Abstract
Endovascular stent grafting is a novel therapeutic technique for repairing aortic aneurysms, and is especially useful for descending aortic aneurysm and aneurysm at the distal arch. However, no effective endovascular approach for the ascending aorta has been reported a remaining site for endovascular repair because of the anatomical restrictions and the presence of vital branches to the head and arms that are present in this region. This report describes an endovascular stent graft repair of a pseudoaneurysm at the ascending aorta using a fenestrated stent graft. The fenestrated stent graft is easy to use and less invasive for the endovascular repair of the ascending aorta.
Keywords: fenestrated stent graft, endovascular repair, ascending aortic aneurysm
Introduction
Endovascular stent grafting is a novel therapeutic technique for the treatment of aortic aneurysms. However, no effective endovascular approach for repairing the ascending aorta and aortic arch have been reported, likely because of the anatomical restrictions and the presence of vital branches to the head and arms that are present in this region. Generally, the repair of these sites with a stent grafts requires an extra anatomical bypass from either the proximal ascending aorta or the femoral artery, with aortic debranching, in order to provide an alternative source of inflow.1) This report presents a case of the endovascular repair of the ascending aorta with customized fenestrations strategically placed for each arch branch without the use of extra anatomical bypass.
Case
An 87-year-old male was found to have an ascending aortic pseudoaneurysm.
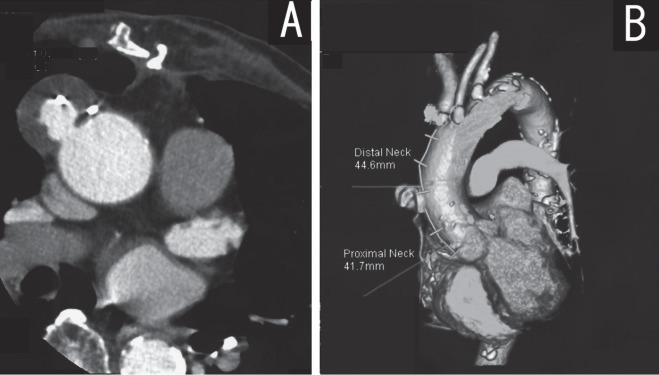
His medical history included mediastinitis following coronary artery bypass grafting (CABG) with the left internal mammary artery (LIMA) to the left anterior descending artery (LAD) and a saphenous vein graft (SVG) from the ascending aorta to the right coronary artery (RCA). He underwent omental transposition and packing for the treatment of post-thoracotomy mediastinitis following CABG. Although he recovered completely, the computed tomography (CT) findings during a routine check-up one year after the coronary surgery detected a pseudoaneurysm of the ascending aorta (Fig. 1A). The pseudoaneurysm originated from the proximal anastomosis of the saphenous vein graft which was located between the coronary ostium and the brachiocephalic artery. Open surgery, which included aortic arch vessels reconstruction, was considered to be very difficult because of the patient's post-mediastinitis status and his advanced age.
Fig. 1.
A: CT revealed a pseudoaneurysm at the anastmosis of the saphenous vein and the ascending aorta.
B: The lesion had a landing zone for the stent graft from the ostium of the coronary arteries and the brachiocephalic artery.
With regard to endovascular surgery, a three dimensional CT (3D-CT) image indicated a sufficient proximal and distal landing zone (about 41 mm) to exclude the pseudoaneurysm without aortic arch vessels debranching (Fig. 1B). In addition, 3D-CT angiography confirmed the patency of the LIMA to the LAD and the SVG to the RCA. The initial coronary angiography demonstrated a total occlusion of the RCA with collateral from the LAD. Therefore, we believed that the RCA would still be maintained via the collateral flow from the LIMA and the LAD when the SVG ostium is occluded by endografting without inducing RCA area ischemia. Moreover, the sufficient landing zone and coronary artery flow suggested that it would be possible to place a stent graft in this ascending aorta case without aortic arch vessel debranching.
The stent graft device was custom-made and was constructed with a self-expandable Z stent and a e-PTFE graft with customized fenestrations strategically adjusted for the patient's vessel anatomy.
The patient's vessel anatomy was measured specifically using 3D-CT (ZIOSTATION, Ziosoft, Inc. Tokyo, Japan) in 0.5 mm slices. This demonstrated that it would be difficult to prevent migration and endoleakage in the placement range unless the graft was placed medially from an area directly above the root of the coronary artery and then to distally apply one or more stents in Zone 4.
The distance between the coronary ostium and the brachiocephalic artery was 85 mm, placing the pseudoaneurysm approximately in the center of the stent. The anatomy indicated that endoleakage could be substantially prevented if an appropriately sized stent graft was placed directly above the coronary arteries to the origin of the brachiocephalic artery.
In addition, the distance from the origin of the brachiocephalic artery to the placement range on the distal side was 110 mm. The design of the SG was therefore configured with five self-expandable Z-stents as the basic specification (Fig. 2A).
Fig. 2.
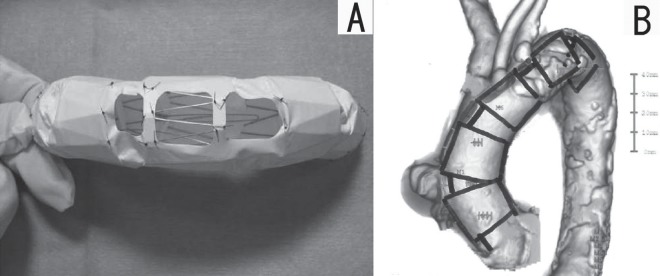
A: The stent graft was constructed with a self-expandable Z stent and an e-PTFE graft with fenestrations for each arch branch.
B: The stent graft device was custom-made so that it could be adjusted to properly fit the patient's vessels. The deployment of the stent graft from the ostia of the coronary arteries to the distal arch was necessary to prevent migration.
The maximum minor axis of the proximal neck was 32 mm, and the maximum minor axis on the distal neck was 28 mm. The diameter of the graft was oversized by 2 mm to achieve effective sealing. Tapered e-PTFE grafts situated 34 mm medially and 30 mm distally were created and sutured with 5-0 polypropylene.
The fenestration was positioned to match the ostium of each arch branch. However, a stent graft placed in the distal aortic arch often rotates due to a distortion of the aorta. Hence, the fenestration was designed to be larger than the diameter of the ostium to prevent occlusion of the branches from the rotation, but not too large to prevent endoleakage and establish a total occlusion of the pseudoaneurysm (Fig. 2B).
The procedure was performed in the operating room under a general anaesthesia while carefully monitoring the arterial blood pressure, electrocardiogram (ECG), and the cardiac output derived from a thermo dilution catheter. OEC9600 (GE health care, USA) was used for fluoroscopic imaging. The patient's right femoral artery was surgically explored, and a 6 Fr sheath was inserted into the right brachial artery. The stent graft was delivered through the right femoral artery with a J-shaped 23 Fr sheath, using the tug-of-wire technique. After delivering the sheath, the stent graft was deployed in the ascending aorta. When the stent graft was deployed, it was pushed along the greater curvature of the aorta in order to achieve accurate deployment, and was gradually deployed utilizing the blood flow force to stick the stent to the aortic wall. The device did not require transient cardiac arrest to use the flow force.
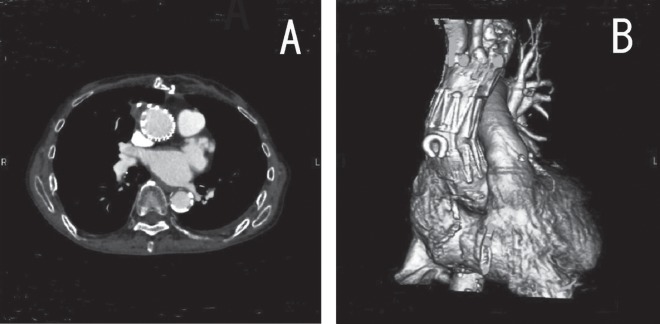
The intra-operative course was uneventful. There were no ECG changes. Although type II endoleakage was detected immediately after the deployment of the stent graft, a follow-up CT taken 7 days after surgery showed the successful exclusion of the pseudoaneurysm without any endoleakage (Fig. 3A), and showed that the stent graft was in a good position (Fig. 3B). There were no significant differences between the upper limb and lower limb blood pressure. The patient was discharged with no complications.
Fig. 3.
A: The pseudoaneurysm was completely occluded.
B: Follow-up CT demonstrated the stent graft to be in a good position and no endoleak was detected.
Discussion
The introduction of endovascular stent graft technology has ushered in a new era in the treatment of diseases affecting the distal aortic arch and the descending thoracic aorta. However, no effective endovascular repair of the ascending aorta and the aortic arch had been reported, likely because of the anatomical restrictions, such as the proximity to the great vessels and arch tortuosity, and there are no adequate commercially available devices available for such a repair.2) Several options have been proposed to overcome these problems. The most popular method is termed ‘hybrid’ (open and endovascular) therapy and involves prophylactic transposition or bypass graft placement to provide flow to the brain and arms.3) Another option is to provide a stent graft with a side branch. In an initial experience with branched stent graft implantations, Inoue and colleagues reported an embolic cerebrovascular accident as the major complication.4) The risk of cerebral infarction seems to be very high in the placement of a stent graft with a side branch, especially when it involves the brachiocephalic artery. McWilliams et al. reported an in-situ fenestrated stent graft designed to preserve the left subclavian artery.5) However, this procedure was very complex, and the long-term stability of in-situ fenestration remains unknown.
The current method uses a custom-made fenestrated stent graft with a J-shaped long sheath. This method does not require surgical transposition of the arch branches or transient cardiac arrest, and it is also widely applicable. However, this option also has some limitations. An adequate space for the landing zone is necessary when using this method, because an inadequate landing zone may lead to graft migration. Migration at the ascending aorta may be associated with a late aneurysm rupture, proximal endoleakage and major cerebral complications due to graft kinking. The key to achieve a successful outcome is a careful preoperative evaluation using MSCT for the custom-made fenestration of the stent graft. Some physicians may think that the insertion of stents or covered stents to the arch vessel is effective for preventing endoleakage and migration. However, these procedures were not applied in this case because cerebral infarction may have occurred. There was no concern about type II endoleakage from the arch vessel, because there was a sufficient distal landing zone. In addition, a covered stent and stents of arch vessels may cause serious side effects if the stent graft migrates at the aortic arch, causing not only stenosis and thrombosis but also occlusion of the arch vessel. Therefore, in order to avoid migration and endoleakage in this case, the stent graft was delivered from the sino-tubular junction of the ascending aorta to the distal aortic arch to obtain a sufficient sealing zone with optimal long term results.
In conclusion, endovascular repair of the ascending aorta and the arch with a fenestrated stent graft can be easily performed, and is less invasive than other proposed procedures. However, it is necessary to conduct a specific analysis using 3D-CT in order to create an accurate stent graft to ensure the patient's safety.
References
- Eskandari MK. Aortic debranching procedures to facilitate endografting. Perspect Vasc Surg Endovasc Ther. 2006; 18: 287–92 [DOI] [PubMed] [Google Scholar]
- Lin PH, Kougias P, Huynh TT, Huh J, Coselli JS. Endovascular repair of ascending aortic pseudoaneuryam. J Endovasc Ther. 2007; 14: 794–8 [DOI] [PubMed] [Google Scholar]
- Bergeron P, Coulon P, De Chaumaray T, Ruiz M, Mariotti F, Gay J, et al. Great vessels transposition and aortic arch exclusion. J Cardiovasc Surg. 2005; 46: 141–7 [PubMed] [Google Scholar]
- Saito N, Kimura T, Odashiro K, Toma M, Nobuyoshi M, Ueno K, et al. Feasibility of the Inoue single-branched stent-graft implantation for thoracic aortic aneurysm or dissection involving the left subclavian artery: short- to medium-term results in 17 patients. J Vascu Surg. 2005; 41: 206–12 [DOI] [PubMed] [Google Scholar]
- McWilliams RG, Murphy M, Hartley D, Lawrence-Brown MM, Harris PL. In situ stent-graft fenestration to preserve the left subclavian artery. J Endovasc Ther. 2004; 11: 170–4 [DOI] [PubMed] [Google Scholar]