Abstract
Intestine has a well-developed lymphatic system that is closely related with its functions, such as mucosal immunological defense or absorption of nutrients. Intestinal lymphoid cells such as lymphocytes, macrophages/monocytes, or dendritic cells are continuously migrating through intestinal mucosa, thereby facilitating their immune responses. Their migrations are well controlled by well-organized molecular mechanisms including adhesion molecules, chemokines, etc. This manuscript will review how dysfunction of lymphoid cell migration is involved in intestinal inflammation, especially in the pathophysiology of intestinal bowel diseases. (*English Translation of J Jpn Coll Angiol 2008; 48: 143-149.)
Keywords: lymphocyte migration, macrophage, dendritic cell, chemokine, intestinal lymphangiectasia
Introduction
The intestine is an organ so rich in lymphoid cells that it is called “a site of controlled inflammation,” and this characteristic is closely related to its immunological defense mechanism. The intestine is also a site of digestion and absorption and characteristically has a dense and diffuse microcirculation network, lined with intestinal epithelial cells, and a very well-developed lymphatic system including the central lymph vessel, which is an important route of lipid absorption. Lymphoid cells consist of lymphocytes, monocytes/macrophages, and dendritic cells, but they do not stay at particular sites but migrate or are washed out into the intestine through the microvessels and lymph vessels, and the disruption of this migration is considered to be closely related to pathologic conditions including inflammatory bowel disease (IBD). In this article, migration abnormalities of intestinal lymphoid cells, their significance in IBD, and protein-losing enteritis, in which the disruption of intestinal lymphatic function is considered to play an important role, are discussed.
Lymphocyte Migration during Intestinal Inflammation and Its Clinical Significance
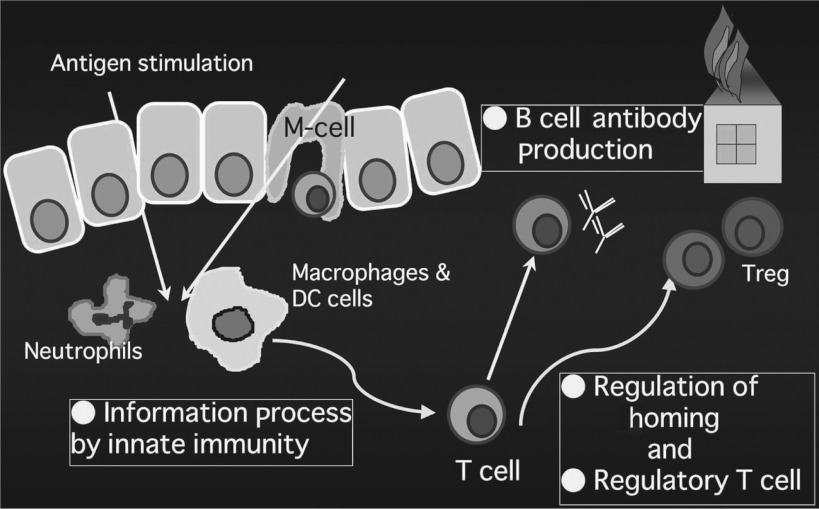
The intestine is continuously exposed to stimulation by food and bacterial antigens from the luminal side, and it not only obtains information about them but also protects itself from these stimuli and maintains negative immune control to prevent excessive immunological responses (Fig. 1). However, in IBD such as Crohn's disease and ulcerative colitis, food allergy, and graft versus host disease (GVHD), this negative control is considered to be disturbed, and, as lymphoid cells respond excessively to antigenic simulation, their aberrant homing occurs at the site of inflammation, causing sustained inflammation and tissue destruction. In the vascular endothelium of the small and large intestinal mucosa, which richly expresses mucosal addressin cell adhesion molecule (MAdCAM)-1, there is a mechanism to selectively accumulate gut-tropic α4β7-integrin-positive effector memory cells. Particularly, MAdCAM-1 is characteristically expressed in the intestinal lamina propria mucosae, and this expression is abnormally enhanced in intestinal inflammation. Homing of lymphocytes in secondary lymphatic apparatuses such as Peyer's patches of the intestine differs from that in the villus mucosa of the intestine and needs combinations of many adhesion molecules including not only α4β7 integrin but also LFA-1 and L-selectin, and they are known to permit homing of naive and central memory T-lymphocytes. Therefore, in chronic enteritis, controlling the interaction between α4β7 integrin-positive T cells and MAdCAM-1 on the vascular endothelial side is speculated to be a possible therapeutic strategy. We induced colitis by orally administering dextran sulfate to mice, and observed the increased expression of MAdCAM-1 in mucosal vascular endothelium of the large intestine and of β7 integrin in lymphocytes infiltrating the mucosa. When neutralizing antibody to MAdCAM-1 was administered to these mice, enteritis was significantly suppressed.1) We also reported a suppressive effect of neutralizing antibody to MAdCAM-1 in a PG-PS colitis model prepared by administering rats with a bacteria-derived toxin.2) In SAMP-1/Yit mice, which are known to be animal models of Crohn's disease as they spontaneously develop ileitis as well as colitis, we also reported that neutralizing antibody of MAdCAM-1 alleviated ileitis.3) Furthermore, in a rat model of chronic inflammation of the small intestine based on food allergy to ovalbumin, neutralizing antibody of MAdCAM-1 showed a suppressive effect.4) Interestingly, in these experimental models, the expression of not only MAdCAM-1 but also vascular cell adhesion molecule (VCAM)-1 was enhanced in the intestinal mucosa during inflammation, and the migration of lymphocytes was increased particularly in venules of the submucosal layer through VCAM-1. Strangely, however, neutralizing antibody to VCAM-1 showed no sufficient suppressive effect against chronic inflammation.
Fig. 1.
Representative players for “negatively regulated immune system” in the intestinal mucosa in response to stimulation by various luminal antigens.
How is it clinically applied to actual IBD patients? α4β7 integrin is markedly expressed in lymphocytes infiltrating the intestinal mucosa of patients with ulcerative colitis or Crohn's disease. In addition, while MAdCAM-1 is slightly expressed in the vascular endothelium of the intestinal lamina propria mucosae of healthy individuals, its expression is reported to be increased nearly 2-fold in patients with ulcerative colitis and up to about 7-fold in patients with Crohn's disease.5) However, since no expression of MAdCAM-1 was noted in the blood vessels near the serosa in patients with Crohn's disease with a fistula, other adhesion molecules are suspected to be involved in fistula formation. Since, as mentioned above, the expression of MAdCAM-1 is specific to the digestive tract, particularly, the intestinal tract, clinical trials of treatment using neutralizing antibody targeted to this route are being conducted abroad. Unfortunately, no human neutralizing antibody to MAdCAM-1 has been developed to the present, and only agents with a suppressive effect against MAdCAM-1 are being developed. The results of a trial using natalizumab, a human type anti-α4-integrin antibody, for the treatment of Crohn's disease were encouraging, and a significant remission-inducing effect was also observed in a large-scale clinical trial (ENACT-1&2).6) However, reports that patients treated with this drug developed progressive multifocal leukoencephalopathy, an opportunistic JC virus infection, have appeared in succession, and its indications and safety are being evaluated further.7) A clinical trial of antibody to α4β7 integrin (Antegren, MLN-02) is also in progress, and encouraging results have been obtained in phase II. For the future, the development of treatments for IBD targeted to adhesion molecules is expected to progress further, and this field is attracting attention as promising.
While clinical trials have not been initiated, treatments targeted to P-selectin, an adhesion molecule closely related to platelets, or designed to block the PSGL-1 route are being evaluated. It has already been reported that neutralizing antibody to PSGL-1 exhibits a therapeutic effect in mouse dextran sulfate sodium (DSS) colitis, and we have also shown that neutralizing antibody to PSGL-1 significantly alleviates naturally occurring ileitis in SAMP-1/Yit mice.8) While various mechanisms have been speculated for this effect, one of the leading hypotheses is that platelets increase the active oxygen-generating ability of leukocytes by adhering to multinucleated white blood cells by this mechanism. It is also possible that, when activated lymphocytes and monocytes/macrophages adhere to the vascular endothelium, integrin is activated by the adhesion of platelets to lymphoid cells, causing an increase in their adhesion ability. Moreover, the mechanism that lymphocytes are further activated through the CD40-CD40L pathway as platelets adhere to activated lymphocytes is also considered possible. The role of platelets in lymphoid cell migration will be investigated further.
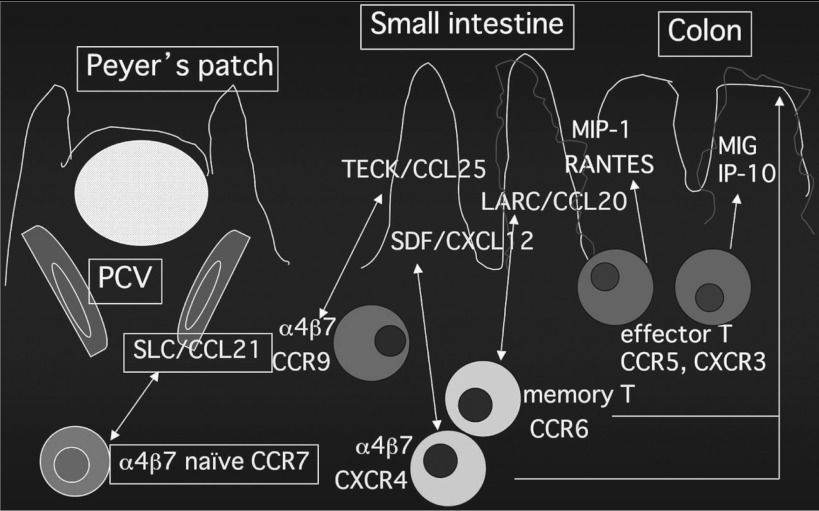
Lymphoid cell migration cannot be explained by adhesion molecules alone, and the roles of chemokines in the tissue transport and tissue selectivity of these cells are considered important (Fig. 2). Chemokines are known not only to induce chemotaxis in tissues but also to play an important role in the promotion of integrin-mediated cell adhesion in the microvascular endothelium. On closer evaluation of gut-associated lymphocytes that express β7-integrin, naive and central memory T lymphocytes express the chemokine receptor CCR7, activate integrin by reacting with CCL21 (SLC) of the high endothelial venule (HEV) of Peyer's patches, and home to Peyer's patches. On the other hand, effector memory T lymphocytes have a CCR5- or CXCR3-positive and CCR7-negaive profile and are considered to migrate to the intestinal mucosa by responding to IP-10, MIG, MIP, and RANTES in the intestinal mucosa at the site of inflammation. Concerning the migration of memory T cells, we have also clarified that the combination of CXCL12 (SDF1-α) and CXCR4 and the combination of CCL20 (LARC, MIP-3α) and CCR6 play important roles regardless of the presence or absence of inflammation and in the presence of inflammation, respectively.9) It has also been reported that chemokines play important roles in not only the difference between activated and non-activated lymphocytes but also the difference in migration to the small and large intestines. Thus, the chemokine CCL25 (TECK) was found to be expressed in the crypts of the small intestine but not in the large intestine, and we demonstrated that it does not function in inflammation.10) The strategy to control enteritis by regulating the migration of lymphoid cells using these chemokines as targets is approaching success in animal models. DSS-induced colitis has been reported to be milder in mice defective in CCR2 and CCR5. Also, a treatment targeted to the IP-10/CXCR3 system, the expression of which is enhanced in the intestinal mucosa of patients with ulcerative colitis, has been reported to be effective in a colitis model. In addition, treatments using CCL20/CCR6 as the target have also been proposed, as mentioned above. CCL20 production in the epithelium is markedly enhanced in the mucosa at inflamed areas of ulcerative colitis, and we demonstrated that the MAdCAM-1-dependent adhesion of T cells to the intestinal mucosal microvascular endothelium can be significantly inhibited by the administration of antibody to CCL20.11) Similarly, it has recently been reported that trinitrobenzene sulfonic acid (TNBS)-induced enteritis could be markedly controlled by the administration of MIP-3α neutralizing antibody.12) However, a specific neutralizing effect is difficult to obtain because of the high structural homology of chemokines. Recently, therefore, specific antagonists of chemokine receptors have been developed, the chemokine receptor antagonist TAK-779, which simultaneously inhibits CCR2, CCR5, and CXCR3, has been reported to suppress DSS enteritis and be a target of treatment, marking a step toward its future clinical application to humans.13)
Fig. 2.
Chemokines which may play a functional role in lymphocyte migration to the intestinal mucosa (Modified from Kunkel EJ, Campbell DJ, Butcher EC: Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation, 2003, 10: 313–323)
Recently, the control of lymphocyte migration using a physiologically active lipid (sphinosine-1-phosphate: S1P) has attracted attention. This attention was triggered by the pharmacological analysis of FYT720, an S1P receptor agonist and a new immunosuppressant, but, today, lymphocyte and lymphoid cell migration is known to be regulated by precise control of the S1P concentration gradient in the body.14) The S1P system controls the movements of lymphocytes from lymphoid apparatus to the lymph vessels with overwhelming power and is also suspected to play a role in IBD. To the present, the S1P system has not been expected to suppress inflammation once it has occurred in consideration of the control of the efflux of inflammatory cells, but it has been reported to alleviate inflammation that has already been induced in animal experiments, and it is attracting attention as an approach different from other migration control measures.
The Significance of Natural Immunocompetent Cells and their Lymphatic Circulation in Digestive Tract Inflammation
If one key player in intestinal immunity is lymphocytes, the other key player is natural immunocompetent cells (neutrophils, monocytes/macrophages, dendritic cells), which initially respond to stimuli from the digestive tract lumen and process the information. Here, monocytes/macrophages and dendritic cells and their migration are focused on, in particular, but, unlike lymphocytes, their pathophysiologic significance and the mechanisms of their migration during inflammation largely remain unclear. Recently, analysis of pattern recognition receptors (PRR) of these cells has advanced, and new information has been obtained about the role of Toll-like receptor (TLR) as a microorganism sensor and signal transmission mechanism. There have also been a number of experimental studies on the involvement of TLR in the pathology of IBD. However, while signals mediated by TLR were reported to induce enteritis, TLR signals are considered to be important for the homeostasis and barrier function of the colon mucosa, because DSS enteritis is exacerbated in TLR2- and 4-defective mice, and the presence of optimal TLR signals is considered to be necessary for controlled inflammation of the intestinal mucosa. Migration of monocytes/macrophages and dendritic cells has also recently attracted attention. In IBD, particularly Crohn's disease, marked monocyte/ macrophage infiltration is observed in the intestinal mucosa and submucosa, and many of the cells are speculated to be newly recruited from blood.15) Geissmann et al. recently reported that two monocyte subsets that home to tissues are present in mouse blood, that they have a relatively short lifespan, that the one that homes to CCR2+ inflamed tissue corresponds to human CD14+ cells, and that the one recruited by fractalkine receptor CX3CR1+ non-inflamed tissue with a relatively long lifespan corresponds to human CD16+ cells.16) According to the results of our experiment on the migration of mouse CD14+ monocytes to the intestine, no significant migration was observed in Peyer's patches or intestinal villus mucosa under physiologic conditions. However, when inflammation is present after the administration of LPS, adhesion of CD14+ monocytes was observed in the microvascular endothelium of both Peyer's patches and intestinal villus mucosa, and we demonstrated that the combinations of PSGL-1 and α4β1 integrin on monocytes with P-selectin and VCAM-1 of the vascular endothelium were markedly involved in this phenomenon.17) In SAMP-1/Yit mice, a model of spontaneous ileitis, a natural increase in the homing of CD14 monocytes to the ileal mucosa was observed, and marked infiltration of CD68+ and PSGL-1+ macrophages was observed with an increase in CD4 lymphocytes in the ileal mucosa. Moreover, as mentioned above, spontaneous occurrence of ileitis could be prevented by the blocking of PSGL-1 in this model, suggesting that the control of tissue infiltration of activated macrophages will be an important therapeutic strategy against IBD.8)
Dendritic cells as well as monocytes/macrophages are important key players in natural intestinal immunity, and their kinetics have recently been attracting attention. Various DCs are known to be localized at specific sites of the intestine. For example, CD11b+CD8α- myeloid DCs are present in the subepithelial dome (SED) of Peyer's patches, and CD11b-CD8α+ lymphoid DCs are present in the interfollicular region (IF). In addition, double negative (DN)-DCs are known to be present in large numbers in Peyer's patches. Myeloid DCs in the SED express CCR6, which is not expressed by other DCs, but lymphoid DCs and DN-DCs express only CCR7. Antigen taken up by M cells is considered to be incorporated into DCs in the SEM, and a mechanism to transport DCs to lymph vessels in the IF region when stimulated by bacteria, etc., is considered to be established.18) CCR7 and lymph vessel ELC (CCL19) are considered important for the entry of DCs into lymph vessels, but cysteinylated leukotriene and β2-integrin are also considered to be necessary. However, according to our evaluation of the properties of DCs in rat intestinal lymph and their migration to mesenteric lymph nodes, relatively immature DCs with a high phagocytic activity migrated to the rat intestinal lymph, and CCR6 was suggested to be more closely related to their transport to the mesenteric lymph nodes than CCR7.19) Since peripheral lymph nodes as well as the skin may be regulated differently compared with intestinal lymph nodes, this point needs further evaluation. Also, in inflammation, CD11c-low, B220+, L-selectin+ plasmacytoid DCs (p-DCs) are transported to the intestinal lymph nodes and, along with other DCs, are considered to be involved in the homing of reg T cells. On the other hand, DCs in the intestinal lamina propria mucosae (LP-DCs) are considered to be mostly myeloid DCs and to include other types only in small numbers. LP-DCs are considered to have special properties, unlike myeloid DCs at other sites.20) Particularly, those that are present in the intestinal lamina propria mucosae express the traits of tight junction protein and are considered to extend projections from the intercellular gaps of the intestinal epithelium and constantly obtain information on antigens. They are reportedly CCR6- and CX3CR1+ and respond to fractalkine signals from the epithelium. Also, DN-DCs of patients with Crohn's disease, particularly those in the ileal mucosa, were reported to markedly express p40 and to be involved in IL-23 production,21) but, recently, Mizoguchi et al. suggested that myeloid DCs of immature phenotypes with monocyte markers are important for granuloma formation in the intestine.22) CD83+CD86+CD40+ mature and activated DCs are known to accumulate at the lesions of Crohn's disease, but new markers including M-DC8+ cells have also been reported. However, much remains unknown about the physiologic gut-lymph circulation of DCs and how it is impaired in IBD, and so further research results are awaited.
Protein-Losing Enteropathy and Disorders of the Intestinal Lymphatic System
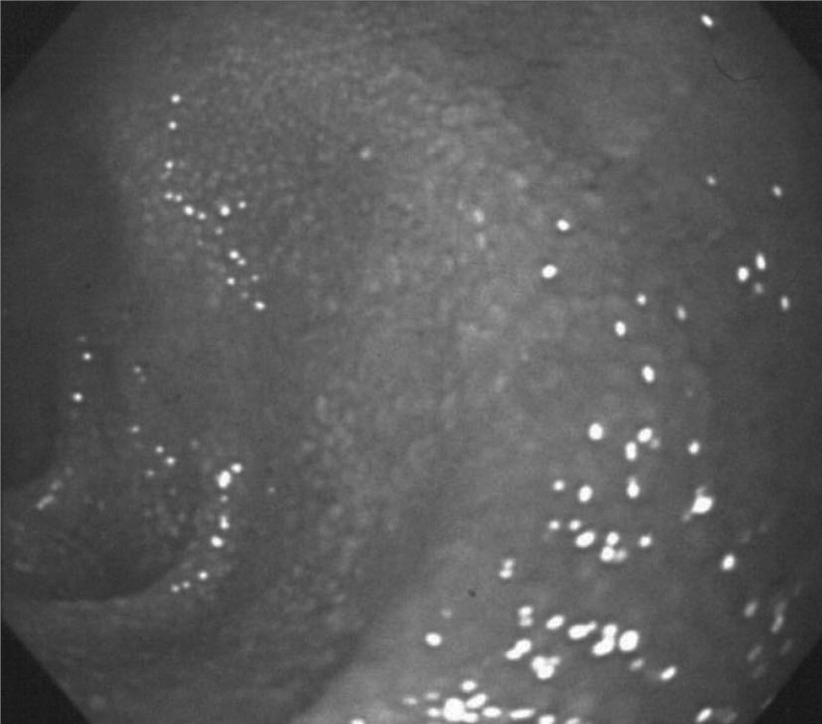
Protein-losing enteropathy is a syndrome with an unknown cause in which hypoproteinemia occurs due to the leakage of protein from the digestive tract. Since the leakage of lymph due to abnormality of the intestinal lymph vessels as well as an increase in the permeability of the intestinal mucosa has been suggested to be a cause of this condition, we would like to touch on this subject on the basis of our recent evaluations as the last topic of this article. While the primary symptoms of protein-losing enteropathy are edema and diarrhea associated with hypoproteinemia, the lymphocyte count and immunoglobulin level are reduced due to the loss of intestinal lymph, and chronic insufficiency of cellular immunity and insufficiency of lipid absorption and deficiency of lipophilic vitamins associated with intestinal lymph congestion also pose clinical problems. Since the condition, which is considered to be caused primarily by blocking of the lymph flow in the intestine, may be regarded as intestinal lymphedema from the viewpoint of lymph circulation, it may be compared with lymphedema of the limbs. Various underlying disorders may cause blocking of the intestinal lymph flow in protein-losing enteropathy, but malignant lymphoma, Crohn's disease, pancreatic cancer, and ovarian cancer are included as secondary causes. However, the disease is often idiopathic, i.e., the cause of lymph vessel impairment cannot be identified, and idiopathic protein-losing enteropathy is categorized as primary intestinal lymphangiectasia if dilation of the central lacteal is demonstrated. While this disease frequently occurs in early childhood, it may also occur in youth or adulthood, exhibiting characteristic endoscopic findings including dilated mucosal lymph vessels observed as white spots and white villi indicating disorder of lipid transfer (Fig. 3).
Fig. 3.
Endoscopic picture of duodenal mucosa in patients with intestinal lymphangiectasia accompanied with protein-losing enteropathy-edematous white villi.
On the basis of the hypothesis that this disease is related to disorders of the lymphangiogenic processes, we evaluated the kinetics of VEGF-C and -D and their receptor VEGFR-3, which are closely associated with lymphangiogenesis, in the intestinal mucosa. As a result, VEGFR-3 and LYVE-1 were expressed in the dilated central lacteals in the patients' duodenal mucosa, but their expression was low in the deep mucosa. On the other hand, the expression of VEGF-C and -D, which are lymph vessel growth factors, was reduced. Moreover, while no change was noted in Prox1 among the transcription factors considered to be important for lymphangiogenesis, the expression of FOXC2 and SOX18 was suppressed compared with healthy individuals.23) These observations suggest that: Although the formation and proliferation of lymph vessels may appear to be increased in the superficial layer of the intestinal mucosa in patients with intestinal lymphangiectasia, a decrease in lymph vessel growth factors and poor lymph vessel formation may be present as essential factors unlike lymphangiogenesis in cancer. We are planning to clarify this point by studying surgical cases. Also, the mechanism of how lymph vessel disorder leads to the enhancement of intestinal permeability to protein is unclear, and further evaluation of the roles of the lymphatic vessels in digestion, absorption, and barrier function of the intestinal mucosa is also considered necessary.
Footnotes
This article is English Translation of J Jpn Coll Angiol 2008; 48: 143-149
References
- Kato S, Hokari R, Matsuzaki K, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther 2000; 295: 183-9 [PubMed] [Google Scholar]
- Hokari R, Kato S, Matsuzaki K, et al. Involvement of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the pathogenesis of granulomatous colitis in rats. Clin Exp Immunol 2001; 126: 259-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Tsuzuki Y, Matsunaga H, et al. In vivo demonstration of T lymphocyte migration and amelioration of ileitis in intestinal mucosa of SAMP1/ Yit mice by the inhibition of MAdCAM-1. Clin Exp Immunol 2005; 140: 22-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Miura S, Tsuzuki Y, et al. Chronic allergy to dietary ovalbumin induces lymphocyte migration to rat small intestinal mucosa that is inhibited by MAdCAM-1. Am J Physiol Gastrointest Liver Physiol 2004; 286: G702-10 [DOI] [PubMed] [Google Scholar]
- Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997; 151: 97-110 [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn's disease. N Engl J Med 2003; 348: 24-32 [DOI] [PubMed] [Google Scholar]
- Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med 2005; 353: 362-8 [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsuzuki Y, Matsuzaki K, et al. Blockade of PSGL-1 attenuates CD14+ monocytic cell recruitment in intestinal mucosa and ameliorates ileitis in SAMP1/Yit mice. J Leukoc Biol 2005; 77: 287-95 [DOI] [PubMed] [Google Scholar]
- Oyama T, Miura S, Watanabe C, et al. CXCL12 and CCL20 play a significant role in mucosal T-lymphocyte adherence to intestinal microvessels in mice. Micro-circulation 2007; 14: 753-66 [DOI] [PubMed] [Google Scholar]
- Hosoe N, Miura S, Watanabe C, et al. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 2004; 286: G458-66 [DOI] [PubMed] [Google Scholar]
- Teramoto K, Miura S, Tsuzuki Y, et al. Increased lymphocyte trafficking to colonic microvessels is dependent on MAdCAM-1 and C-C chemokine mLARC/ CCL20 in DSS-induced mice colitis. Clin Exp Immunol 2005; 139: 421-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchar K, Kelly CP, Keates S, et al. MIP-3alpha neutralizing monoclonal antibody protects against TNBS-induced colonic injury and inflammation in mice. Am J Physiol Gastrointest Liver Physiol 2007; 292: G1263-71 [DOI] [PubMed] [Google Scholar]
- Tokuyama H, Ueha S, Kurachi M, et al. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-Mucosal Immunity in Gut and Lymphoid Cell Trafficking mediated colitis. Int Immunol 2005; 17: 1023-34 [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005; 309:1735-9 [DOI] [PubMed] [Google Scholar]
- Burgio VL, Fais S, Boirivant M, et al. Peripheral monocyte and naive T-cell recruitment and activation in Crohn's disease. Gastroenterology 1995; 109: 1029-38 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71-82 [DOI] [PubMed] [Google Scholar]
- Ishii N, Tsuzuki Y, Matsuzaki K, et al. Endotoxin stimulates monocyte-endothelial cell interactions in mouse intestinal Peyer's patches and villus mucosa. Clin Exp Immunol 2004; 135: 226-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med 2000; 191: 1381-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Miura S, Nagata H, et al. In situ demonstration of dendritic cell migration from rat intestine to mesenteric lymph nodes: relationships to maturation and role of chemokines. J Leukoc Biol 2004; 75: 434-42 [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001; 2: 361-7 [DOI] [PubMed] [Google Scholar]
- Becker C, Wirtz S, Blessing M, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest 2003; 112: 693-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Ogawa A, Takedatsu H, et al. Dependence of intestinal granuloma formation on unique myeloid DC-like cells. J Clin Invest 2007; 117: 605-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokari R, Kitagawa N, Watanabe C, et al. Changes in regulatory molecules for lymphangiogenesis in intestinal lymphangiectasia with enteric protein loss. J Gastroenterol Hepatol 2008; 23: e88-95 [DOI] [PubMed] [Google Scholar]