Introduction
In 1885 Osler, first used the term mycotic aneurysm to describe an infected aneurysm in a patient with subacute bacterial endocarditis.1) This term may create considerable confusion, since mycotic is typically used to define true fungal infections or infections related with endocarditis. Mycotic aneurysm is used for all extracardiac or intracardiac aneurysms caused by bacterial and fungal infections. The term infected aneurysm is clarified and more appropriate, since few infections involve fungi.
Infected aortic aneurysms are rare; they represent only 1– 2.6% of all aortic aneurysms.2–4) For the clinician, early diagnosis is the cornerstone of effective treatment. Without medical or surgical management, catastrophic hemorrhage or uncontrolled sepsis may occur. However, symptomatology is frequently nonspecific during the early stages, so a high index of suspicion is required to make the diagnosis.
Pathogenesis
The source of infection in infected aneurysms is either intravascular or extravascular.
1. Primary infected aneurysms arise from adjacent surrounding areas of infection or trauma, either from direct contact or via lymphatic spread. Pre-existing abdominal aortic aneurysms or post-traumatic false aneurysms may become secondarily infected.2) Alternatively, a local extravascular infectious focus, such as osteomyelitis of the spinal vertebrae, may penetrate directly or via lymphatic tissue into the aorta, leading to necrosis of the wall, false aneurysm formation and subsequent rupture.5–7)
2. Secondary infected aneurysms arise from septic embolization, either through the vasa vasorum or intraluminally into areas of abnormal intima, such as preexisting aneurysms or atherosclerotic plaques. Organisms may colonise the intact vascular wall through the vasa vasorum, where the arterial wall is weakened by a local suppurative process which results in Microbial arteritis with aneurysm formation.3, 8, 9)
Pathology
The bacteriology of infected aneurysms has significantly changed since the original description in the 1800s. Before the antibiotics era, nearly 90% of infected aneurysms were associated with bacterial endocarditis,8) occurring predominantly in the ascending aorta and arch. The bacteria most commonly grown from the walls of the infected aneurysms were a reflection of those responsible for bacterial endocarditis and included Streptococcus (viridians and faecalis), Staphylococcus (aureus and epidermidis), Haemophilus and Pneumococcus. The introduction and widespread use of antibiotics resulted in a sharp decline in the frequency of bacterial endocarditis.8, 9) Currently, about 80% of mycotic aneurysms are the result of microbial aortitis; 3% are estimated to involve infection of a preexisting aneurysm. Fungal infections are rare which common species are Hitoblastoma capsulatum, Candida and Aspergillus.10, 11)
In non-endocarditis bacteremia, the most commonly reported site of infected aneurysms was the abdominal aorta due to infection of an existing atherosclerotic plaque.12) Salmonella species in particular have a strong predilection to infect damaged aortic intima and abnormal arterial intima, especially arteries harboring atherosclerotic plaque.13, 14) In some series, Salmonella was responsible for more than 50% of infected aneurysms.13) The virulence species, Salmonella typhimurium and Salmonella chloeraesuis, account for over 50% of the reported cases of Salmonella infected aneurysms.14, 15) Other common organisms are Streptococcus, Bacteroides, Escherichia coli, and Staphylococcus aureus.16–20)
The changes in bacterial species and arterial location of infected aneurysms are the result of the increasing incidence of arterial catheterizations, as well as injecting drug users. Predominance of gram positive bacteria such as Staphylococcus and Streptococcus are isolated, gram negative species such as Escherichia coli and Pseudomonas are also seen.16) Intact arterial intima is highly resistant to infection. Therefore, areas of intimal disruption are preferred sites for bacterial seeding, and infected aneurysms are seen in areas of atherosclerotic plaques, bifurcations, trauma, and immediately distal to coarctations.13, 21) Staphylococcal species are usually associated with infected false aneurysms secondary to trauma and also being the major organism seen in vertebral osteomyelitis.7)
Acquired immunosuppression, such as in renal failure patients on dialysis or those treated with steroids, may predispose to the development of infected aneurysms, especially by uncommon organisms.22, 23) Methicillin-resistant Staphlococcus aureus (MRSA) is rapidly emerging as a common pathogen, especially in those patients who are immunocompromised, have prolonged hospital stay, or undergo invasive procedures.12, 16, 22) Zoonosis infections from organisms such as Streptococcus suis, Yersinia pseudotuberculosis, and Pasteurella multocida are causative agents of infected aneurysms.24–26)
The virulence of the infecting organism may also determine outcome. In a small study showed that four organisms, notably Salmonella species, Bacteroides fragilis, Staphylococcus aureus, and Pseudomonas aeruginosa, accounted for all the deaths, ruptures, and suprarenal aneurysm infections which occurred in 10 (77%) out of 13 patients.9, 13–15) Characterisation of the different bacteria is important, since gram-negative sepsis results in higher rupture rates than infection with gram-positive bacteria.9)
Anatomical Location
Infected aneurysms secondary to septic emboli usually involve the large muscular and elastic arteries. The aorta is the most common site due to the higher incidence of underlying atherosclerotic plaques and aneurysms with a larger vasa vasorum where infected emboli may dislodge.
Infected aneurysms can affect almost any artery in the body. With the advent of invasive monitoring and interventional procedures or as a result of drug misuse, the femoral artery has recently become the most common location of all infected aneurysms (56%), followed in frequency by the thoracic and abdominal aorta (33%), intra or extracranial (5%), innominate (2%), iliac (2%), and splanchnic arteries (1%). Amongthe splanchnic arteries, the superior mesenteric artery is the most frequently involved, and the hepatic artery is the next most commonsite.27)
Clinical Presentation
There is no distinct clinical presentation for infected aortic aneurysms. Presentation varies from an individual patient to another depending on several factors including the underlying etiology, type of organism, duration of infection, and the anatomic location of the aneurysm. Classic manifestations include abdominal pain, fever, and a pulsatile abdominal mass. The presence of bacterial endocarditis or other bacterial illness, rheumatic fever, a recent operation or arterial catheterization, and lumbar osteomyelitis are all risk factors for a subsequent infected aneurysm.
Comorbid factors are common, 70% of the patients had at least one comorbid condition associated with some degree of Immunosuppressive disorders.20) Comorbid conditions included diabetes (33%), chronic renal failure (30%), chronic steroid use (16%), and chronic disease (16%), such as rheumatoid arthritis, non-Hodgkin lymphoma, multiple myeloma, neutropenia, and monoclonal gammopathy.
History of a recent febrile gastrointestinal illness is common. Other distant sources for infection include pneumonia, urinary tract sepsis, osteomyelitis and soft tissue infection. Up to 20% of cases present with frank aneurysm rupture. The first presentation may be of gastrointestinal bleeding from primary aortoenteric fistula (10%). Rupture into the vena cava (aortocaval fistula) is a less common presentation (4%).11, 20, 28, 29)
Diagnosis
The diagnosis of infected aneurysms can be very difficult. Fever and leukocytosis are usually the first findings in 70% of cases,30) with a palpable aneurysm or back pain constituting the third part of a classic triad of symptoms. The presence of leukocytosis, elevated sedimentation rate and positive blood cultures strengthens the surgeon's suspicion of the diagnosis of infected aneurysm. The symptoms of sepsis may be discrete and may easily go unrecognized, especially in the early stages. The onset of both mycotic aneurysm and osteomyelitis can be insidious. Pain and fever may be the only presenting symptoms. Untreated osteomyelitis of the spine may extend anteriorly into the soft tissues, as in our patient, or it may extend posteriorly into the vertebral canal and result in a chronic epidural abscess.6, 7) Arterial blood culture is necessary. If blood cultures are positive, they are helpful to signal the need for the specific antibiotic therapy. However blood cultures, are negative in up to 50% of cases, negative blood cultures do not rule out the presence of an infected aneurysm. Gram stains and cultures obtained from the aneurysm wall at the time of surgery are also not uniformly positive. The most commonly isolated organisms include Salmonella species, Streptococcus, Bacteroides, Escherichia coli, and Staphylococcus aureus. Generally, gram negative infections tend to be more virulent than gram positive infections.15)
Ultrasonography, computed tomography (CT) including spiral CT, and magnetic resonance imaging (MRI) can all be helpful. CT scan is the cornerstone in establishing the diagnosis of infected aortic aneurysm. Classic findings include periaortic edema fluid with thickening of the aneurysm wall and infiltration of the periaortic tissues. The presence of gas bubbles, seen with certain bacterial infections, is diagnostic. CT scan also defines the location and morphology of the aneurysm. While any part of the aorta can be involved, there is a tendency for infected aneurysms to involve the paravisceral segment of the aorta.31) Three-dimensional reconstruction shows infected aneurysms to be more commonly saccular than fusiform (80% vs 20%). Furthermore, CT scan offers an opportunity to obtain by puncture periaortic fluid for gram staining and culture preoperatively.10) MRI provides essentially similar findings to those obtained by CT scan, probably with better definition of the periaortic soft tissue reaction.32) Radioisotope imaging techniques using indium111-labeled leukocytes or a gallium-67 citrate scan have traditionally been used to confirm the presence of an infective process. Results however are not always conclusive and are not popular for clinical use in emergency cases.33) Angiography, while not necessary in all cases, is useful in defining the accurate location and shape of the aneurysm, especially in the splanchnic artery aneurysm.34) More importantly, involvement of the visceral vessels is determined providing essential preoperative data for planning the appropriate form of reconstruction. It is important to note that the operative findings, namely infection and suppuration of the periaortic tis- sues, remain the ultimate diagnostic criteria even in the absence of positive cultures or classic imaging findings.
Transesophageal echocardiography may also assist in the early diagnosis of infected aneurysms. Because of the close proximity of the esophagus to the aorta, it provides detailed views of the descending aorta. Color Doppler echocardiography can demonstrate the flow from the aorta into the abscess cavity.35)
Management Strategies
Management of infected aneurysms is a challenging clinical problem for the vascular surgeon. Infected aneurysms continue to be associated with both high morbidity and high mortality. They are frequently associated with complicating factors, such as late or delay in diagnosis, rupture, sepsis, and paravisceral location, infected aneurysms tend to occur in immunocompromised patients that result in increased morbidity and mortality.1, 2) The fundamental aspects of treatment are control or eradication of infection and establishment of arterial reconstruction.
Control of Infection
1. Antibiotics
Broad-spectrum antibiotics should be started pre-operatively after taking blood cultures. Antibiotics should not be used as the sole line of therapy as infected aneurysms have a definite risk of rupture and should be continued until the source of bacteremia is removed.38) The duration of antibiotic therapy is not well established but most authors recommend a minimum of 6 weeks intravenously and orally for another 6 weeks.30, 38) The antibiotics can be discontinued if there is no clinical, hematological or radiological evidence of ongoing sepsis. Longer durations and even life-long antibiotic-therapy have been recommended by others.3, 19, 39, 40) Some authors believe that patients with a prosthetic reconstruction should continue on low-dose antibiotics for life. However, the advantage of a more prolonged therapy has not been confirmed.
2. Debridement
All infected arterial tissue and necrotic tissue must be debrided until reach the point where the tissue is healthy to prevent subsequent recurrence of infection and disruption of the arterial suture line. After debridement the whole area should be irrigated with antibiotic solution and normal saline.
Establishment of Arterial Reconstruction
The principles of surgical treatment for infected aneurysm include: control of hemorrhage, debridement of infected tissue, and arterial reconstruction of vital arteries through uninfected tissue planes.
Surgical managements include extra-anatomical bypass followed by aneurysm resection, aneurysm resection followed by extra-anatomical bypass, aneurysm resection alone or aneurysm resection with in situ reconstruction.
1. Aneurysm resection and debridement
Excision followed by proximal and distal ligation is the treatment of choice for the area with good collaterals. Monofilament sutures should be used for suturing and ligation because of lower risk of recurrent infection. The ligated arterial stump should be covered with healthy tissue. In he abdomen, omentum or prevertebral fascia should be used; in the groin transposed Sartorius muscle is useful for covering the arterial stumps.5)
2. Arterial reconstruction
The virulence of the organism and severity of the arterial infection are more important determinants than any single operative procedure or method of arterial reconstruction.12, 15) When there is gross contamination from infected aneurysm, excision and extra-anatomical bypass is the treatment of choice. The associated mortality was still high, ranging from 25% to 30%.3, 41, 44) Death is usually related to persistent sepsis with multi-organ failure. The magnitude and long duration of the operation especially in shocked and unstable patients is a major contributing factor to the perioperative mortality. Disruption of the aortic stump is reported in about 20% of cases.3, 28, 41–44) When contamination is less severe, the aorta may be replaced in-situ. Extra-anatomical reconstruction is not suitable for cases of suprarenal or supraceliac aneurysms with visceral artery involvement, in situ aortic replacement is necessary.3, 4, 38, 39) There are various types of in situ repair describe: cryopreserved human arterial or venous allografts, arterial or venous homografts, prosthetic grafts, and animal xenografts. However, the published data are derived from series with a small number of patients and a large number of variables.
Synthetic Grafts
Review of the literature shows rather poor results from direct replacement of infected aneurysms with prosthetic grafts. There is an approximately 25% early mortality and a similar incidence of aortic sepsis complications and vascular re-interventions. In-situ reconstruction with prosthetic graft is associated with 16–20% risk of recurrent infection, requiring late extra-anatomical bypass.4, 19, 38, 39, 46) Antibiotic-coated Dacron grafts presented an attractive adjunct. Prolonged anti-staphylococcal activity of rifampin-bonded, gelatin impregnated Dacron grafts has been demonstrated after implantation in the arterial circulation in experimental and human studies. Infection rates, as well as mortality and morbidity rates, are much lower for rifampin-treated grafts than for plain in situ graft replacement.42, 47) Encouraging results have also been reported with the use of silver coated polyester grafts.48)
Autologous Tissue
Vein grafts tend to be superior to synthetic grafts as they are more resistant to infection. The 5-year primary and secondary patency rates for the use of autologous superficial femoropopliteal veins as a conduit for in-situ reconstruction of infected aortic aneurysms were 83% and 100% respectively, with excellent limb salvage and minimal long term lower extremity venous congestion.49) Operative mortality rates similar to prosthetic graft repair with low amputation rates and recurrent infection. The use of autogenous material is certainly appealing in these cases but the technique has the disadvantage of significantly lengthening the procedure by the extra time needed to harvest the deep veins and tailor them to match the size of the aorta. This generally requires between one and a half to two hours, making the procedure unsuitable for shocked or unstable patients. Furthermore, there is always the concern about post operative leg edema and long term venous insufficiency. Advocates of this approach however report the incidence of this complication to be minimal.49, 50)
Allografts
The allografts may be more resistant to infection because they allow transfer of antibiotics and immunocompetent cells across the wall and also into the perigraft space. However, it is not widely practiced however because of the high cost and the complicated preservation procedure required. Furthermore graft deterioration with significant dilatation, mural thrombus formation, distal stenosis or graft occlusion has been reported in up to 25% of cases, some of which may be histologically related to chronic rejection.51–53)
Endovascular Treatment
In the recent years with the expanding use of endovascular aortic aneurysm repair (EVAR), the indications of the procedure have been extended to include infected aneurysms. Endovascular treatment with stent-grafts has been introduced as an alternative, with the anticipation that lesser surgical trauma, especially in the septic patient with considerable co-morbidity, will reduce the risk of cardiopulmonary, neurological and renal complications. It is becoming a real alternative to open surgery for the treatment of mycotic aneurysm involving the descending thoracic and abdominal aorta, including penetrating aortic ulcers, and.54–62) This could be a temporising treatment prior to definitive open surgical repair, or a therapeutic alternative in critically ill patients who may not survive open surgery. The potential benefits of endovascular repair include small incisions, minimal aortic cross-clamping time with reduction in end-organ ischemia, avoidance of general anesthesia, full heparinsation, single lung ventilation and cardiopulmonary bypass. Endoluminal repair should reduce the length of stay in intensive care units, high dependency units and in hospital, with an earlier return to activities of daily living and consequent improvement in the quality of life.63–66) An additional drainage procedure is usually required either via CT guided drainage or by open surgical approach (Fig. 1a, 1b).
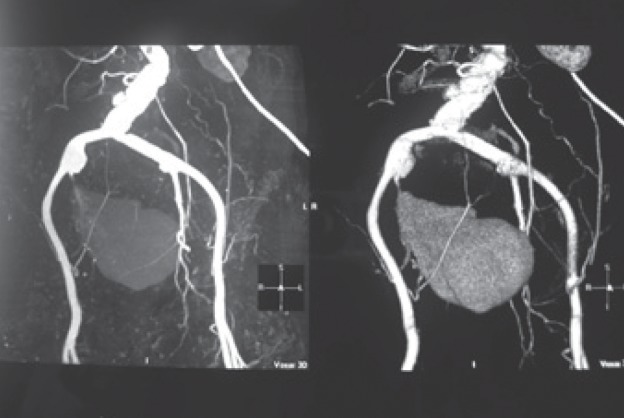
Fig. 1a.
Aortogram demonstrates a large saccular infected aneurysm of the right internal iliac artery. Hemoculture results revealed Burkholderia pseudomallei.
Fig. 1b.
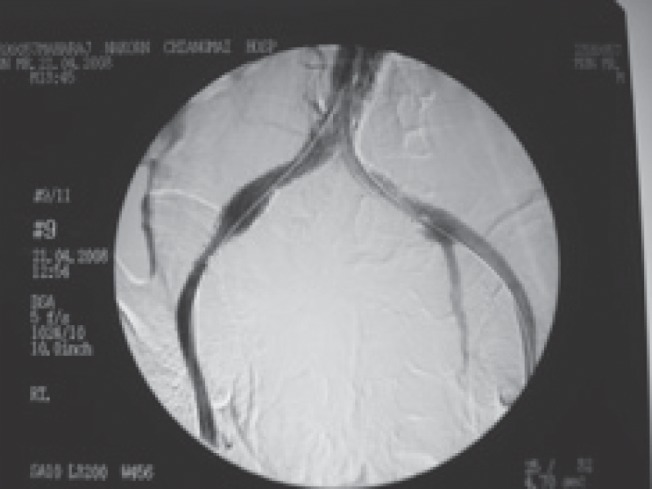
Aortogram demonstrates satisfactory endoluminal occlusion of the aneurysm with the EVAR stent-graft covering right internal iliac artery. The patient underwent percutaneous drainage of the abscess pocket on the following day.
Persistent infection after EVAR treatment of infected aortic aneurysms is closely associated with a poor prognosis. The endoprostheses may become infected so compounding the problem over a greater length of aorta, and they may cause rupture of a fragile vessel because of the necessary oversizing used to hold the device in place. From the results of the analysis, we identified aneurysm rupture and fever at operation as the most significant variables associated with the occurrence of persistent infection in these patients. When patients present with rupture or have fever, the EVAR method should be considered as a temporary measure to achieve hemodynamic stability.63–67) Additionally, if the fever persists after the EVAR, This would be an indication to keep the patient on long-term broad-spectrum antibiotics for a long-term until a definite surgical treatment is considered. However, further multi institutional and registry data are required to clarify the long-term outcomes of EVAR and to determine whether EVAR use in infected aortic aneurysms is as effective as or better than standard operation.68)
Conclusion
Infected aneurysms are associated with high morbidity and mortality. They are frequently associated with complicating factors, such as late or delay in diagnosis, rupture, sepsis, and paravisceral location, infected aneurysms tend to occur in immunocompromised patients that result in increased morbidity and mortality. A high index of suspicion is required for early diagnosis. The fundamental aspects of treatment are control or eradication of infection and establishment of arterial reconstruction. Surgical options should be tailored to individual patients. Inteventions such as in-situ replacement with antibiotic-impregnated grafts and endovascular stenting remain unproven in the long term.
References
- Osler W. The Gulstonian lectures on malignant endocarditis. Br Med J. 1885; 1: 467–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DJ, Shepard AD, Evans JR, Wright DJ, Smith RF, Ernst CB. Management of infected aortoiliac aneurysms. Arch Surg. 1991; 126: 873–9 [DOI] [PubMed] [Google Scholar]
- Muller BT, Wegener OR, Grabitz K, Pillany M, Thomas L, Sandman W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries : Experience with anatomic and extraanatomic repair in 33 cases. J Vasc Surg. 2001; 33: 106–13 [DOI] [PubMed] [Google Scholar]
- Chan FY, Crawford ES, Coselli JS, Safi HJ, Williams TW. In- situ prosthetic graft replacement for mycotic aneurysm of the aorta. Ann Thorac Surg. 1989; 47: 193–203 [DOI] [PubMed] [Google Scholar]
- McCready RA, Bryant MA, Divelbiss JL, et al. Arterial infections in the new millennium: an old problem revisited. Ann Vasc Surg. 2006; 20: 590–5 [DOI] [PubMed] [Google Scholar]
- Naktin J, DeSimone J, Lumbar Vertebral osteomyelitis with mycotic abdominal aortic aneurysm caused by highly penicillin-resistant Streptococcus pneumoniae. J Clin Microbiol. 1999; 12: 4198–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberry PT, Smith MD, Cammisa FP, et al. Mycotic aortic aneurysm in patients who have lumbar vertebral osteomyelitis. J Bone Joint Surg. 1995; 77-A: 1729–32 [DOI] [PubMed] [Google Scholar]
- Stengel A, Wolferth CC. Mycotic bacterial aneurysms of intravascular origin. Arch Intern Med. 1923; 31: 527–54 [Google Scholar]
- Brown SL, Busuttil RW, Baker JD, et al. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg. 1984; 1: 541–7 [PubMed] [Google Scholar]
- Gomes MN, Choyke PL, Wallace RB. Infected aortic aneurysms: a changing entity. Ann Surg. 1992; 215: 435–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides GK, Simmons RL, Najarian JS. Mycotic aneurysms in transplant patients. Arch Surg. 1976; 111: 472–6 [DOI] [PubMed] [Google Scholar]
- Hsu PJ, Lee CH, Lee FY, Liu JW. Clinical and microbiological characteristics of mycotic aneurysms in a medical center in southern Taiwan. J Microbiol Immunol Infect. 2008; 41: 318–24 [PubMed] [Google Scholar]
- Batt M, Magne J-L, Pierre A, Fernandez Guerrero ML, Aguado JM, Arribas A, et al. The spectrum of cardiovascular infections due to Salmonella enterica: a review of clinical features and factors determining outcome. Medicine. 2004; 83: 123–38 [DOI] [PubMed] [Google Scholar]
- Cohen PS, O Brien TF, Schoenbaum SC, Medeiros AA. The risk of endothelial infection in adults with Salmonella bacteremia. Ann Intern Med. 1978; 89: 931–2 [DOI] [PubMed] [Google Scholar]
- Zak FG, Strauss L, Saphra I. Rupture of diseased large arteries in the course of enterobacterial (Salmonella) infections. N Engl J Med. 1958; 258: 824–8 [DOI] [PubMed] [Google Scholar]
- Reddy DJ, Ernst CB. Infected aneurysms. In: Rutherford RB. ed. Vascular surgery. Philadelphia: WB Saunders; 2005: 1581–96 [Google Scholar]
- Moneta GL, Taylor LM, Yeager RA, et al. Surgical treatment of infected aortic aneurysm. Am J Surg. 1998; 175: 396–9 [DOI] [PubMed] [Google Scholar]
- Sriussadaporn S, Pak-Art R, Chiamananthapong S, Tangchai W, Nivatvongs S, Sirichindakul B, et al. Surgery of the abdominal aorta: experience of a university hospital in Thailand. J Med Assoc Thai. 2001; 84: 1655–60 [PubMed] [Google Scholar]
- Hsu RB, Tsay YG, Wang SS, Chu SH. Surgical treatment for primary infected aneurysm of the descending thoracic aorta, abdominal aorta, and iliac arteries. J Vasc Surg. 2002; 36: 746–50 [DOI] [PubMed] [Google Scholar]
- Oderich GS, Panneton JM, Bower TC, Cherry KJ, Jr, Rowland CM, Noel AA, et al. Infected aortic aneurysm : aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001; 34: 900–8 [DOI] [PubMed] [Google Scholar]
- Bennett DE, Cherry JK. Bacterial infection of aortic aneurysms: a clinicopathologic study. Am J Surg. 1967; 113: 321–6 [DOI] [PubMed] [Google Scholar]
- Paccalin M, Amuora Z, Brocheriou I, Hernigou A, Deliangle MH, Lesco BM, et al. Infectious aneurysm due to Listeria monocytogenes : a new case and review of the literature. Rev Med Interne. 1998; 19: 661–5 [DOI] [PubMed] [Google Scholar]
- Dupont JR, Bnavita JA, Digiovanni RJ, Spector HB, Nelson SC. Acquired immunodeficiency syndrome and mycotic abdominal aortic aneurysms : a new challenge ? Report of a case. J Vasc Surg, 1989; 10: 254–7 [PubMed] [Google Scholar]
- Laohapensang K, Rutherford BR, Aworn S. Infected Abdominal Aortic Aneurysm due to Streptococcus suis. Surg Infect. 2009; 10: 1–3 [DOI] [PubMed] [Google Scholar]
- Koelemay MJ. Pasteurella multocida infection, a rare cause of mycotic abdominal aortic aneurysm. J Vasc Surg. 2009; 50: 1496–8 [DOI] [PubMed] [Google Scholar]
- Hadou T, Elfarra M, Alauzet C, Guinet F, Lozniewski A, Lion C. Abdominal aortic aneurysm infected by Yersinia pseudotuberculosis. J Clin Microbiol. 2006; 44: 3457–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WS, Malone JM. Mycotic aneurysms. In: Aneurysms. New Findings and Treatment, Yao JST, Pearce WH. Eds. Appleton and Lange, Norwalk, Connecticut: 1994; 389–410 [Google Scholar]
- Conolly JE, Kwaan JH, McCart PM, Brownell DA, Levine EF. Aortoenteric fistula. Ann Surg. 1981; 194: 402–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C, Safi H, Beall AC., Jr Primary aortoduodenal fistula caused by salmonella aortitis. J Vasc Surg. 1987; 6: 415–41 [PubMed] [Google Scholar]
- Hsu RB, Chen RJ, Wang SS, Chu SH. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg. 2004; 40: 30–5 [DOI] [PubMed] [Google Scholar]
- Blair RH, Resnik MD, Polga JP. CT appearance of mycotic abdominal aortic aneurysms. J Comput Assist Tomogr. 1989; 13: 101–4 [DOI] [PubMed] [Google Scholar]
- Katz BH, Black RA, Colley DP. CT-guided fine needle aspiration of a periaortic collection. J Vasc Surg. 1987; 5: 762–4 [PubMed] [Google Scholar]
- Chen P, Lamki L, Raval B. Indium-111 leukocyte appearance of Salmonella mycotic aneurysm. Clin Nucl Med. 1994; 19: 646–8 [DOI] [PubMed] [Google Scholar]
- Walsh DW, Ho VB, Haggerty MF. Mycotic aneurysm of the aorta: MRI and MRA feature. J Magn Reson Imaging. 1997; 7: 312. [DOI] [PubMed] [Google Scholar]
- Joffe II, Emmi RP, Oline J, et al. Mycotic aneurysm of the descending thoracic aorta: the role of transesophageal echocardiography. J Am Soc Echocardiogr. 1999; 9: 663–7 [DOI] [PubMed] [Google Scholar]
- Wang H, Rammos S, Elwook P. Successful endovascular treatment of a ruptured mycotic intracavernous carotid artery aneurysm in an AIDS patient. Neurocrit Care. 2007; 7: 156–9 [DOI] [PubMed] [Google Scholar]
- Lawrence GH. Surgical management of infected aneurysms. Am J Surg. 1962; 104: 355–64 [DOI] [PubMed] [Google Scholar]
- Fichelle JM, Tavet J, Cormier P, et al. Infected infrarenal aortic aneurysms: when is in situ reconstruction safe?. J Vasc Surg. 1993; 17: 635–45 [DOI] [PubMed] [Google Scholar]
- Kuliu KA, Heikkinen L, Salo J. One stage vascular surgery for abdominal aortic aneurysm infected by Salmonella. Eur J Vasc Surg. 1989; 3: 173–5 [DOI] [PubMed] [Google Scholar]
- Blackett RL, Hill SF, Bowler I, Morgan JR, HeardEARD GE. Mycotic aneurysm of the aorta due to group B streptococcus. Eur J Vasc Surg. 1989; 3: 177–9 [DOI] [PubMed] [Google Scholar]
- Kyriakides C, Kan Y, Kerle M, Cheshire NJ, Mansfield AO, Wolfe JH. 11-year experience with anatomical and extra-anatomical repair of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg. 2004; 27: 585–9 [DOI] [PubMed] [Google Scholar]
- Naylor AR, Clarks S, London NJM, Sayers RD, MacPherson DS, Barrie WW, et al. Treatment of major aortic graft infection: preliminary experience with total graft excision and in situ replacement with a rifampin-bonded prosthesis. Eur J Vasc Endovasc Surg. 1995; 9: 252–6 [DOI] [PubMed] [Google Scholar]
- Oz MC, Brener BJ, Buda JA, et al. A ten-year experience with bacterial aortitis. J Vasc Surg. 1989; 10: 439–49 [DOI] [PubMed] [Google Scholar]
- Bacourt F, Koskas F. Axillobifemoral artery bypass and aortic exclusion for vascular septic lesions: a multi-centered retrospective study of 98 cases. Ann Vasc Surg. 1992; 6: 119–26 [DOI] [PubMed] [Google Scholar]
- Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009; 32: 488–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CY, Ko WC, Kan CD, Lin PY, Yang YJ. In situ reconstruction of septic aortic pseudoaneurysm due to Salmonella or Streptococcus microbial aortitis: long-term follow-up. J Vasc Surg. 2003; 38: 975–82 [DOI] [PubMed] [Google Scholar]
- Chervu A, Moore WS, Gelabert HA, Colburn MD, Chvapil M. Prevention of graft infection by use of prostheses bonded with a rifampin/collagen release system. J Vasc Surg. 1991; 14: 521–5 [PubMed] [Google Scholar]
- Batt M, Magne JL, Alric P, Muzj A, Ruotolo C, Ljungstrom KG, et al. In situ revascularization with silver-coated polyester grafts to treat aortic infection: Early and midterm results. J Vasc Surg. 2003; 38: 983–9 [DOI] [PubMed] [Google Scholar]
- Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg. 1997; 25: 255–70 [DOI] [PubMed] [Google Scholar]
- Wells JK, Hagino RT, Bargmann KM, Jackson MR, Vvalentine RJ, Kakish HB, et al. Venous morbidity after superficial femoral-popliteal vein harvest. J Vasc Surg. 1999; 29: 282–91 [DOI] [PubMed] [Google Scholar]
- Kieffer E, Gomes D, Chiche L, Fléon MH, Kaskas F, Bahini A. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004; 39: 1009–17 [DOI] [PubMed] [Google Scholar]
- Noel AA, Gloviczki P, Cherry KJ, Jr, et al. Abdominal aortic reconstruction in infected fields: early results of the United States Cryopreserved Aortic Allograft Registry. J Vasc Surg. 2002; 35: 847–52 [DOI] [PubMed] [Google Scholar]
- Leseche G, Castier Y, Petit MD, et al. Long-term results of cryopreserved arterial allograft reconstruction in infected prosthetic grafts and mycotic aneurysms of the abdominal aorta. J Vasc Surg. 2001; 34: 616–22 [DOI] [PubMed] [Google Scholar]
- Guerrero ML, Urbano J, Ortiz A, Caramelo C, De Górgolas M. Endovascular repair of mycotic aneurysms of the aorta: an alternative to conventional bypass surgery in patients with acute sepsis. Scand J Infect Dis. 2007; 39: 268–71 [DOI] [PubMed] [Google Scholar]
- Berchtold C, Eibl C, Seelig MH, Jakob P, Schonleben K. Endovascular treatment and complete regression of an infected abdominal aortic aneurysm. J Endovasc Ther. 2002; 9: 543–8 [DOI] [PubMed] [Google Scholar]
- MitchellIL RS, Miller DC, Dake MD. Stent-graft repair of thoracic aortic aneurysms. Semin Vasc Surg. 1997; 10: 257–71 [PubMed] [Google Scholar]
- Demers P, Miller DC, MitchellI RS, Kee ST, Chagonjian L, Dake MD. Stent-graft repair of penetrating atherosclerotic ulcers in the descending thoracic aorta: mid-term results. Ann Thorac Surg. 2004; 77: 81–6 [DOI] [PubMed] [Google Scholar]
- Stanley BM, Semmens JB, Lawrence-Brown MM, Denton M, Grosser D. Endoluminal repair of mycotic thoracic aneurysms. J Endovasc Ther. 2003; 10: 511–5 [DOI] [PubMed] [Google Scholar]
- Smith JJ, Taylor PR. Endovascular treatment of mycotic aneurysms of the thoracic and abdominal aorta: the need for level I evidence. Eur J Vasc Endovasc Surg. 2004; 27: 569–70 [DOI] [PubMed] [Google Scholar]
- Madhavan P, McDonnell CO, Dowd MO, Sultan SA, Doyle M, Colgan MP, et al. Suprarenal mycotic aneurysm exclusion using a stent with a partial autologous covering. J Endovasc Ther. 2000; 7: 404–9 [DOI] [PubMed] [Google Scholar]
- Kinney EV, Kaebnick HW, Mitchell RA, Jung MT. Repair of mycotic paravisceral aneurysm with a fenestrated stent-graft. J Endovasc Ther. 2000; 7: 192–7 [DOI] [PubMed] [Google Scholar]
- Bond SE, McGuinness CL, Reidy JF, Taylor PR. Repair of secondary aortoesophageal fistula by endoluminal stent-grafting. J Endovasc Ther. 2001; 8: 597–601 [DOI] [PubMed] [Google Scholar]
- Bell RE, Taylor PR, Aukett M, Evans GH, Reidy JF. Successful endoluminal repair of an infected thoracic pseudoaneurysm caused by methicillin-resistant Staphylococcus aureus. J Endovasc Ther. 2003; 10: 29–32 [DOI] [PubMed] [Google Scholar]
- Bell RE, Taylor PR, Aukett M, Sabharwal T, Reidy JF. Results of Urgent and Emergency Thoracic Procedures Treated by Endoluminal Repair. Eur J Vasc Endovasc Surg. 2003; 25: 527–31 [DOI] [PubMed] [Google Scholar]
- Krohg-Sorensen K, Hafsahl G, Fosse E, Geiran OR. Acceptable short-term results after endovascular repair of diseases of the thoracic aorta in high risk patients. Eur J Cardiothorac Surg. 2003; 24: 379–87 [DOI] [PubMed] [Google Scholar]
- Jones KG, Bell RE, Sabharwal T, Aukett M, Reidy JF, Taylor PR. Treatment of mycotic aortic aneurysms with endoluminal grafts. Eur J Vasc Endovasc Surg. 2005; 29: 139–44 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fajardo JA, Gutierrez V, Martin-Pedroza M, Del Rio L, Carrera S, Vaquero C. Endovascular repair in the presence of aortic infection. Ann Vasc Surg. 2005; 19: 94–8 [DOI] [PubMed] [Google Scholar]
- Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: A systematic review. J Vasc Surg. 2007; 46: 906–12 [DOI] [PubMed] [Google Scholar]