Abstract
Aim: To assess the utility of skin perfusion pressure (SPP) measurement in evaluating the outcome of vascular constructions for critical limb ischemia (CLI) patients.
Methods: We retrospectively studied 19 lower limbs in 18 patients who underwent arterial reconstruction for CLI from whom SPP measurements had been obtained pre- and postoperatively between 2008 and 2010. Six limbs whose ulcers had healed postoperatively were classified into group H, 7 limbs whose ulcers had not healed into group U, and 6 limbs without ulcers into group N. SPP values were compared among these groups.
Results: The preoperative SPP values in all groups were <30 mmHg, without significant differences among the groups. The SPP values in groups H and N significantly improved after operation, and those in group U were significantly lower than those in the other groups.
Conclusions: SPP measurement before and after arterial reconstruction is useful to assess improvement in tissue circulation and to predict the likelihood of wound healing. An SPP value ≥30 mmHg was considered necessary for wound healing, supporting the findings of the few reports in the literature on the usefulness of SPP for assessing vascular reconstruction effects on ulcer wound healing.
Keywords: skin perfusion pressure, peripheral artery disease, arterial reconstruction, wound healing
Introduction
The number of patients with critical limb ischemia (CLI) due to peripheral artery disease (PAD) is increasing worldwide, and the need for arterial reconstruction surgery has, therefore, become more frequent. When considering arterial reconstruction in such cases, there are several important clinical points which vascular surgeons attempt to clarify: the extent to which ischemia affects symptoms such as pain or ulcers; whether the indications for arterial reconstruction are certain or not; the extent to which the peripheral circulation of the patient had improved with the arterial reconstruction, and the safe perfusion level required for the optimal healing of ischemic ulcers.
For the assessment of these points, skin perfusion pressure (SPP) measurement is considered to be reliable and useful.1–5) SPP measurement is a noninvasive method of estimating microcirculation of the limbs at a depth of 1.5 to 2.0 mm beneath the skin using a Doppler laser probe. The ankle-brachial pressure index (ABI) is internationally considered to be the optimum method to estimate peripheral circulation. However, patients with diabetes mellitus or chronic kidney disease often have severely calcified peripheral arteries. A blood pressure cuff cannot sufficiently compress the ankle arteries, and, therefore, their ABI often shows false high values. Moreover, if the patient has additional lesions below the ankle, these cannot be evaluated using ABI. However, in such patients accurate evaluation of peripheral circulation is possible with SPP measurement and is unaffected by arterial calcification of arteries.6, 7) SPP measurement can also be performed below the ankle and near the ulcer, can evaluate the insufficiency of smaller arteries,3) and can predict the outcome of ulcers and wound healing.2) Previous studies have concluded that ulcers and wounds in which the SPP decreases to <30 or 40 mm Hg do not heal.1, 2, 4–6)
We started SPP measurements following the establishment of a vascular laboratory at our institution in August 2008. Although the number of examinations of SPP is still small in our institution at present, we deemed it useful to have an initial evaluation of its effectiveness by retrospectively assessing the changes in SPP values obtained between the pre- and postoperative periods8, 9) and the relationship between the SPP values and ulcer healing, in order to assess the utility of SPP measurement in evaluating the outcome of arterial reconstructions in patients with CLI caused by arteriosclerosis obliterans (ASO).
Materials and Methods
Between 2008 and 2010, we performed 84 vascular reconstructions in 74 patients with ASO at our institution. SPP was measured before and after arterial reconstruction in 19 lower limbs of 18 patients (15 men and 3 women, average age, 73.2 ± 6.7 years) with CLI (Fontaine stages 3 and 4). SPP was measured at the center of the distal portion of the plantar and/or dorsal area of the foot, pre- and postoperatively.
SPP was measured using the SensiLase PAD 3000 (Vasamed Inc., Eden Prairie, MN, USA) at room temperature with the patient in the supine position. We used a laser Doppler probe positioned underneath a 5.8-cm-wide blood pressure cuff (laser pressure transducer (LPT) cuff) wrapped around the patient's foot. The specific dates of SPP measurement pre- and postoperatively were not decided, but the usual date for postoperative examination was after the pain and swelling in the foot had subsided.
We divided the patients into 3 groups. Those whose ischemic ulcers had healed after arterial reconstruction were classified into group H. Those whose ischemic ulcers had not healed were classified into group U. Those without ischemic ulcers at any time during the observation period were classified into group N.
All statistical analyses were performed with JMP statistical software (version 7.0.1; SAS Institute, Cary, NC, USA). Results are shown as means ± standard deviation (SD). Multiple comparisons of average values among the 3 groups were analyzed using the Tukey-Kramer honestly significant difference (HSD) test. P values less than 0.05 were considered to represent a statistically significant difference among the groups. Comparisons of average SPP values pre- and postoperatively were analyzed using the Wilcoxon signed-rank test. The Pearson chi-square test was used to analyze the ratio of underlying atherosclerotic risk factors.
Results
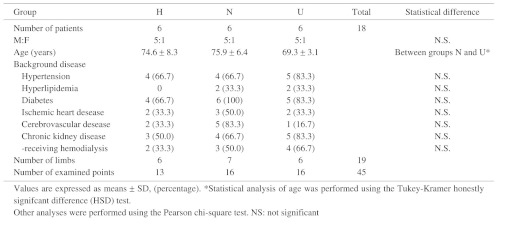
Among a total of 19 lower limbs of 18 evaluable patients, 6 limbs were classified into group H (measurements obtained at 13 points), 7 into group N (measurements obtained at 16 points), and 6 into group U (measurements obtained at 16 points). One limb in group U had a deep ulcer with exposed toe bones and methicillin-resistant Staphylococcus aureus (MRSA) infection. Another limb in group U had a large ulcer on the entire heel with MRSA infection. Other ulcers in groups H and U were located on the skin surface of the toe of each limb. The demographic characteristics and atherosclerotic risk factors of the patients are shown in Table 1. The number of patients did not differ significantly among the 3 groups in terms of any diseases; however, there were more patients with cerebral vascular disease in group N than in the other groups. Moreover, although not significantly different, the number of patients receiving hemodialysis was the largest in group U, followed by group N, and then finally group H, reflecting the prevalence rate of renal disorder in these groups.
Table 1. Demographic characteristics and atherosclerotic risk factors of patients, and examined SPP points.
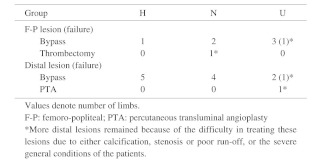
The arterial reconstruction procedures performed on the limbs are shown in Table 2. The number of successful distal bypass procedures was high in group H. Almost half of the limbs in groups N and U had undergone arterial reconstruction only in the femoro-popliteal area. One patient in group N underwent thrombectomy because of acute exacerbation of the lesion in the superficial femoral artery; however, we could not repair severe distal lesions because of the severe general condition of the patient. In all limbs of the patients in group U, more distal lesions remained because of the difficulty in treating these lesions due to either calcification, stenosis or poor run-off, or the severe general conditions of the patients. Failed arterial reconstruction was recognized at 5 points in 2 limbs in group U, and among the remaining 4 limbs in group U, 1 limb required minor amputation and 1 limb required major amputation, despite the patency of the reconstructed arteries. New ulcers did not occur postoperatively in any group. Rest pain disappeared in all patients in group N.
Table 2. Arterial reconstruction procedures performed for the lower limbs.
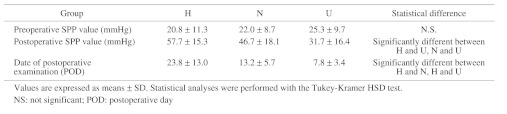
The SPP values are shown in Table 3. The preoperative SPP values in all groups were <30 mmHg, without a statistically significant difference. The postoperative SPP values increased by 36.9 ± 17.0 mmHg in group H (P = 0.002), 24.7 ± 12.0 mmHg in group N (P <0.0001), and 6.6 ± 12.7 mmHg in group U (N.S.). In these groups, the actual postoperative SPP values were 57.7 ± 15.3 mmHg in group H, 46.7 ± 18.1 mmHg in group N, and 31.7 ± 16.4 mmHg in group U; the postoperative SPP value of group U was markedly lower than those of the other 2 groups. The date of postoperative examination was later in group H than in the other 2 groups.
Table 3. SPP values before and after arterial reconstructions.
Postoperative demographics of the numbers of limbs divided by mean SPP values in each group are shown in Fig 1. In group H, no limb had a mean SPP value of <30 mmHg and 83% of the limbs had a value of ≥40 mmHg. In group N, 57% of the limbs had a mean SPP value of <40 mmHg. In group U, 66% of the limbs had a mean SPP value of <40 mmHg and 50% of the limbs had a mean SPP value of <30 mmHg.
Fig. 1.
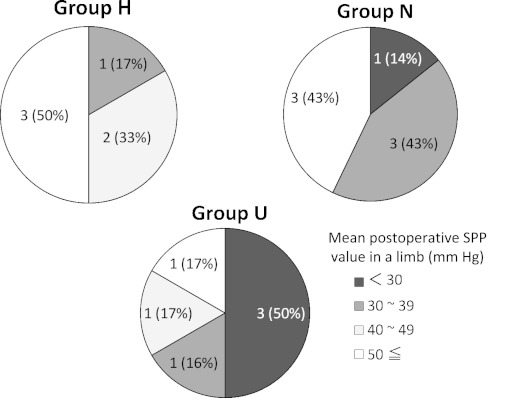
Postoperative demographics of the numbers of limbs divided by the mean SPP values in each group. {number of limbs (%)}
Almost all (89%) points with SPP values of <30 mmHg were in group U. For SPP values of 30–39 mm Hg, group U showed a decrease of points to 33%, group N showed 56% and group H showed 11%. In SPP values of 40–49 mmHg, each of the 3 groups was nearly equivalent at 33%. Finally, only 11% of the points with SPP values of ≥50 mmHg were in group U, while 50% of the points with SPP values of ≥50 mmHg were in group H.
Discussion
In this study, we set out to assess the utility of SPP measurement in the evaluation of the outcome of vascular constructions for patients with CLI.
Regarding the backgrounds of the patients in the present study in relation to wound healing, half of the limbs of the patients in group U had undergone arterial reconstruction in the proximal area above the knee. However, graft failure occurred in 2 limbs in this group. On the other hand, the operative results in groups H and N were acceptable, despite similar or high ratios of limbs that underwent distal bypass surgery.
The principle of arterial reconstruction is to cure the lesions successively, starting from the most proximal lesion.10, 11) With reconstruction of the proximal lesion, an increase in peripheral blood perfusion can be expected even if remnants of the obliterative lesion remain on the distal side. However, the postoperative SPP values in group U indicated that postoperative perfusion was insufficient in this group. Additionally, in 2 limbs in which an increase in peripheral blood perfusion was expected in this group, the procedure resulted in arterial reconstruction failure and eventual amputation. It is possible that some patients in group U with severe lesions in the distal arteries had poor run-off, despite the success of proximal arterial reconstruction. The postoperative SPP values in group N were better than those in group U but less than those in group H. Compared with group H, group N featured more patients with chronic kidney disease and group U had even more. Additionally, groups N and U included more patients with diabetes than group H; therefore, the patients in groups N and U could have had more advanced distal lesions than did the patients in group H.12–14)
Previous studies have suggested a value of 30 mmHg1, 2, 6) or 40 mmHg4, 5) to be the critical SPP threshold necessary for wound healing. In the present study, 50% of the limbs had a mean postoperative SPP value of <30 mmHg in group U, consistent with the results of previous reports. Therefore, we considered an SPP value of <30 mmHg to indicate an unfavorable condition for wound healing. However, there was no limb in group H that showed a mean SPP value of <30 mmHg postoperatively. This reinforced our assumption that an SPP value of ≥30 mmHg was necessary for wound healing. On the other hand, 88% of the limbs in group H showed a mean SPP value of ≥40 mmHg postoperatively. Yamada et al. reported that 69% of patients with an ischemic ulcer and with an SPP value of ≥40 mmHg showed wound healing, and when a cut-off SPP value of 40 mm Hg for wound healing was set, the sensitivity was 72% and the specificity was 88%.5) Based on the above results, it is desirable to have a minimum SPP value of at least 30 mmHg, and ideally higher than 40 mmHg, for wound healing. In group N, SPP improved to a lesser extent than in group H; however, rest pain disappeared in all patients. It is generally known that greater blood perfusion is required to promote wound healing than to improve rest pain. An SPP value of 30 mmHg may be the borderline for the development of rest pain.
In group U, 1 limb with postoperative SPP values of ≥60 mmHg in 2 points were observed. This patient had used steroids for a long period for polyneuropathy, thus the drug or the underlying disease could have negatively influenced wound healing. Another study reported a patient who required major amputation, despite showing a high SPP value, due to an uncontrolled infection in the foot.15) This indicates that high SPP values do not necessarily guarantee wound healing. SPP measurement can evaluate perfusion only to a depth of about 1.5 to 2.0 mm below the skin surface. Therefore, not only SPP, but also many other factors such as muscle perfusion, oxygen partial pressure of blood, presence of infection, size of ulcer, and cellular capacity for regeneration, may influence wound healing, therefore wound healing cannot be predicted by SPP value alone. The control of all other factors is necessary to achieve wound healing.
The limitations of this study were as follows. First, the number of patients was too small to make a definitive conclusion. However, the present results add evidence supporting the usefulness of SPP examination for assessing the effects of vascular reconstruction on wound healing, although additional studies involving a larger number of patients as well as more data are needed. Second, the timing of postoperative SPP measurement was not fixed partly because of the influence of the physical condition of the patient and partly because this was a study of retrospective data, not a planned prospective study. Since all limbs were examined only once after surgery, data on changes in the SPP value over time were not available. Evaluation and comparison of SPP at a similar time would be ideal in future studies for a more appropriate comparison.
Conclusion
SPP measurement before and after arterial reconstruction is useful to assess improvement in tissue circulation and to predict to some extent the possibility of wound healing. In support of the findings of limited reports in the literature on the usefulness of SPP for assessing the effects of vascular reconstruction on ulcer would healing, an SPP value of ≥30 mmHg is considered necessary for wound healing, but it does not guarantee it. It is desirable to have a minimum SPP value of 30 mmHg and ideally greater than 40 mmHg to achieve wound healing.
Disclosure Statement
The authors declare that they have no conflicts of interest associated with this study.
Acknowledgements
We are indebted to Mr. Roderick J. Turner, Assistant Professor Edward F. Barroga and Professor J. Patrick Barron, Chairman of the Department of International Medical Communications at Tokyo Medical University for their review of the English manuscript.
References
- 1.Castronuovo JJ, Adera HM, Smiell JM.Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg 1997; 26: 629-37 [DOI] [PubMed] [Google Scholar]
- 2.Lo T, Sample R, Moore P.Prediction of wound healing outcome using skin perfusion pressure and transcutaneous oximetry: a single-center experience in 100 patients. Wounds 2009; 21: 310-6 [PubMed] [Google Scholar]
- 3.Miyashita Y, Saito S, Miyamoto A.Cilostazol increases skin perfusion pressure in severely ischemic limbs. Angiology 2011; 62: 15-7 [DOI] [PubMed] [Google Scholar]
- 4.Urabe G, Yamamoto K, Onozuka A.Skin perfusion pressure is a useful tool for evaluating outcome of ischemic foot ulcers with conservative therapy. Ann Vas Dis 2009; 2: 21-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada T, Ohta T, Ishibashi H.Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs - Comparison with other noninvasive diagnostic methods. J Vasc Surg 2008; 47: 318-23 [DOI] [PubMed] [Google Scholar]
- 6.Adera HM, James K, Castronuovo JJ., JrPrediction of amputation wound healing with skin perfusion pressure. J Vasc Surg 1995; 21: 823-8 [DOI] [PubMed] [Google Scholar]
- 7.Shimazaki M, Matsuki T, Yamauchi K.Measurement of skin perfusion pressure in hemodialyzed patients: Association with toe/brachial index. Dialysis & Transplantation 2008; 37: 431-8 [Google Scholar]
- 8.Kawarada O, Yokoi Y. Native chronic total occlusion recanalization after lower limb bypass graft occlusion: a series of nine cases. Catheter Cardiovasc Interv 2010; 76: 214-9 [DOI] [PubMed] [Google Scholar]
- 9.Iida O, Nanto S, Uematsu M.Importance of the angiosome concept for endovascular therapy in patients with critical limb ischemia. Catheter Cardiovasc Interv 2010; 75: 830-6 [DOI] [PubMed] [Google Scholar]
- 10.Hirsch AT, Haskal ZJ, Hertzer NR.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic)--A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery,* Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). Circulation 2006; 113: E463-654 [DOI] [PubMed] [Google Scholar]
- 11.Norgren L, Hiatt WR, Dormandy JA.Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 45: S5-67 [DOI] [PubMed] [Google Scholar]
- 12.Hoshino J, Fujimoto Y, Naruse Y.Characteristics of revascularization treatment for arteriosclerosis obliterans in patients with and without hemodialysis. Circ J 2010; 74: 2426-33 [DOI] [PubMed] [Google Scholar]
- 13.Kimura H, Miyata T, Sato O.Infrainguinal arterial reconstruction for limb salvage in patients with end-stage renal disease. Eur J Vasc Endovasc Surg 2003; 25: 29-34 [DOI] [PubMed] [Google Scholar]
- 14.Ramdev P, Rayan SS, Sheahan M.A decade experience with infrainguinal revascularization in a dialysis-dependent patient population. J Vasc Surg 2002; 36: 969-74 [DOI] [PubMed] [Google Scholar]
- 15.Komoda T. Study on the usefufness of skin perfusion pressure (SPP) in patients with foot lesions. J Jpn PRS 2009; 29: 73-82 [Google Scholar]


