Abstract
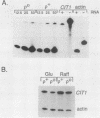
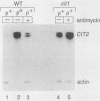
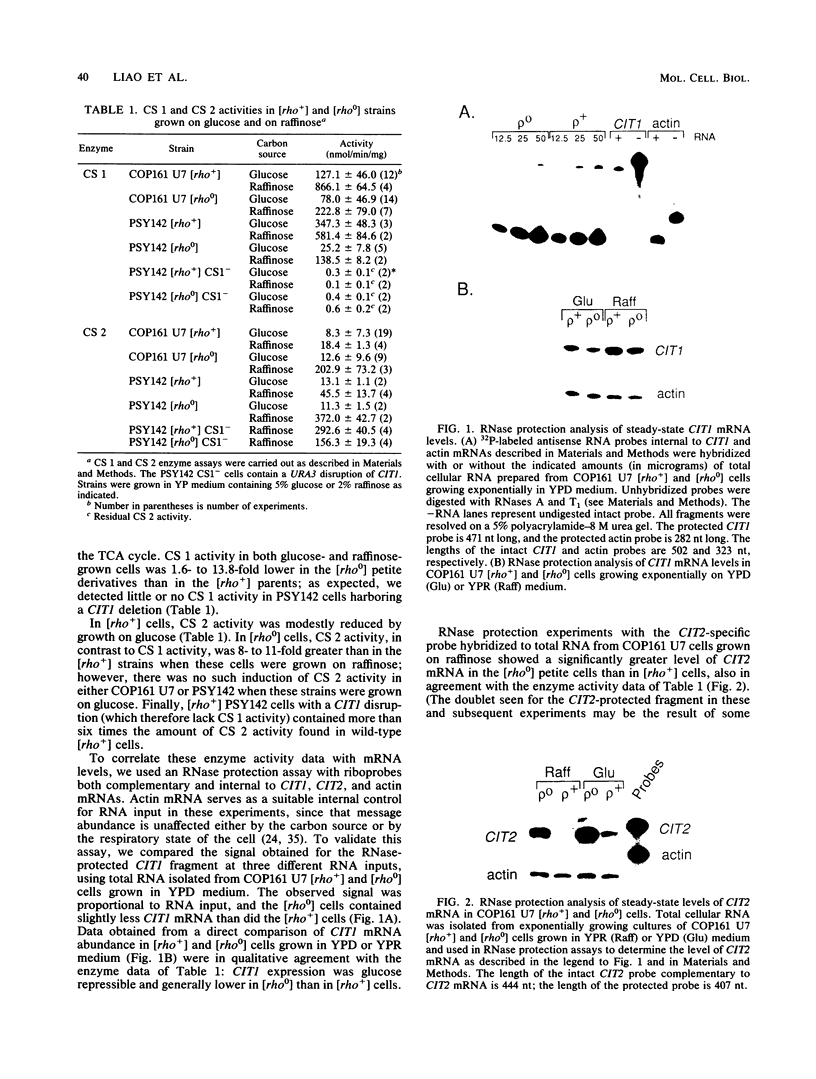
We have examined the effects of perturbation of mitochondrial function on expression of two nuclear genes encoding the mitochondrial and peroxisomal forms of citrate synthase in Saccharomyces cerevisiae, CIT1 and CIT2. CIT2 expression was as much as 30-fold higher in [rho0] petites, than in isochromosomal [rho+] cells, whereas CIT1 expression was slightly down regulated in [rho0] cells. CIT2 expression was also increased in [rho+] cells by inhibition of respiration with antimycin A or in [rho+] cells containing a disruption of the CIT1 gene. These effects were additive, and together they approached the level of CIT2 expression seen in [rho0] cells. Experiments using heterologous gene fusions showed that all of the effects leading to increased expression of CIT2 were transcriptionally controlled through 5'-flanking CIT2 DNA sequences. Analysis of [rho+] and [rho0] cells containing disruptions of CIT1 and CIT2, singly and in combination, showed that the peroxisomal citrate synthase could partially spare the mitochondrial isoform for growth yield in [rho+] but not in [rho0] cells. These studies suggest a physiological role for increased expression of CIT2 in cells with altered mitochondrial function. They also provide additional evidence for a retrograde path of communication from mitochondria to the nucleus in yeast cells.
Full text
PDF

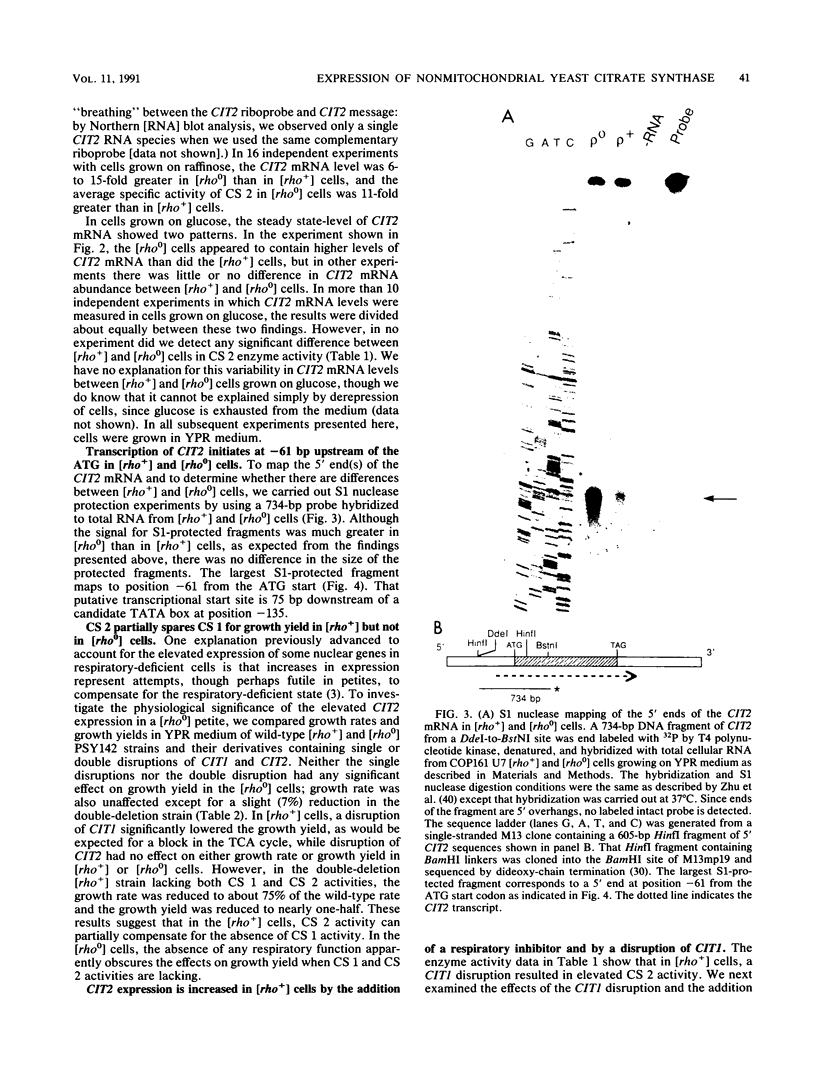
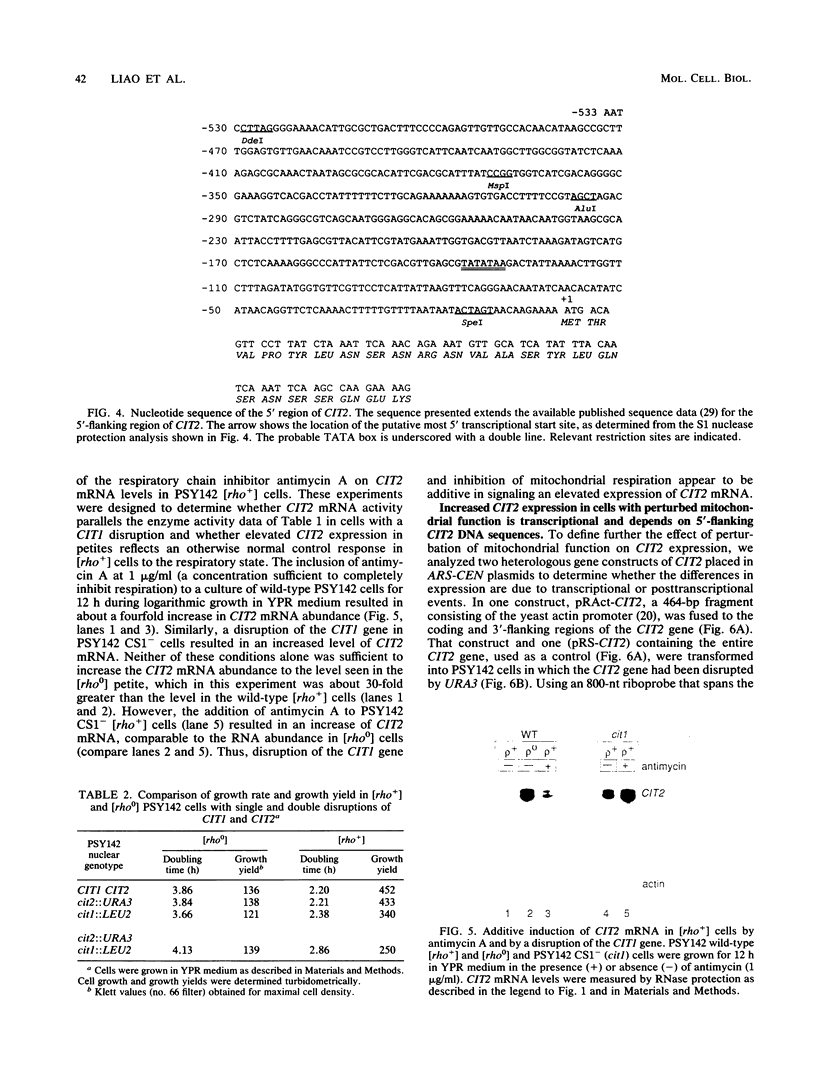




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butow R. A., Docherty R., Parikh V. S. A path from mitochondria to the yeast nucleus. Philos Trans R Soc Lond B Biol Sci. 1988 May 31;319(1193):127–133. doi: 10.1098/rstb.1988.0037. [DOI] [PubMed] [Google Scholar]
- Dang H., Franklin G., Darlak K., Spatola A. F., Ellis S. R. Discoordinate expression of the yeast mitochondrial ribosomal protein MRP1. J Biol Chem. 1990 May 5;265(13):7449–7454. [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Douglas M. G., Geller B. L., Emr S. D. Intracellular targeting and import of an F1-ATPase beta-subunit-beta-galactosidase hybrid protein into yeast mitochondria. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3983–3987. doi: 10.1073/pnas.81.13.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion E. A., Warner J. R. The major promoter element of rRNA transcription in yeast lies 2 kb upstream. Cell. 1984 Dec;39(3 Pt 2):663–673. doi: 10.1016/0092-8674(84)90473-2. [DOI] [PubMed] [Google Scholar]
- Fearon K., Mason T. L. Structure and regulation of a nuclear gene in Saccharomyces cerevisiae that specifies MRP7, a protein of the large subunit of the mitochondrial ribosome. Mol Cell Biol. 1988 Sep;8(9):3636–3646. doi: 10.1128/mcb.8.9.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Guarente L. Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol. 1989;5:153–180. doi: 10.1146/annurev.cb.05.110189.001101. [DOI] [PubMed] [Google Scholar]
- Guarente L., Lalonde B., Gifford P., Alani E. Distinctly regulated tandem upstream activation sites mediate catabolite repression of the CYC1 gene of S. cerevisiae. Cell. 1984 Feb;36(2):503–511. doi: 10.1016/0092-8674(84)90243-5. [DOI] [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Rosenkrantz M. S., Guarente L. Saccharomyces cerevisiae contains two functional citrate synthase genes. Mol Cell Biol. 1986 Jun;6(6):1936–1942. doi: 10.1128/mcb.6.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G., Evans C. T., Malloy C., Srere P. A. Metabolic studies on citrate synthase mutants of yeast. A change in phenotype following transformation with an inactive enzyme. J Biol Chem. 1989 Jul 5;264(19):11204–11210. [PubMed] [Google Scholar]
- Kispal G., Rosenkrantz M., Guarente L., Srere P. A. Metabolic changes in Saccharomyces cerevisiae strains lacking citrate synthases. J Biol Chem. 1988 Aug 15;263(23):11145–11149. [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A. S., Hines V., Small G. M. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990 Apr;10(4):1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczynski G. T., Schultz P. W., Jaehning J. A. Use of yeast nuclear DNA sequences to define the mitochondrial RNA polymerase promoter in vitro. Mol Cell Biol. 1989 Aug;9(8):3193–3202. doi: 10.1128/mcb.9.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez de castro I., Arias de Saavedra J. M., Machado A., Mayor F. Regulation of the level of yeasts citrate synthase by oxygen availability. Mol Cell Biochem. 1976 Sep 30;12(3):161–169. doi: 10.1007/BF01741714. [DOI] [PubMed] [Google Scholar]
- Olesen J., Hahn S., Guarente L. Yeast HAP2 and HAP3 activators both bind to the CYC1 upstream activation site, UAS2, in an interdependent manner. Cell. 1987 Dec 24;51(6):953–961. doi: 10.1016/0092-8674(87)90582-4. [DOI] [PubMed] [Google Scholar]
- Parikh V. S., Conrad-Webb H., Docherty R., Butow R. A. Interaction between the yeast mitochondrial and nuclear genomes influences the abundance of novel transcripts derived from the spacer region of the nuclear ribosomal DNA repeat. Mol Cell Biol. 1989 May;9(5):1897–1907. doi: 10.1128/mcb.9.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V. S., Morgan M. M., Scott R., Clements L. S., Butow R. A. The mitochondrial genotype can influence nuclear gene expression in yeast. Science. 1987 Jan 30;235(4788):576–580. doi: 10.1126/science.3027892. [DOI] [PubMed] [Google Scholar]
- Partaledis J. A., Mason T. L. Structure and regulation of a nuclear gene in Saccharomyces cerevisiae that specifies MRP13, a protein of the small subunit of the mitochondrial ribosome. Mol Cell Biol. 1988 Sep;8(9):3647–3660. doi: 10.1128/mcb.8.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickey T. M., Lewin A. S. Extramitochondrial citrate synthase activity in bakers' yeast. Mol Cell Biol. 1986 Feb;6(2):488–493. doi: 10.1128/mcb.6.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Botstein D. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Methods Enzymol. 1983;101:167–180. doi: 10.1016/0076-6879(83)01012-5. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz M., Alam T., Kim K. S., Clark B. J., Srere P. A., Guarente L. P. Mitochondrial and nonmitochondrial citrate synthases in Saccharomyces cerevisiae are encoded by distinct homologous genes. Mol Cell Biol. 1986 Dec;6(12):4509–4515. doi: 10.1128/mcb.6.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa M., Suda K., Schatz G. Isolation of the nuclear yeast genes for citrate synthase and fifteen other mitochondrial proteins by a new screening method. EMBO J. 1984 Aug;3(8):1773–1781. doi: 10.1002/j.1460-2075.1984.tb02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely E., Montgomery D. L. Glucose represses transcription of Saccharomyces cerevisiae nuclear genes that encode mitochondrial components. Mol Cell Biol. 1984 May;4(5):939–946. doi: 10.1128/mcb.4.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawick J. D., Wright R. M., Poyton R. O. Transcription of yeast COX6, the gene for cytochrome c oxidase subunit VI, is dependent on heme and on the HAP2 gene. J Biol Chem. 1989 Apr 25;264(12):7005–7008. [PubMed] [Google Scholar]
- Tzagoloff A., Myers A. M. Genetics of mitochondrial biogenesis. Annu Rev Biochem. 1986;55:249–285. doi: 10.1146/annurev.bi.55.070186.001341. [DOI] [PubMed] [Google Scholar]
- Winkler H., Adam G., Mattes E., Schanz M., Hartig A., Ruis H. Co-ordinate control of synthesis of mitochondrial and non-mitochondrial hemoproteins: a binding site for the HAP1 (CYP1) protein in the UAS region of the yeast catalase T gene (CTT1). EMBO J. 1988 Jun;7(6):1799–1804. doi: 10.1002/j.1460-2075.1988.tb03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Conrad-Webb H., Liao X. S., Perlman P. S., Butow R. A. Functional expression of a yeast mitochondrial intron-encoded protein requires RNA processing at a conserved dodecamer sequence at the 3' end of the gene. Mol Cell Biol. 1989 Apr;9(4):1507–1512. doi: 10.1128/mcb.9.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]