Although aortic dissection was previously a disease with an extremely poor prognosis, recent advances in diagnostic imaging and therapeutic modalities may improve its prognosis.1, 2) However, much of its etiology and pathogenesis remains unknown. In addition, new pathological variants of aortic dissection, such as intramural hematoma (IMH) and penetrating atherosclerotic ulcer, and related diseases have been identified, raising a new problem. Against this background, this paper revisits the basic pathology of aortic dissection, and discusses the structural abnormalities of the aortic media and mechanism of aortic dissection, as extrapolated from them.
Medial Weakness: A Pathological Problem with Aortic Dissection
Aortic dissection used to be clearly defined as a pathological condition characterized by the presence of an aortic intimal tear and medial dissection, that is, a condition in which blood flows from the entry site into the false lumen.3) However, advances in diagnostic imaging have resulted in the identification of aortic dissection with no entry tear or false lumen flow, and these variants were included in the category of aortic dissection.4) For the differentiation between the two types of aortic dissection, the former is referred to as classic or communicating aortic dissection, and the latter as IMH or non-communicating aortic dissection. In IMH, no entry tear or false lumen flow is observed, strongly suggesting that no intimal tear exists anatomically. However, the limitations of diagnostic imaging make it difficult to establish its presence or absence. Therefore, it is doubtful whether IMH should be treated as the same entity as classic aortic dissection. However, in some cases, IMH has a poor prognosis in that it progresses to classic aortic dissection, or ruptures. Thus, clinically, it is believed that it is not contradictory to treat IMH as a variant of aortic dissection.5, 6) In contrast, pathologically, since the diagnosis can be confirmed by autopsy, IMH is clearly defined as a dissection without an intimal tear.7, 8) However, at the same time, the important question of whether dissection with a tear (classic aortic dissection) and that without a tear (IMH) is the same disease arises. This is related to the long-standing debate over which occurs first, intimal tear or medial dissection, in the development of aortic dissection. The former is the hypothesis that a crack (intimal tear) first forms in the intima overlying a site of aortic medial weakness, followed by blood inflow from the lumen, resulting in medial dissection.9) This is a very natural way of thinking in terms of the direction of blood flow. However, from this viewpoint, it follows that no dissection occurs without an intimal tear, making it contradictory to include IMH in the category of aortic dissection. On the other hand, the latter is based on the fact that no intimal tears were identified in about 10% of autopsied patients with aortic dissection, leading to a hypothesis that bleeding from the medial vasa vasorum at a site of aortic medial weakness results in aortic dissection.7) This hypothesis suggests that medial dissection is sometimes followed by the development of an intimal tear, and sometimes not. Therefore, there is no contradiction in regarding classic aortic dissection and IMH as the same disease. However, one may raise an objection that since the pressure in the aortic lumen is higher than that in the vasa vasorum, bleeding from the latter cannot occur. The question of which occurs first, intimal tear or medial dissection, has not been fully resolved to date. What is pathologically important is that both events assume the pre-existence of a medial weakness. In other words, there is a common recognition that no extensive aortic dissection occurs without an aortic medial weakness, whether an intimal tear or bleeding from the vasa vasorum occurs first. Thus, various studies focusing on medial weakness have been conducted to date. In the following paragraphs, we will present the results and concepts derived from these and our studies.
Cystic Medial Necrosis (Degeneration) and Limitations of Histological Examination
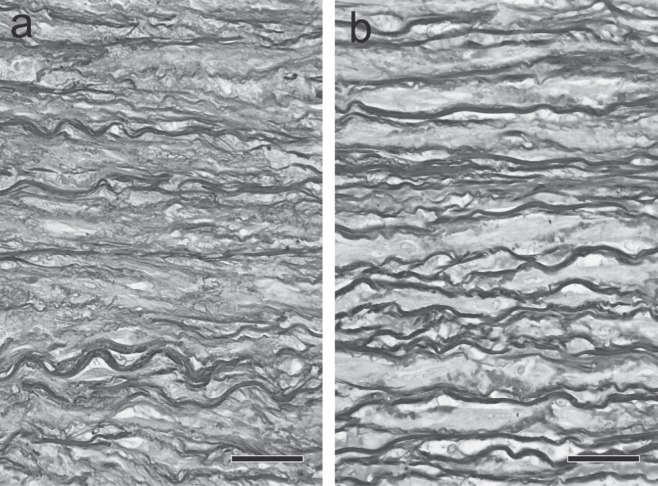
For many years, it has been reported that cystic medial necrosis (CMN) is observed in the aortic media of patients with aortic dissection. As shown in Fig. 1, CMN is a pathological condition characterized by the local disappearance of elastic fibers in the arterial media, a reduction in smooth muscle cells (SMCs), and an increase in proteoglycans. The histological appearance suggests a medial weakness,9) but the incidence of CMN is not as high as expected. Wilson and Hutchin 10) and Larson and Edwards 11) identified CMN in only 10 and 18% of autopsy cases of aortic dissection, respectively. In a histological study of autopsy material from 111 patients with aortic dissection, we observed CMN in a high percentage (82%) of patients with inherited connective tissue disease (ICTD) typified by Marfan syndrome, but in only 19% of non-ICTD patients, and the degree of CMN in non-ICTD patients was milder than that in ICTD patients.12) A recent study of surgical specimens has also reported a difference in the incidence of CMN between ICTD and non-ICTD patients.13) This strongly suggests that CMN is a major factor involved in the development of aortic dissection in ICTD patients, and that it is difficult to fully explain the pathogenesis of aortic dissection in non-ICTD patients by CMN alone. Then, what changes are observed in the media of aortic dissection in non-ICTD patients? Unfortunately, histological examination is useless for this question, and often fails to detect significant changes. Close examination under a high magnification seems to show fewer dark brown-stained elastic fibers in the aorta of aortic dissection patients than in that of normal subjects (Fig. 2 a & b); however, unfortunately, these figures give no more than an impression, indicating the limitations of light microscopy. In such a case, detailed electron microscopic examination may provide useful information. The following paragraphs describe the ultrastructure of the aortic media of normal subjects and aortic dissection patients, and discuss the difference in aortic function inferred from ultrastructural observations.
Fig. 1.

Cystic medial necrosis observed in a patient with aortic dissection. There was a focal loss of elastic fibers in the aortic media, accompanied by a decrease in smooth muscle cells and an increase in proteoglycans (Elastica van Gieson stain). Scale bar = 500 µm (Reproduced from Ref. 12).
Fig. 2.
Histological appearance of the human aortic media. a: Normal subject. b: Aortic dissection patient. Elastic fibers appear dark brown (Elastica van Gieson stain). Upper, intimal side. Lower, adventitial side. Scale bar = 50 µm. Elastic fibers appear dark brown (Elastica van Gieson stain).
Ultrastructure of the Normal Aortic Media
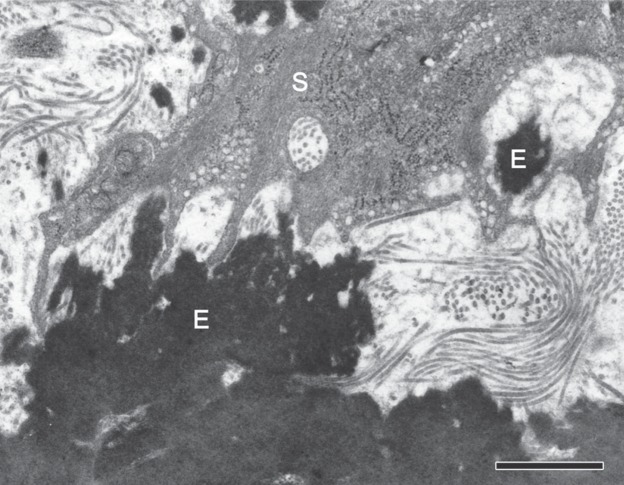
Electron microscopic studies by the research group of Glagov showed et al. showed that the normal aortic media consists of lamellar units comprising concentric elastic laminae and intervening smooth muscle cells, elastic fibers, collagen fibers, and ground substance.14, 15) The lamellar structure apparently gives the impression of weakness, but, actually, the tunica media is very tough. For example, it has been reported that a pressure as high as 600 mmHg is required to tear the media, that is, to cause dissection by infusing physiological saline into the media.16) Much of the structure that confers the medial strength remains unknown, but it is believed to be derived from the interconnection of the components of the lamellar units. First, let us focus on the most important components, elastic fibers and smooth muscle cells. When the human aortic media is treated with formic acid to remove the components other than the elastic fibers, and the structure of the remaining elastic fibers is examined by electron microscopy, the elastic laminae (running horizontally in Fig. 3a) can be seen to be mutually connected by interlaminar elastic fibers (running vertically in the figure).17) We can clearly see that elastic fibers, which appear to be a mere overlapping of layers on a two-dimensional image as obtained by light microscopy microscopy, actually assume a three-dimensional framework or network structure. In addition, transmission electron microscopy has shown that smooth muscle cells present in this framework structure are densely adherent and connected with elastic fibers.18, 19) The aorta receives blood from the heart and dilates passively during systole, and, conversely, contracts passively during diastole to smoothly pump the blood accumulated in the lumen to the periphery (Windkessel effect). Moreover, as described below, the thoracic aorta moves up and down and twists during systole and diastole. In other words, the aorta repeatedly expands and contracts passively like rubber in rhythm with the heart beat. It seems that the integrity of the media is preserved against the repeated motion of the aorta because elastic fibers and smooth muscle cells are strongly interconnected to function as a whole structure. Such interconnections between elastic fibers and between elastic fibers and smooth muscle cells (Fig. 4) are observed not only in humans but also in other animals; therefore, this structure seems to be a universal one necessary to maintain medial integrity against various forces.15, 20)
Fig. 3.
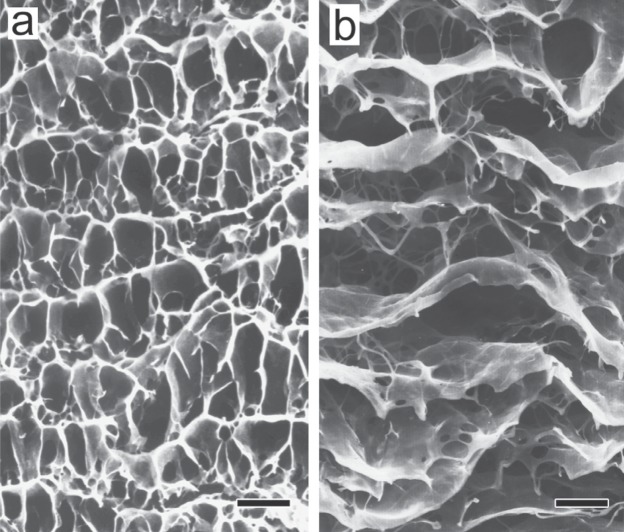
Three-dimensional architecture of elastic fibers in the human aortic media. a: Normal subject. b: Aortic dissection patient. The aorta was treated with formic acid to remove the components other than the elastic fibers, and then examined by scanning electron microscopy. Upper, intimal side. Lower, adventitial side. Scale bar = 20 µm (Reproduced from Ref. 12).
Fig. 4.
Interconnections between elastic fibers and smooth muscle cells. Black-appearing elastic fibers (E) and the processes of smooth muscle cells are connected with one another at many sites. The rat aortic media was examined by transmission electron microscopy after tannic acid staining. Scale bar = 1 µm (Reproduced from Ref. 17).
Changes in the Architecture of Medial Elastic Fibers in Aortic Dissection: Implications for the Mechanism of Aortic Dissection
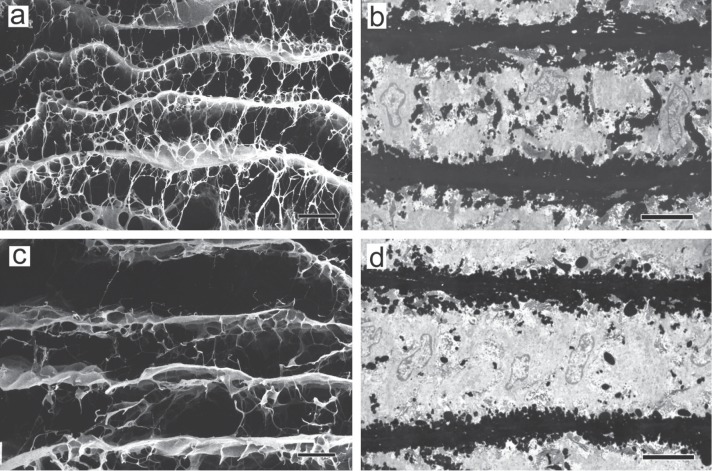
As shown in Fig. 3b, a three-dimensional scanning electron microscopic study of the structure of medial elastic fibers in aortic dissection patients using the same method as that for the normal aortic media revealed no significant changes in concentric elastic laminae (running horizontally in the figure) compared with those in normal subjects (Fig. 3a), but a marked reduction in interlaminar elastic fibers (running vertically in the figure) .17) Identical findings were observed in rats with aortic dissection induced experimentally by β-aminopropionitrile (BAPN).20) Fig. 5a shows the structure of elastic fibers in the normal rat aortic media, composed basically of concentric elastic laminae with connecting interlaminar elastic fibers, as in the normal human aortic media. Transmission electron microscopy confirmed this structure, showing the presence of many black interlaminar elastic fibers between horizontally running black elastic laminae, as shown in Fig. 5b. On the other hand, in the aortic media of BAPN-treated rats, scanning (Fig. 5c) and transmission (Fig. 5d) electron microscopy showed that the structure of concentric elastic laminae was almost normal, but interlaminar elastic fibers were markedly reduced. Thus, the observation of similar, species-independent changes strongly suggests that changes in the architecture of elastic fibers play a very important role in the development of aortic dissection.
Fig. 5.
The architecture of elastic fibers in experimental aortic dissection. a & b: Normal rat. c & d: β-aminopropionitrile-treated rat. a & c, The aorta was treated with formic acid to remove the components other than the elastic fibers, and then examined by scanning electron microscopy. Scale bar = 20 µm. b & d, The aorta was examined by transmission electron microscopy after tannic acid staining. Scale bar = 5 µm. Upper, intimal side. Lower, adventitial side (Reproduced from Ref. 20).
We can extrapolate interesting mechanical changes from the structural changes of elastic fibers. That is, a reduction in interlaminar elastic fibers may result in the weakening of the connection between elastic laminae and that between elastic fibers and smooth muscle cells, thereby reducing the integrity of the tunica media. In extreme terms, this architecture can be likened to a deck of cards, that is, a structure with no vertical interconnection. Such a structure has little resistance to a tearing (Fig. 6a) or shear force (Fig. 6b). Little resistance to a tearing force means little resistance to a dissection-promoting force involved in aortic dissection. Blanton et al. developed a dog model of aortic dissection by creating an artificial intimal tear in the normal dog aorta to cause medial dissection.21) This model shows that medial dissection can occur even when the architecture of the aorta is normal, that is, the components of the lamellar units are normally interconnected. However, a review of the literature shows that the dissection is narrow and localized. On the other hand, in humans, aortic dissection is often extensive, probably as a result of the weakened connection between the elastic laminae in the tunica media, as described above. At autopsy on patients with aortic dissection, we often accidentally increase the extent of medial dissection by touching the aorta, which also suggests that the tunica media has little resistance to tearing forces.
Fig. 6.
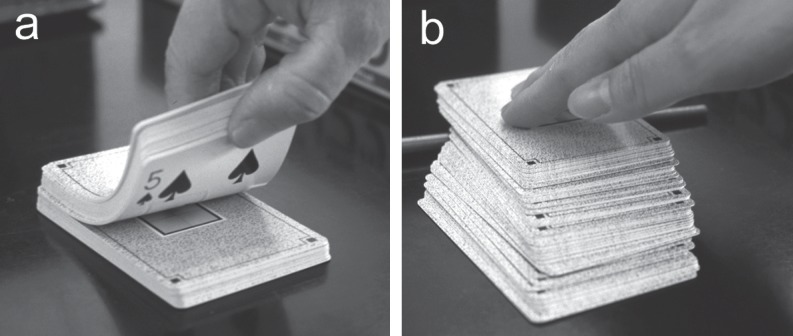
The weakness of lamellar structures is simulated by a stack of playing cards. a: A tearing force is applied. b: A shear force is applied.
On the other hand, what we should think of the decreased resistance to a shear force is a difficult question. First, let us consider the force sustained by the aorta. The thoracic aorta is known to move in the long axis direction on every heart beat, and it has been noted that this force may play an important role in the development of dissection.9, 22) In a recent analysis using a finite element model, the largest stress increase due to aortic root displacement was identified approximately 2 cm above the sinotubular junction,22) which, interestingly, is the most common site for the development of an intimal tear.12) On the other hand, Anagnostopoulos noted the possibility that the medial strain produced by stress from blood flow in the aortic lumen strongly influences the development of an intimal tear.23) These points of view are very attractive in suggesting that the stress on the aortic wall is sufficiently great to cause aortic dissection. In other words, it is possible to extrapolate that, in the presence of architectural changes in elastic fibers, as shown in Figs. 3b and 5c, a force associated with the motion of the aorta or that of blood flow in the aortic lumen produces a strain (Fig. 6b), which causes medial dissection. What is important is that this extrapolation applies to both classic aortic dissection and IMH. So, we consider that if medial strain affects the intima, dissection with intimal tear occurs, resulting in classic aortic dissection. On the other hand, when medial strain tends to extend in the lateral direction, medial dissection alone may occur, leading to IMH.
Elastic Fiber and Elastin Abnormalities in Aortic Dissection: Literature Review
Studies using other animal models have also reported that abnormalities in elastin, the main component of elastic fibers, have an important significance in the development of aortic dissection. The research group of Daugherty et al. reported that the administration of angiotensin II to apolipoprotein E-deficient mice resulted in the development of localized aortic dissection, followed by the dilation of the lumen and subsequent development of an aneurysm.24) The importance of this report is that medial elastic fiber degeneration and macrophage accumulation were observed before the development of aortic dissection, suggesting that the structural abnormalities of elastic fibers induced aortic dissection. Also, it has been reported that, in fibrillin-1-deficient mice, elastic fiber fragmentation occurs, and aortic clamping results in the development of dissection.25) Since fibrillin-1 is required for the assembly of microfibrils around elastin, their findings suggest that microfibril abnormalities are involved in elastic fiber fragmentation. In addition, aortic dissection was also reported to occur in biglycan-deficient mice26); however, unlike human aortic dissection, the aortic dissection in this model occurred between the media and adventitia, and no histological changes were observed in elastic fibers. However, since biglycan is believed to be involved in elastogenesis and elastic fiber stabilization, the possibility cannot be excluded that ultrastructural abnormalities of elastic fibers were produced, leading to the development of aortic dissection.
On the other hand, it has been suggested that, in humans, some molecules induce abnormalities of elastic fibers and elastin, thereby being involved in the development of aortic dissection. It is well-known that aortic dissection occurs in Marfan syndrome, in which microfibril abnormalities due to fibrin-1 gene mutations are believed to cause abnormalities in elastic fibers.27) The research group of Wang et al. reported that fibulin-5 expression in the aortic wall was down-regulated in patients with aortic dissection.28) Fibulin-5 is known to have an important function in elastin polymerization, and the possibility has been suggested that decreased fibulin-5 expression is associated with abnormalities in elastin assembly. Peng et al. reported that the amount of medin-derived amyloid deposited in the aortic media was lower, and that of non-amyloid medin was higher, in aortic dissection patients than in a control group.29) Medin amyloid is commonly found in the aortic media of individuals older than 50 years of age, and is a molecule in close contact with elastic fibers. Medin, an immature amyloid, has been suggested to be cytotoxic, and the possibility has been noted that medin may act on smooth muscle cells to increase the expression of matrix metalloproteinase (MMP)-2 in aortic dissection patients, thereby promoting the degradation of elastin and collagen. Koullias et al. investigated MMP expression in thoracic aortic aneurysm and aortic dissection patients, and reported that MMP-2 and -9 expression levels in the aortic media were higher in the aortic dissection than in the thoracic aortic aneurysm patients.30) Since MMP-2 and -9 are involved in the degradation of both collagen and elastin, these results suggest that elastin degradation is increased in aortic dissection. In addition, aortic dissection patients are often complicated by hypertension,7, 10, 11, 12) suggesting that hypertension is closely involved in the development of aortic dissection by causing abnormalities in the architecture of elastic fibers in the aortic media.17) These abnormalities may have resulted from some changes in the metabolism of elastin and myofibrils, but much of their cellular and molecular biological mechanisms is unknown.
In addition to elastic fibers, other extracellular matrix components such as collagen fibers and glycosaminoglycans are present in the aortic media, and are involved in maintenance of the aortic wall integrity. Abnormalities in these components have also been noted in aortic dissection patients. For example, the composition of collagen fibers has been reported to vary with the location of the aorta.31) Among glycosaminoglycans, the content of dermatan sulfate and hyaluronate has been reported to be increased in the areas of aortic dissection, suggesting that these localized increases are related to the development of aortic dissection.32) In electron microscopic and immunohistochemical studies of cases of aortic dissection, Ishii et al. observed the presence of spirally thickened collagen fibers, thinning and loss of the basement membrane of smooth muscle cells, and marked expression of MMP-1, -2, and -9 and tissue inhibitors of metalloproteinase (TIMP)-1 and -2, suggesting that overall changes in extracellular matrix components have an important significance in the development of aortic dissection.33)
Conclusion
Despite marked advances in the diagnostic imaging and treatment of aortic dissection, pathological research is lagging behind. This seems to be mainly due to the fact that pathological knowledge is not necessarily required for the diagnosis and treatment of acute aortic dissection. However, basic pathological, biochemical, and physiological knowledge lay the foundations for better diagnosis and treatment. Needless to say, when considering disease prevention, it is necessary to recognize abnormalities in the aortic wall before the occurrence of the catastrophic event of aortic dissection, and address them. Undoubtedly, medial weakness forms a basis for aortic dissection, and the weakness is most likely to be due to the structural abnormalities of elastic fibers, the main component of the media. In the future, it will be important to conduct a detailed study of the pathogenesis of their structural abnormalities. For example, considering the close association between aortic dissection and hypertension, it is an important problem to elucidate the effects of hypertension on elastin and microfibrils and their mechanism employing pathological methods. In addition, the pathophysiological study of the force generated by blood flow, blood pressure, or the motion of the aorta is also necessary.
References
- Nienaber CA, Eagle KA. Aortic dissection: New frontiers in diagnosis and management. Part I: From etiology to diagnostic strategies. Circulation. 2003; 108: 628–35 [DOI] [PubMed] [Google Scholar]
- Nienaber CA, Eagle KA. Aortic dissection: New frontiers in diagnosis and management. Part II: Therapeutic management and follow-up. Circulation. 2003; 108: 772–778 [DOI] [PubMed] [Google Scholar]
- DeBakey ME, Henly WS, Cooley DA, Morris GC, Jr., Crawford ES, Beall AC., Jr.Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965; 49: 130–149 [PubMed] [Google Scholar]
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation. 1999; 99: 1331–1336 [DOI] [PubMed] [Google Scholar]
- von Kodolitsch Y, Csösz SK, Koschyk DH, Schalwat I, Loose R, Karck M, Dieckmann C, Fattori R, Haverich A, Berger J, Meinertz T, Nienaber CA. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation. 2003; 107: 1158–1163 [DOI] [PubMed] [Google Scholar]
- Sawhney NS, DeMaria AN, Blanchard DG. Aortic intramural hematoma: An increasingly recognized and potentially fatal entity. Chest. 2001; 120: 1340–1346 [DOI] [PubMed] [Google Scholar]
- Gore I, Hirst AE., Jr.Dissecting aneurysm of the aorta. Cardiovasc Clin. 1973; 5: 239–260 [PubMed] [Google Scholar]
- Vilacosta I, San Román JA, Ferreirós J, Aragoncillo P, Méndez R, Castillo JA, Rollán MJ, Batlle E, Peral V, Sánchez-Harguindey L. Natural history and serial morphology of aortic intramural hematoma: a novel variant of aortic dissection. Am Heart J. 1997; 134: 495–507 [DOI] [PubMed] [Google Scholar]
- Wheat MW, Palmer RF. Dissecting aneurysms of the aorta. Curr Probl Surg. 1971: 1–43 [PubMed] [Google Scholar]
- Wilson SK, Hutchins GM. Aortic dissecting aneurysms: causative factors in 204 subjects. Arch Pathol Lab Med. 1982; 106: 175–180 [PubMed] [Google Scholar]
- Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984; 53: 849–855 [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Kurozumi T, Sueishi K, Tanaka K. Dissecting aneurysm: a clinicopathologic and histopathologic study of 111 autopsied cases. Hum Pathol. 1990; 21: 291–296 [DOI] [PubMed] [Google Scholar]
- Homme JL, Aubry MC, Edwards WD, Bagniewski SM, Pankratz VS, Kral CA, Tazelaar HD. Surgical pathology of the ascending aorta: A clinicopathologic study of 513 cases. Am J Surg Pathol. 2006; 30: 1159–1168 [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967; 20: 99–111 [DOI] [PubMed] [Google Scholar]
- Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis. 1985; 5: 19–34 [DOI] [PubMed] [Google Scholar]
- Carson MW, Roach MR. The strength of the aortic media and its role in the propagation of aortic dissection. J Biomech. 1990; 23: 579–588 [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Shiokawa Y, Sueishi K. Alterations of elastic architecture in human aortic dissecting aneurysm. Lab Invest. 1990; 62: 751–760 [PubMed] [Google Scholar]
- Dingemans KP, Jansen N, Becker AE. Ultrastructure of the normal human aortic media. Virchows Arch A Pathol Anat Histol. 1981; 392: 199–216 [DOI] [PubMed] [Google Scholar]
- Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000; 258: 1–14 [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Sueishi K. Alteration of elastic architecture in the lathyritic rat aorta implies the pathogenesis of aortic dissecting aneurysm. Am J Pathol. 1992; 140: 959–969 [PMC free article] [PubMed] [Google Scholar]
- Blanton FS, Muller WH, Warren WD. Experimental production of dissecting aneurysms of the aorta. Surgery. 1959; 45: 81–90 [PubMed] [Google Scholar]
- Beller CJ, Labrosse MR, Thubrikar MJ, Robicsek F. Role of aortic root motion in the pathogenesis of aortic dissection. Circulation. 2004; 109: 763–769 [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos CE. Acute aortic dissection. University Park Press; 1975: 1–99 [Google Scholar]
- Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003; 23: 1621–1626 [DOI] [PubMed] [Google Scholar]
- Matt P, Huso DL, Habashi J, Holm T, Doyle J, Schoenhoff F, Liu G, Black J, Van Eyk JE, Dietz HC. Murine model of surgically induced acute aortic dissection type A. J Thorac Cardiovasc Surg. 2010; 139: 1041–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007; 115: 2731–2738 [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation. 2005; 111: e150–e157 [DOI] [PubMed] [Google Scholar]
- Wang X, LeMaire SA, Chen L, Carter SA, Shen YH, Gan Y, Bartsch H, Wilks JA BA, Utama B, Ou H, Thompson RW, Joseph S, Coselli JS, Wang XL. Decreased expression of fibulin-5 correlates with reduced elastin in thoracic aortic dissection. Surgery. 2005; 138: 352–359 [DOI] [PubMed] [Google Scholar]
- Peng S, Larsson A, Wassberg E, Gerwins P, Thelin S, Fu X, Westermark P. Role of aggregated medin in the pathogenesis of thoracic aortic aneurysm and dissection. Lab Invest. 2007; 87: 1195–1205 [DOI] [PubMed] [Google Scholar]
- Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2004; 78: 2106–2111 [DOI] [PubMed] [Google Scholar]
- Whittle MA, Robins SP, Hasleton PS, Anderson JC. Biochemical investigation of possible lesions in human aorta that predispose to dissecting aneurysms: pyridinoline crosslinks. Cardiovasc Res. 1987; 21: 161–168 [DOI] [PubMed] [Google Scholar]
- Cattell MA, Hasleton PS, Anderson JC. Glycosaminoglycan content is increased in dissecting aneurysms of human thoracic aorta. Clin Chim Acta. 1994; 226: 29–46 [DOI] [PubMed] [Google Scholar]
- Ishii T, Asuwa N. Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum Pathol. 2000; 31: 640–646 [DOI] [PubMed] [Google Scholar]