus and excretory ducts in the acute phase and remit in the recovery phase.41)
Testicular vascular lesions and capsulitis were observed in cases in the acute phase, but no interstitial or parenchymal inflammation was noted in the testis.42)
In nerve tissue, aseptic chorio and/or leptomeningitis was noted in about half of autopsy cases of Kawasaki disease, and mild or moderate inflammatory cell infiltration by lymphocytes, monocytes/macrophages, and a few neutrophils was observed. Edema in perivascular or perineuronal areas and a localized spongy state were occasionally noted. Regarding cerebral blood vessels, perivascular inflammatory cell infiltration was observed, but panangiitis was not.43)
Pathogenic Hypothesis Recently Attracting Attention
Rowley et al. observed that IgA plasma cells infiltrated vasculitis lesions with many monocytes/macrophages and CD8 T lymphocytes in autopsy cases of Kawasaki disease.44) These IgA plasma cells also infiltrated non-vascular tissues throughout the body, such as the bronchus, pancreas, and renal tissue.45) They hypothesized that a pathogen, probably a virus, which invades via the respiratory or digestive organs is processed by the lymph apparatus in the organ, local B lymphocytes differentiate into precursors of IgA plasma cells, and then IgA-producing plasma cells reach not only the coronary artery and heart muscle but also various organs throughout the body. IgA plasma cells are oligoclonal or antigen-driven.46) Synthetic antibodies were produced in vitro by cloning α and κ variable-region genes prevalent in the Kawasaki disease arterial wall into immunoglobulin expression vectors and producing the antibodies in tissue culture. Employing this antibody, autopsy preparations from Kawasaki disease patients were immunohistochemically investigated. In addition to monocytes/macrophages in vascular lesions, a substance reactive with the antibody was present in the cytoplasm of bronchial ciliated epithelial cells.47) The cytoplasmic inclusion bodies in bronchial epithelium could be identified by H&E staining and observed as an electron-dense non-structured spheroid substance under an electron microscope.48) Analysis of the structure of this cytoplasmic inclusion body is underway.
Nagata et al.49) immunohistochemically investigated biopsy specimens of small intestinal mucosa, and assumed that antigen which strongly activates CD4-positive cells in intestinal mucosa and intestinal epithelial cells is associated with the development of Kawasaki disease. To investigate this hypothesis, they analyzed bacteria isolated from the oral cavity and duodenal mucosa, and clarified that gram-positive cocci with superantigen activity and gram-negative bacteria, which produce heat shock protein 60 and induce inflammatory cytokine production in peripheral blood monocytes in pediatric patients, were isolated from children with Kawasaki disease at a high rate, suggesting that these microorganisms act together and induce Kawasaki disease.50)
References
- Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of fingers and toes in children. Clinical observation of 50 patients. Jpn J Allergy. 1967; 16: 178–222 (in Japanese) [PubMed] [Google Scholar]
- Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph-node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974; 54: 271–6 [PubMed] [Google Scholar]
- Tanaka N, Sekimoto K, Naoe S. Kawasaki disease. Relationship with infantile periarteritis nodosa. Arch Pathol Lab Med. 1976; 1: 81–6 [PubMed] [Google Scholar]
- Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. 2005; 47: 232–4 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yashiro M, Uehara R, Sadakane A, Chihara I, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: Results of the 2007–2008 nationwide survey. J Epidemiol. 2010; 20: 302–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984; 2: 1055–8 [DOI] [PubMed] [Google Scholar]
- Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007; 356: 663–75 [DOI] [PubMed] [Google Scholar]
- Inoue Y, Okada Y, Shinohara M, Kobayashi T, Kobayashi T, Tomomasa T, et al. A multicenter prospective randomized trial of corticosteroids in primary therapy for Kawasaki disease: clinical course and coronary artery outcome. J Pediatr. 2006; 149: 336–41 [DOI] [PubMed] [Google Scholar]
- Mori M, Imagawa T, Katakura S, Miyamae T, Okuyama K, Ito S, et al. Efficacy of plasma exchange therapy for Kawasaki disease intractable to intravenous gamma-globulin. Mod Rheumatol. 2004; 14: 43–7 [DOI] [PubMed] [Google Scholar]
- Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005; 146: 662–7 [DOI] [PubMed] [Google Scholar]
- Naoe S. Pathology of Kawasaki disease, excluding cardiac changes. J Soc Kinki Area Kawasaki Dis Res. 1987; 9: 1–3 (in Japanese) [Google Scholar]
- Amano S, Hazawa F, Hamashima Y. Pathology of Kawasaki disease, II. Distribution and incidence of the vascular lesions. Jpn Circ J. 1979; 43: 741–8 [DOI] [PubMed] [Google Scholar]
- Amano S, Hazawa F, Kubagawa H, et al. : General pathology of Kawasaki disease on the morphological alterations corresponding to the clinical manifestations. Acta Pathol Jpn. 1980; 30: 681–94 [PubMed] [Google Scholar]
- Hamashima Y: Kawasaki disease. Trans Soc Pathol Jpn. 1977; 66: 59–92 (in Japanase) [Google Scholar]
- Landing BH, Larson E. Pathological features of Kawasaki disease (mucocutaneous lymph node syndrome). Am J Cardiovasc Pathol. 1987; 1: 215–29 [PubMed] [Google Scholar]
- Takahashi K, Oharaseki T, Yokouchi Y, Naoe S, Jennette JC : Kawasaki disease arteries and polyarteritis nodosa. Pathol Case Rev. 2007; 12: 193–9 [Google Scholar]
- Shibuya K, Atobe T, Masuda H, Tanaka N: The histological study on pulmonary vascular lesions in Kawasaki diease. J Jpn Coll Angiol. 1987; 27: 293–304 (in Japanese) [Google Scholar]
- Shibuya K, Atobe T, Naoe S, Masuda H, Tanaka N, Kusawaka S: The histological study on pulmonary lesions in autopsy cases of Kawasaki disease. Prog Med. 1986; 6: 35–42 (in Japanese) [Google Scholar]
- Asaji A, Shibuya H, masuda H, Tanaka N: The histological study on the kidney involved with Kawasaki disease with a comparative study on the coronary aerterial lesions. J Jpn Coll Angiol. 1989; 29: 453–60 (in Japanese) [Google Scholar]
- Ogawa H: Kidney pathology in muco-cutaneous lymphnode syndrome. Jpn J Nephrol. 1985; 27: 1229–37 [PubMed] [Google Scholar]
- Takeuchi E, Ohshio G, Shimizu J, Yoshioka H, Hamashima Y, Fujiwara K, et al. Pathology of the kidney in Kawasaki disease. J Soc Kinki Area Kawasaki Dis Res. 1987; 9: 26–9 (in Japanese) [Google Scholar]
- Morishita S, Mawatari K, Yoshimi K, Kurosawa O, Sakai I, Nishibatake M, et al. Catheter intervention and surgical treatment of renovascular hypertension due to Kawasaki disease. In: Kato H. ed. Kawasaki Disease. Amsterdam: Elsevier, 1995 [Google Scholar]
- Salcedo JR, Greenberg L, Kapur S. Renal histology of mucocutaneous lymph node syndrome (Kawasaki disease). Clin Nephrol. 1988; 29: 47–51 [PubMed] [Google Scholar]
- Tanaka T, Koike M, Minami Y. Pathology of liver injury in Kawasaki disease. Shoni-Naika. 1984; 16: 2393–7 (in Japanese) [Google Scholar]
- Ohshio G, Furukawa F, Fujiwara H, Hamashima Y. Hepatomegaly and splenomegaly in Kawasaki disease. Pediatr Pathol. 1985; 4: 257–64 [DOI] [PubMed] [Google Scholar]
- Suddleson EA, Reid B, Woolley MM, Takahashi M. Hydrops of the gallbladder associated with Kawasaki syndrome. J Pediatr Surg. 1987; 22: 956–9 [DOI] [PubMed] [Google Scholar]
- Masuda H, Naoe S, Tanaka N. pathological study on initial arterial lesions of Kawasaki disease, observation of removed gallbladders. Research Committee of Intractable Vasculitis Syndromes of the Ministry of Health and Welfare of Japan, Annual Report for 1981, 1982: 274–9 (in Japanese)
- Ando M, Asaji A, Naoe S, Masuda H, Tanaka N. Pathology of pancreatic arterial lesions in Kawasaki disease. Research Committee of Intractable Vasculitis Syndromes of the Ministry of Health and Welfare of Japan, Annual Report for 1985, 1986: 117–22 (in Japanese)
- Yoshioka H, Miyake T, Oshio G, Shimizu J, et al. Clinicopathological study on the pancreas in 26 autopsy cases of Kawasaki disease. J Soc Kinki Area Kawasaki Dis Res. 1986; 8: 30–3 [Google Scholar]
- Kurashige M, Naoe S, Masuda H, Tanaka N. A morphological study of the digestive tract in Kawasaki disease—31 autopsies—. J Jpn Coll Angiol. 1984, 24: 407–18 (in Japanese) [Google Scholar]
- Marsh WL, Jr, Bishop JW, Koenig HM. Bone marrow and lymph node findings in a fatal case of Kawasaki's disease. Arch Pathol Lab Med. 1980, 104: 563–7 [PubMed] [Google Scholar]
- Giesker DW, Pastuszak WT, Forouhar FA, Krause PJ, Hine P. Lymph node biopsy for early diagnosis in Kawasaki disease. Am J Surg Pathol. 1982; 6: 493–501 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Naoe S, masuda H, et al. Pathological study of lymph node lesion in Kawasaki disease. Prog Med. 1986; 6: 21–7 (in Japanese) [Google Scholar]
- Hirose S, Hamashima Y. Morphological observations on the vasculitis in the mucocutaneous lymphnode syndrome, A skin biopsy of 27 patients. Eur J Pediatr. 1978; 129: 17–27 [DOI] [PubMed] [Google Scholar]
- Histopathological findings of skin in acute and convalescent stage of patients with mucocutaneous lymp-node syndrome. Igaku-no-ayumi. 1984; 128: 805–6 (in Japanese) [Google Scholar]
- Sugawara T, Hattori S, Hirose S, Furukawa S, Yabuta K, Shirai T. Immunopathology of the skin lesion of Kawasaki disease. Prog Clin Biol Res. 1987; 250: 185–92 [PubMed] [Google Scholar]
- Kuniyuki S, Asada M. An ulcerated lesion at BCG vaccination site during the course of Kawasaki disease. J Am Acad Dermatol. 1997; 37: 303–4 [PubMed] [Google Scholar]
- Sato N, Sagawa K, Sasaguri Y, Inoue O, Kato H. Immunopathology and cytokine detection in the skin lesions of patients with Kawasaki disease. J Pediatr. 1993; 122: 198–203 [DOI] [PubMed] [Google Scholar]
- Masuda H, Shozawa T, Naoe S, Tanaka N. The intercostal artery in Kawasaki disease. A pathologic study of 17 autopsy cases. Arch Pathol Lab Med. 1986; 110: 1136–42 [PubMed] [Google Scholar]
- Burns JC, Wright JD, Newburger JW, Schneeberger EE, Mierau GW, Smith LE. Conjunctival biopsy in patients with Kawasaki disease. Pediatr Pathol Lab Med. 1995; 15: 547–53 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Ohshio G, Takeuchi E, Hamashima Y. Severe inflammation of salivary glands and ducts in Kawasaki disease: A histopathological study of nine autopsy cases. Ann Paediatr Jpn. 1986; 32: 24–8 [Google Scholar]
- Ko T, Fujiwara H, Shimizu J, et al. pathological study of testis in Kawasaki disease. J Soc Kinki Area Kawasaki Dis Res. 1983; 5: 7–9 (in Japanese) [Google Scholar]
- Amano S, Hazama F. Neutral involvement in Kawasaki disease. Acta Pathol Jpn. 1980; 30: 365–73 [DOI] [PubMed] [Google Scholar]
- Rowley AH, Eckerley CA, Jäck HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997; 159: 5946–55 [PubMed] [Google Scholar]
- Rowley AH, Shulman ST, Mask CA, Finn LS, Terai M, Baker SC, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis. 2000; 182: 1183–91 [DOI] [PubMed] [Google Scholar]
- Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001; 166: 1334–43 [DOI] [PubMed] [Google Scholar]
- Rowley AH, Baker SC, Shulman ST, Garcia FL, Guzman-Cottrill JA, Chou P, et al. Detection of antigen in bronchial epithelium and macrophages in acute Kawasaki disease by use of synthetic antibody. J Infect Dis. 2004; 190: 856–65 [DOI] [PubMed] [Google Scholar]
- Rowley AH, Baker SC, Shulman ST, Fox LM, Takahashi K, Garcia FL, et al. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis. 2005; 192: 1757–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Yamashiro Y, Maeda M, Ohtsuka Y, Yabuta K. Immunohistochemical studies on small intestinal mucosa in Kawasaki disease. Pediatr Res. 1993; 33: 557–63 [DOI] [PubMed] [Google Scholar]
- Nagata S, Yamashiro Y, Ohtsuka Y, Shimizu T, Sakurai Y, Misawa S, Ito T. Heat shock proteins and superantigenic properties of bacteria from the gastrointestinal tract of patients with Kawasaki disease. Immunology. 2009; 128: 511–20 [DOI] [PMC free article] [PubMed] [Google Scholar]