Abstract
We report the successful treatment of thoracoabdominal dissection, which extended into the left iliac artery, despite two independent complications: graft infection and a relatively rare, delayed postoperative paraplegia. The paraplegia suddenly occurred on postoperative day 10, and after an intravenous infusion of heparin and methylprednisolone, it gradually subsided. Moreover, graft infection was diagnosed on postoperative day 27, and with continuous irrigation of antibiotic treatment it was cured without recurrence of infection. Although anticoagulation therapy is not indicated for paraplegia, we suppose that it might be used as an adjunct therapeutic.
Keywords: delayed postoperative paraplegia, graft infection, thoracoabdominal dissection
Introduction
Spinal cord injury is the most critical complication in the treatment of descending thoracic and thoracoabdominal aneurysms, either involving open surgical repair or endovascular techniques.1–3) In contrast to immediate spinal cord deficits, delayed neurologic injury has attracted little attention and remains poorly understood.4) Graft infection is a life-threatening and treatment-resistant complication, especially after thoracoabdominal aneurysm repair. We report successful treatment of thoracoabdominal dissection, which extended into left iliac artery, despite two independent complications of relatively rare, delayed postoperative paraplegia and graft infection.
Case
A 54-year-old man with a history of hypertension had sudden-onset back pain in May 2009 and was referred to the Tottori Red Cross Hospital, Tottori, Japan. The patient was prescribed antihypertensive medication and was discharged; however, one month later, he returned to the hospital with another episode of sudden-onset back pain. Enhanced chest and abdominal computed tomography (CT) on the initial admission revealed thoracoabdominal dissection, which extended into the left common iliac artery (CIA) measuring 55 × 68 mm in maximum diameter. Physical examination was unremarkable except for being overweight (height, 172 cm; weight, 90 kg). The results of blood examination, electrocardiogram, and echocardiography were normal. The CT on the second admission revealed another dissection and expansion of the aneurysm measuring 61 × 71 mm in diameter (Fig. 1). All abdominal branches originated from the true lumen. After the dissection and expansion of the aneurysm, we decided to operate by employing a four-branched graft for the proximal part and a bifurcated graft for the distal part. During surgery, we drained the cerebrospinal fluid (CSF) and monitored transcranial motor evoked potentials (MEP). We repositioned the patient in the right lateral decubitus position and made the incision at the seventh intercostal space to the costal cartilage, proceeded to cut obliquely to the umbilicus and then to above the symphysis pubis. A retroperitoneal approach exposed the aorta. After the distal sites of the bilateral external iliac arteries were anastomosed to the bifurcated grafts, we established a percutaneous cardiopulmonary system (PCPS) by inserting a cannula into the arterial branch of the bifurcated graft and then through the left renal vein to the right atrium. We anastomosed the two grafts, celiac trunk, superior mesenteric artery (SMA), two left renal arteries (RAs), right RA, the proximal sites of the descending aorta at the level of Th8, and inferior mesenteric artery (IMA) by sequential segmental repair under normothermic partial cardiopulmonary bypass without cardiac arrest. We also attempted the reattachment of the intercostal arteries, but the aortic wall was too fragile to reconstruct the intercostal arteries. During the operation, the CSF and MEP were normal. The clamp time of the CA, SMA, one left RA, another RA, a right RA, and the descending aorta was 22, 16, 18, 16, 73, and 81 min, respectively. The total PCPS time was 394 min. The lowest temperature during cardiopulmonary bypass was 34.7°C.
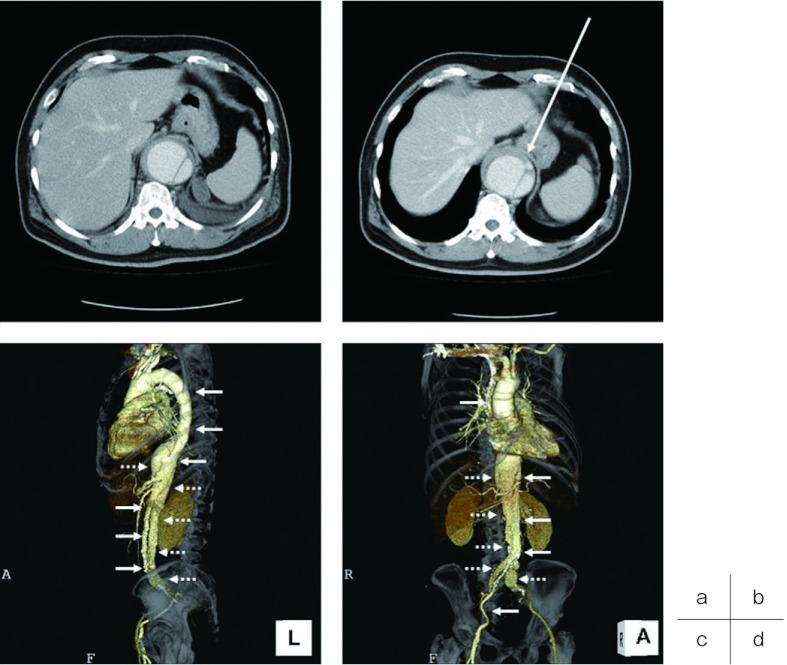
Fig. 1.
Preoperative enhanced CT.
a) Enhanced CT on the first admission reveals a dissecting thoracoabdominal aortic aneurysm measuring 55 × 68 mm in maximum diameter. b) Enhanced CT on the second admission shows another dissection and expansion of the aneurysm, measuring 61 × 71 mm in diameter. c) Lateral view of three-dimensional CT (3DCT) showing a true (solid arrow) and false (dotted arrow) lumen. d) Right anterior oblique view of 3DCT showing a true (solid arrow) and false (dotted arrow) lumen.
CT, computed tomography
The postoperative CT before the onset of delayed paraplegia showed the occlusion of the right RA and IMA, as well as occlusion of intercostal arteries (Fig. 2). On postoperative day 2, we removed the CSF drain, and the patient received intravenous infusion of heparin (15000 units/day) and 1500 mg of methylprednisolone for three days, followed by the administration of warfarin (3 mg/day) and ticlopidine (200 mg/day). On day 10, motor and sensory functions of the lower extremities were impaired, but fortunately, the paraplegia gradually recovered within 6 hours. Moreover, on day 27, the patient developed a fever and the abdomen was somewhat distended. The CT showed massive fluid accumulation in the left retroperitoneal space (Fig. 3a). Thus, we placed two drainage tubes into the space with continuous irrigation (saline solution of 250 ml/hour) for 14 days and intermittent irrigation (saline solution of 1000 ml / 4 hours, twice a day) for 5 days (Fig. 3b). Since the space was infected with Klebsiella oxytoca (KSBL), sulbactam/ cefoperazone (SBT/ CPZ, 2 g/day for 6 days) and cefmetazole (CMZ, 1 g/day for 2 weeks) were administered intravenously. Closure of the wound was achieved without any residual sign of infection, and the patient was discharged on the postoperative day 60. Later, as a preventative measure, the wound was irrigated daily with minocycline (MINO, 200 mg/day for 1 year).
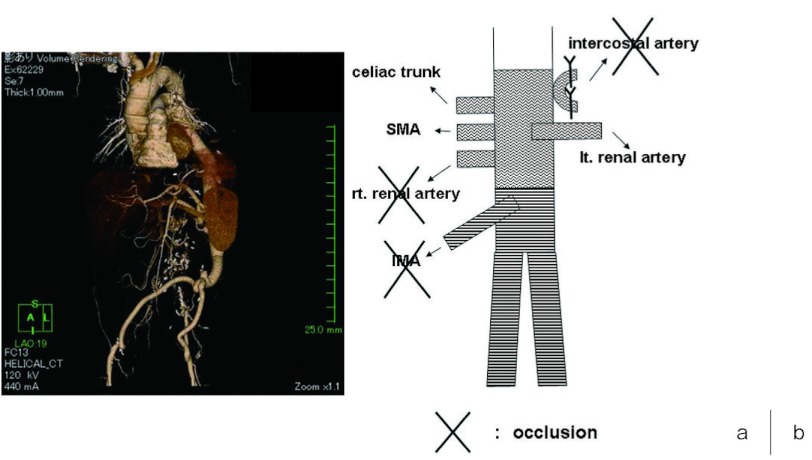
Fig. 2.
a) Postoperative CT scan before the onset of delayed paraplegia.
Scan shows occlusion of the right renal artery and IMA as well as intercostal arteries, patency of celiac trunk, SMA and left renal artery.
b) The illustration of the postoperative CT.
CT, computed tomography; IMA, inferior mesenteric artery; SMA, superior mesenteric artery
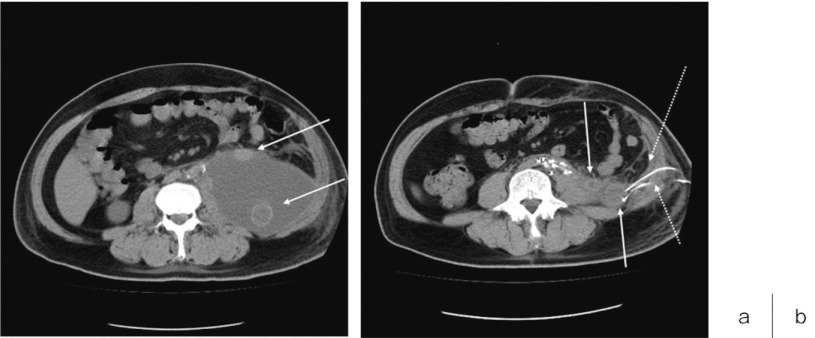
Fig. 3.
a) Postoperative CT scan of postoperative day 27 of two grafts (solid arrow) in the massive fluid accumulation in the left retroperitoneal space.
b) CT scan of resolved fluid accumulation in the left retroperitoneal space after two drainage tubes (dotted arrow) were placed for the closed irrigation.
CT, computed tomography
Discussion
Spinal cord injury is the most critical complication in the treatment of descending thoracic and thoracoabdominal aneurysms that either involve open surgical repair or endovascular strategies.1–3) In contrast to immediate spinal cord deficits, delayed neurologic injury has attracted little attention in the literature and remains poorly understood.4) Wong et al.5) in their review, reported that the rates of immediate and delayed paraplegia after major TAA repair are 1.7–8.1 and 0.8%–8.1%, respectively. They also described the therapies used to treat delayed paraplegia or paraparesis after thoracoabdominal aortic aneurysm repair in 34 patients: corticosteroids (82%), osmotic diuresis (76%), vasopressor agents (21%), and red blood cell transfusion and intravenous naloxone (6%), as well as CSF drainage (continuation with an existing drain) (9%), drain reinsertion (29%), and the insertion of a new drain (29%).5) Safi HJ and collegues6) reported that they keep the mean arterial pressure greater than 90 to 100 mmHg, hemoglobin greater than 10 mg/dL, and cardiac index greater than 2.0 L/min to optimize postoperative spinal cord perfusion and oxygen delivery. As we retrospectively found that the blood pressure (systolic pressure: 100–110 mmHg) was lower than normal from postoperative day 5 after taking antihypertensive medication, the present case may be consistent with the previous reports that delayed paraplegia probably results from a state of malperfusion and postoperative hypotension, leading to spinal cord malperfusion.7, 8) However, when we consider why it took until postoperative day 5 for the paraplegia to occur, we agree with the report that hypotension is associated with some, but not all, delayed-onset deficits, and other factors may have been important, including rheologic and thrombotic factors.5) Moreover, the perfusion of the spinal cord depends on the stabilization of the collateral network of remaining segmental arteries (SAs) after the serial surgical sacrifice of SAs during aneurysm resection.7) In the present case, as we can immediately get methylprednisolone and heparin even on the bed side, we chose 1500 mg of methylprednisolone and the intravenous perfusion of heparin (15000 units/day), along with the administration of warfarin (3 mg/day) and ticlopidine (200 mg/day) to keep a rich collateral network. Although the exact mechanism of delayed paraplegia is unknown and anticoagulation therapy is not popular for the paraplegia, we speculate that anticoagulation therapy may be one of the adjunctive therapeutic strategies to maintain a rich collateral network.
Graft infection is a life-threatening and treatment-resistant complication, especially after thoracoabdominal aneurysm repair. However, in some cases, resection and re-replacement of the infected graft is almost impossible.9) Wound irrigation techniques are classified into closed and open irrigation.10) In the present case, we resolved the infection with appropriate antibiotics by a closed irrigation.
References
- 1.Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg 2007; 83: S865-9 [DOI] [PubMed] [Google Scholar]
- 2.Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg 2007; 83: S856-61 [DOI] [PubMed] [Google Scholar]
- 3.Roselli EE, Greenberg RK, Pfaff K, Francis C, Svensson LG, Lytle BW. Endovascular treatment of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2007; 133: 1474-82 [DOI] [PubMed] [Google Scholar]
- 4.LeMaire SA, Miller CC, 3rd, Conklin LD, Schmittling ZC, Coselli JS. Estimating group mortality and paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2003; 75: 508-13 [DOI] [PubMed] [Google Scholar]
- 5.Wong DR, Coselli JS, Amerman K, Bozinovski J, Carter SA, Vaughn WK.Delayed spinal cord deficits after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2007; 83: 1345-55 [DOI] [PubMed] [Google Scholar]
- 6.Safi HJ, Huynh TT, Estrera AL, Miller CC., III2005: 1490–511. [Google Scholar]
- 7.Etz CD, Luehr M, Kari FA, Bodian CA, Smego D, Plestis KA.Paraplegia after extensive thoracic and thoracoabdominal aortic aneurysm repair: does critical spinal cord ischemia occur postoperatively? J Thorac Cardiovasc Surg 2008; 135: 324-30 [DOI] [PubMed] [Google Scholar]
- 8.Azizzadeh A, Huynh TT, Miller CC, 3rd, Estrera AL, Porat EE, Sheinbaum R.Postoperative risk factors for delayed neurologic deficit after thoracic and thoracoabdominal aortic aneurysm repair: a case-control study. J Vasc Surg 2003; 37: 750-4 [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya M, Taketani T, Kubota H, Ohtsuka T, Takamoto S. Open retroperitoneal irrigation for graft infection after thoracoabdominal aortic aneurysm repair. Jpn J Thorac Cardiovasc Surg 2003; 51: 37-40 [DOI] [PubMed] [Google Scholar]
- 10.Acinapura AJ, Godfrey N, Romita M, Cunningham J, Jr, Adams PX, Jacobowitz IJ.Surgical management of infected median sternotomy: closed irrigation vs. muscle flaps. J Cardiovasc Surg (Torino) 1985; 26: 443-6 [PubMed] [Google Scholar]