Abstract
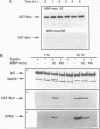
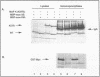
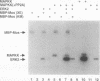
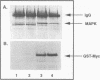
Several protein kinases, including Mos, maturation-promoting factor (MPF), mitogen-activated protein (MAP) kinase, and MAP kinase kinase (MAPKK), are activated when Xenopus oocytes enter meiosis. De novo synthesis of the Mos protein is required for progesterone-induced meiotic maturation. Recently, bacterially synthesized maltose-binding protein (MBP)-Mos fusion protein was shown to be sufficient to initiate meiosis I and MPF activation in fully grown oocytes in the absence of protein synthesis. Here we show that MAP kinase is rapidly phosphorylated and activated following injection of wild-type, but not kinase-inactive mutant, MBP-Mos into fully grown oocytes. MAP kinase activation by MBP-Mos occurs within 20 min, much more rapidly than in progesterone-treated oocytes. The MBP-Mos fusion protein also activates MPF, but MPF activation does not occur until approximately 2 h after injection. Extracts from oocytes injected with wild-type but not kinase-inactive MBP-Mos contain an activity that can phosphorylate MAP kinase, suggesting that Mos directly or indirectly activates a MAPKK. Furthermore, activated MBP-Mos fusion protein is able to phosphorylate and activate a purified, phosphatase-treated, rabbit muscle MAPKK in vitro. Thus, in oocytes, Mos is an upstream activator of MAP kinase which may function through direct phosphorylation of MAPKK.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Seger R., Krebs E. G. The mitogen-activated protein kinase activator. Curr Opin Cell Biol. 1992 Dec;4(6):992–999. doi: 10.1016/0955-0674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Barrett C. B., Schroetke R. M., Van der Hoorn F. A., Nordeen S. K., Maller J. L. Ha-rasVal-12,Thr-59 activates S6 kinase and p34cdc2 kinase in Xenopus oocytes: evidence for c-mosxe-dependent and -independent pathways. Mol Cell Biol. 1990 Jan;10(1):310–315. doi: 10.1128/mcb.10.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. Related proteins are phosphorylated at tyrosine in response to mitogenic stimuli and at meiosis. Mol Cell Biol. 1989 Jul;9(7):3143–3147. doi: 10.1128/mcb.9.7.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct 16;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Daar I., Nebreda A. R., Yew N., Sass P., Paules R., Santos E., Wigler M., Vande Woude G. F. The ras oncoprotein and M-phase activity. Science. 1991 Jul 5;253(5015):74–76. doi: 10.1126/science.1829549. [DOI] [PubMed] [Google Scholar]
- Daar I., Zhou R., Shen R. L., Nebreda A., Oskarsson M., Santos E., da Silva P. P., Masui Y., Vande Woude G. F. Mos and Ras: two oncoproteins that display M-phase activity. Cold Spring Harb Symp Quant Biol. 1991;56:477–488. doi: 10.1101/sqb.1991.056.01.054. [DOI] [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Dominguez I., Marshall M. S., Gibbs J. B., García de Herreros A., Cornet M. E., Graziani G., Diaz-Meco M. T., Johansen T., McCormick F., Moscat J. Role of GTPase activating protein in mitogenic signalling through phosphatidylcholine-hydrolysing phospholipase C. EMBO J. 1991 Nov;10(11):3215–3220. doi: 10.1002/j.1460-2075.1991.tb04884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. S., Meyer A. N., Li J., Donoghue D. J. Phosphorylation of conserved serine residues does not regulate the ability of mosxe protein kinase to induce oocyte maturation or function as cytostatic factor. J Cell Biol. 1992 Feb;116(3):725–735. doi: 10.1083/jcb.116.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. S., Pickham K. M., Kanki J. P., Lee B. A., Pena S. V., Donoghue D. J. Xenopus homolog of the mos protooncogene transforms mammalian fibroblasts and induces maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5805–5809. doi: 10.1073/pnas.86.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Moriyama K., Matsuda S., Okumura E., Kishimoto T., Kawasaki H., Suzuki K., Yahara I., Sakai H., Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991 Sep;10(9):2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hattori S., Fukuda M., Yamashita T., Nakamura S., Gotoh Y., Nishida E. Activation of mitogen-activated protein kinase and its activator by ras in intact cells and in a cell-free system. J Biol Chem. 1992 Oct 5;267(28):20346–20351. [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Kosako H., Takenaka K., Moriyama K., Sakai H., Akiyama T., Gotoh Y., Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992 Mar;11(3):973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. M., Masui Y. Cytostatic factor (CSF) activity in cytosols extracted from Xenopus laevis eggs. Exp Cell Res. 1990 Jan;186(1):66–73. doi: 10.1016/0014-4827(90)90211-r. [DOI] [PubMed] [Google Scholar]
- Nakielny S., Cohen P., Wu J., Sturgill T. MAP kinase activator from insulin-stimulated skeletal muscle is a protein threonine/tyrosine kinase. EMBO J. 1992 Jun;11(6):2123–2129. doi: 10.1002/j.1460-2075.1992.tb05271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Okazaki K., Furuno N., Watanabe N., Sagata N. The 'second-codon rule' and autophosphorylation govern the stability and activity of Mos during the meiotic cell cycle in Xenopus oocytes. EMBO J. 1992 Jul;11(7):2433–2446. doi: 10.1002/j.1460-2075.1992.tb05308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori M., L'Allemain G., Weber M. J. Regulation of tetradecanoyl phorbol acetate-induced responses in NIH 3T3 cells by GAP, the GTPase-activating protein associated with p21c-ras. Mol Cell Biol. 1992 Mar;12(3):936–945. doi: 10.1128/mcb.12.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. MAP kinases: charting the regulatory pathways. Science. 1992 Sep 4;257(5075):1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Posada J., Sanghera J., Pelech S., Aebersold R., Cooper J. A. Tyrosine phosphorylation and activation of homologous protein kinases during oocyte maturation and mitogenic activation of fibroblasts. Mol Cell Biol. 1991 May;11(5):2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. J., Cheng M., Zhen E., Vanderbilt C. A., Feig L. A., Cobb M. H. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6924–6928. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy L. M., Singh B., Gautier J., Arlinghaus R. B., Nordeen S. K., Maller J. L. The cyclin B2 component of MPF is a substrate for the c-mos(xe) proto-oncogene product. Cell. 1990 Jun 1;61(5):825–831. doi: 10.1016/0092-8674(90)90192-h. [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar I., Oskarsson M., Showalter S. D., Vande Woude G. F. The product of the mos proto-oncogene as a candidate "initiator" for oocyte maturation. Science. 1989 Aug 11;245(4918):643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya E. K., Polverino A. J., Chang E., Wigler M., Ruderman J. V. Oncogenic ras triggers the activation of 42-kDa mitogen-activated protein kinase in extracts of quiescent Xenopus oocytes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9831–9835. doi: 10.1073/pnas.89.20.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N., Moriguchi T., Ohta K., Gotoh Y., Nishida E. Regulation of a major microtubule-associated protein by MPF and MAP kinase. EMBO J. 1992 Nov;11(11):3977–3984. doi: 10.1002/j.1460-2075.1992.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992 Mar 20;68(6):1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Yew N., Mellini M. L., Vande Woude G. F. Meiotic initiation by the mos protein in Xenopus. Nature. 1992 Feb 13;355(6361):649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]
- Zhou R. P., Oskarsson M., Paules R. S., Schulz N., Cleveland D., Vande Woude G. F. Ability of the c-mos product to associate with and phosphorylate tubulin. Science. 1991 Feb 8;251(4994):671–675. doi: 10.1126/science.1825142. [DOI] [PubMed] [Google Scholar]
- Zhou R. P., Shen R. L., Pinto da Silva P., Vande Woude G. F. In vitro and in vivo characterization of pp39mos association with tubulin. Cell Growth Differ. 1991 May;2(5):257–265. [PubMed] [Google Scholar]
- de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992 Jun 18;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]