Abstract
Purpose: We have reviewed ruptured and nonruptured infected aortoiliac aneurysms to study the clinical presentation, management and eventual outcome of patients managed with in situ prostheses, axillofemoral prostheses grafts and endovascular reconstruction.
Design: A retrospective chart review of 16 cases treated at a single institution.
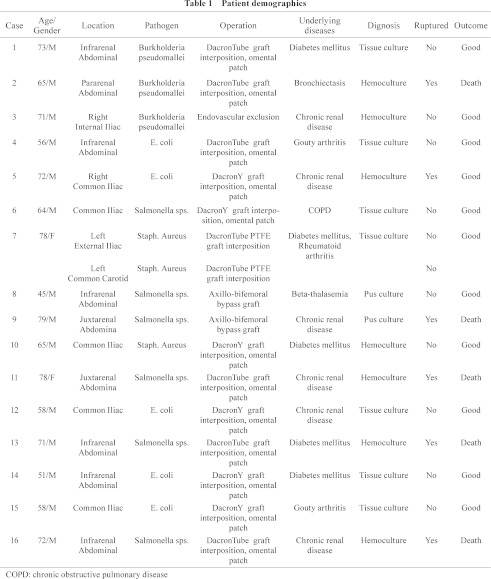
Methods: From January 2007 to March 2008, a total of 93 patients with aortoiliac aneurysms underwent surgical repair at our institution. Among these, 16 patients (17.2%) were shown to be infected aneurysms of the infrarenal (n = 6), juxtarenal (n = 2), and pararenal aorta (n = 1); the others were 5 common, 1 external, and 1 internal iliac arteries. Fourteen patients were male and 2 were female with the mean age of 66 years (range, 45–79). In all cases, the diagnosis was confirmed by abdominal computed tomography and empirical parenteral antibiotics were administered at least 1 week, unless in patients need emergency operations. At the time of an operation, all were saccular and were classified as primary infected aortoiliac aneurysms. Thirteen patients had surgical debridement with in situ graft interposition and omental wrapping, 2 underwent aneurysm exclusion and extra-anatomic (axillo-femoral) bypass, 1 underwent aneurysmectomy of left external iliac artery and polytetrafluoroethylene (PTFE) graft interposition, and 1 underwent endovascular exclusion. The parenteral antibiotics were continued in the postoperative period for 4–6 weeks. Chronic renal disease was present in 37.5% (6/16), with diabetes mellitus present in 31.25% (5/16). The most common pathogen was Salmonella sp. (n = 6) and E. coli (n = 5). Thirty-seven percent (6/16) of the patients presented late, with a 37.5% (6/16) incidence of ruptured (4 contained, 2 free ruptured) that needed emergency surgery.
Results: Disease-specific mortality was 31.25% (5/16). The 30-day mortality rate of ruptured cases is high 67% (4/6), because patients present late in the course of the disease. One patient who underwent aneurysm exclusion and extra-anatomic (axillo-femoral) bypass died 6 months later from burst aortic stump. Salmonella and E. coli are the most common pathogens.
Conclusions: Early diagnosis followed by surgical intervention with proper antibiotic coverage provides the best results. Mortality rate was still high in patients with sepsis and rupture. An in situ graft interposition and omental wrapping is a safe option for revascularization of infected aneurysms of the iliac arteries and infrarenal aorta.
Keywords: infected aortoiliac aneurysms, open repair, endovascular repair
Introduction
Management of infected aortoiliac aneurysms is a challenging and difficult clinical problem for the vascular surgeons. In the West, Infected aortoiliac aneurysms are rare; they represent only 1% to 2% of all aortic aneurysms.1) In an Asian population, infected abdominal aortic aneurysms may raise up to 13.6%.2) Infected aortoiliac aneurysms are associated with high morbidity and high mortality 21%–44% because they are frequently associated with complicating factors, such as late or delay in diagnosis, rupture, sepsis, and paravisceral location.1,2) The goals for treatment of infected aortoiliac aneurysms are aneurysm resection, extensive soft-tissue debridement, remote arterial reconstruction out of the field of infection, omental patch and antibiotics. However, aneurysms located in the paravisceral aortas are not amenable to conventional extra-anatomic reconstruction. In situ aortic replacement is necessary, potentially exposing the patient to an increased risk of graft infections.1–6)
In this report, we describe our experience with the management of 16 patients with infected aneurysms of the infrarenal aorta and iliac arteries, 12 of whom underwent in situ aortic replacement, 2 underwent extra-anatomical bypass, 1 underwent aneurysmectomy of left external iliac artery and PTFE graft interposition, and 1 underwent endovascular exclusion.
Materials and Methods
A retrospective review of medical records of cases admitted to the Division of Vascular and Endovascular Surgery of the Department of Surgery of the Chiang Mai University Hospital for a 15-month period from January 2007 to March 2008 with the diagnosis of infected aneurysm. Included were patients with infected aneurysms of the abdominal aorta and iliac arteries. We excluded patients with infected prosthetic aortic grafts and infections of the thoracic aorta.
The presence of an infected aneurysm was defined by clinical evidence of infection (e. g., fever, leukocytosis), operative findings of gross contamination (inflammation and purulence), and positive aortic wall cultures. A patient with negative culture results was considered to have an infected aneurysm if he or she had previously had clinical signs of infection, suggestive intraoperative findings (e. g., periaortic abscess), and had received at least 1 week of antibiotics.1) Other evidence such as blood culture results and other imaging studies including abdominal ultrasound and CT angiography, if performed, were taken into consideration. Medical records of the 16 patients were reviewed for demographic data, presenting symptoms, operative details, and postoperative outcomes. Patient demographics were assessed for age, gender, aneurysm size, aneurysm site (suprarenal, juxtarenal or infrarenal), fever (temperature >38°C), tachycardia (pulse rate >100 per minute), hypotension (systolic blood pressure <100 mmHg), leukocytosis (white blood cell count >10 × 109/L), neutrophilia (neutrophil count >75%), and results of blood and operative cultures. Their comorbidities and risk factors for infection (e. g., recent or concurrent infection, recent invasive procedures, and steroid use) and atherosclerosis (e. g., diabetes mellitus, systemic hypertension, smoking, hyperlipidemia, and peripheral vascular disease), complications, early and late postoperative survival, and long-term surveillance of graft patency were also recorded (Table 1).
Preoperatively, intravenous ceftriaxone and metronidazole combination was administered empirically upon clinical diagnosis. This was changed when cultures were available.
All patients who underwent surgery, 12 had surgical debridement with in situ graft interposition and omental wrapping, 2 had extra-anatomic bypass, 1 underwent aneurysmectomy of left external iliac artery and PTFE graft interposition, and 1 underwent endovascular exclusion. The location of each aneurysm was documented after careful review of radiologic tests and operative notes. Aneurysms were classified as pararenal, juxtarenal, and infrarenal or iliac according to published standards. Segments of resected infected aortic wall or pus were sent for bacteriological culture, the type of repair performed and the nature of the graft inserted were recorded. Postoperative outcomes and cause of death in those patients who died were determined from the medical records. The course of preoperative antibiotics was continued in the postoperative period for 4–6 weeks. The mean follow-up period was 40 months (range, 32–48 months). Follow up of survivors included a review of postdischarge clinic visits and telephone interviews.
Results
From January 2007 to March 2008, 93 patients underwent surgical repair of aortoiliac aneurysms at our institution. Sixteen patients (17.2%) had infected aneurysms, based on the criteria of imaging studies and culture reports; this group comprised 14 men and 2 women of mean age 66 years (range, 45–79 years). The diagnosis of infected aneurysm was made preoperatively in all 16 patients. All of the patients presented with abdominal or back pain, and five had recurrent fevers. Seven patients had positive blood cultures (43.7%), 7 patients had positive tissue cultures (43.7%) and 2 (12.7%) had positive pus cultures. Broad spectrum antibiotics were used in all 16 patients. Chronic renal disease was present in 37.5% (6/16), with diabetes mellitus present in 31.2% (5/16). None of the 16 patients had evidence of immune deficiency. The diagnosis of aortic aneurysm was confirmed by abdominal CT scan and by CT angiography (Figs. 1 and 2). Four patients had two or more CT scans from outside hospitals, which demonstrated rapid aneurysm expansion within 1 week. Four patients had focal aortic pseudoaneurysms with contained ruptures (25%) and 2 had free ruptures (12.5%); all of these were confirmed during the operation. The aneurysm location was in the infrarenal aorta in six patients (37.5%), in the pararenal aorta in one patient, and in the juxtarenal aorta in two patients; the location in others were 5 common (37.5%), 1 external, and 1 internal iliac artery. Seven patients had positive hemoculture results (Table 1) and two patients had signs of hemodynamic instability due to free ruptures.
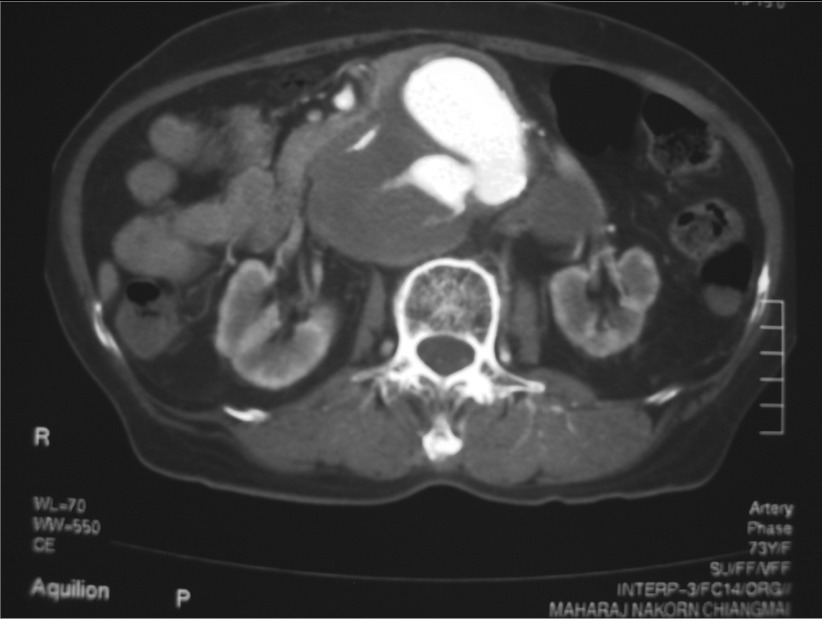
Fig. 1.
CT scan of a large pararenal infected aortic aneurysm.
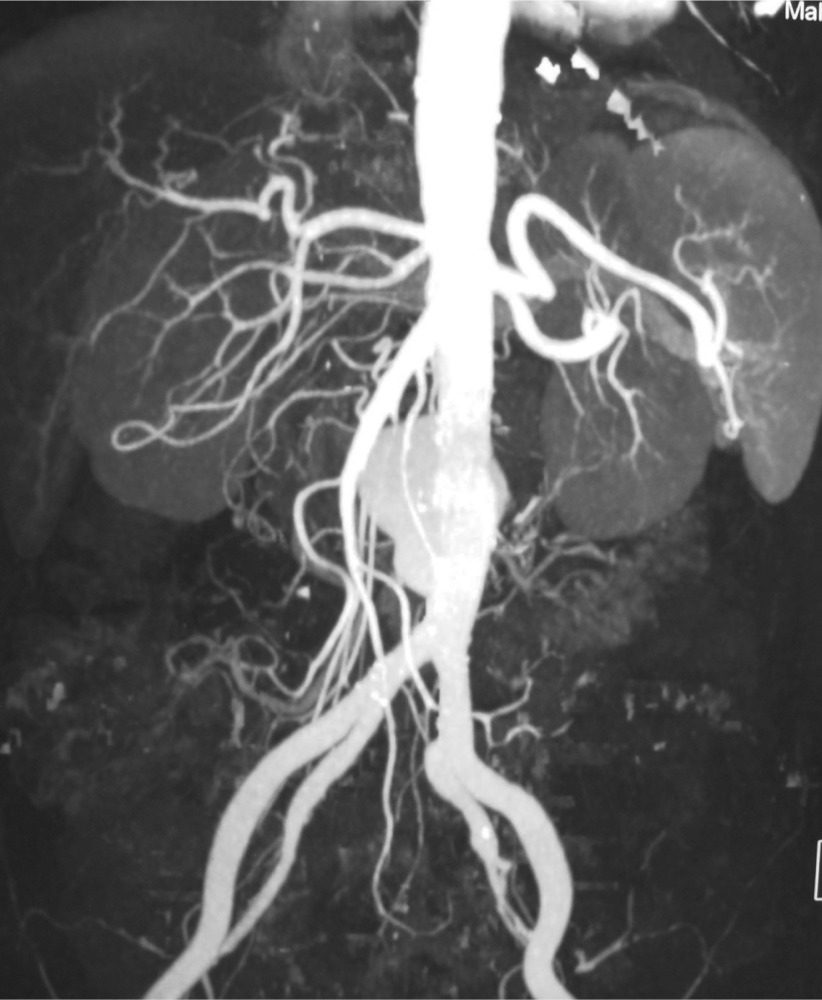
Fig. 2.
Aortograms showing infrarenal saccular aneurysms of the aorta.
Operative details
Surgery was performed after an interval of 1–16 days since admission. Emergency surgery was performed in 37.5% (n = 6) of patients due to rupture. The mean operating time was 4 hours and 46 minutes. Midline abdominal incisions were used for all 16 patients. In all cases, the infected aorta, including the aneurysm and any grossly abnormal segments, was excised. Surrounding tissue was widely debrided. The choice of conduit depended on the location and extent of the aneurysm. Metallic Silver Vaporized Dacron prostheses (Silver Graft/B Braun, Munsingen, Germany) were used to replace infected aneurysms in seven patients with aneurysmal disease extending above and below the level of the renal arteries. Grafts were anastomosed to aortic segments free of gross contamination, using the inclusion technique or extensive posterior graft extensions to revascularize the renal arteries. Omental grafting around the in situ vascular conduit was done in all of 12 patients. Two other patients with a frank pus contamination underwent extra-anatomic bypass (Axillo-bifemoral) and excision of the infected aneurysm segment. One patient underwent endovascular exclusion and percutaneous drainage of infected due to hostile abdomen and unfit for a major operation. One patient had infected aneurysms both left common carotid and external iliac artery with Staph. aureus, underwent aneurysmectomy and PTFE graft interposition.
Postoperative course
Cultures of arterial wall specimens were positive in seven patients (43.7%), blood cultures were positive in 7 (43.7%) and pus cultures, in 2 patients (12.5%).
Salmonella sp. was responsible in 37.5% (n = 6) of patients (Table 1). E. coli 31.2% (n = 5), Burkholderia pseudomallei 18.7% (n = 3) and Staph. aureus 12.5% (n = 2). Gram-negative organisms were by far the most common isolates from aortic wall cultures. Six of the seven patients with preoperative blood cultures had positive aortic wall cultures. The blood and aortic wall culture results correlated in every instance. One patient died of sepsis 4 days postoperatively. The patient had signs of rapidly progressive multisystem organ failure and succumbed to overwhelming cardiopulmonary insufficiency. All four patients had preoperative evidence of clinical sepsis. All six surviving patients had prolonged postoperative hospital stays. The mean duration of postoperative hospital stay for the survivors was 23 ± 12 days (range, 8 to 41 days). Four of the six patients had prolonged ventilator dependence because of pulmonary insufficiency (ARDS). Two had other serious postoperative complications, including renal insufficiency in one and multiple complications (upper gastrointestinal bleed, intestinal obstruction requiring laparotomy, and fungal sepsis from a central line) in the second. The 30-day mortality rate of ruptured cases was high, 67% (4/6); one patient who underwent aneurysm exclusion and extra-anatomic (axillo-femoral) bypass died 6 months later from a burst aortic stump. The mean follow up of all patients was 40 months (range, 32 to 48 months). There were no signs of infection in the eleven patients; all are currently living at home and have resumed their normal, premorbid level of activity.
Overall, the 30-day in-hospital mortality rates for aortoiliac and infected aortoiliac aneurysms were 4.7% and 25%, respectively. There were 4 deaths in the infected group: 2 patients with free rupture, who initially presented with unstable vital signs and hemorrhagic shock, died 11 and 14 days after the operation. One patient with a concealed, ruptured aneurysm of the paravisceral abdominal aorta developed acute renal failure postoperatively, despite a successful graft, and the patient died 17 days after the operation. In the other patient with a concealed, ruptured aneurysm, despite a successful surgical repair, the patient died 23 days after the operation due to marked sepsis and multiple organ failure. The mean follow-up time for the remaining 12 patients was 40 months (range, 32 to 48 months). There was 1 late death: 1 patient died from a burst aortic stump 6 months after the aneurysm exclusion and extra-anatomic (axillo-femoral) bypass. There were no late grafts or aortic infections in the 11 surviving patients, from ultrasound examinations and leukograms.
Discussion
Primary arterial infections have been classified into 4 types1) to which a fifth type can be added.6)
Mycotic aneurysm due to septic emboli (embolomycotic); usually from endocarditis
Microbial arteritis − Bacteremia causes infection in an atheromatous plaque leading to destruction of the wall and aneurysm formation; this is most common in the aorta
Infection of a pre-existing aneurysm
Post traumatic false aneurysm, such as following penetrating injury, invasive intra-arterial cannulation or drug abuse
Aortic infection from a contiguous organ with sepsis − from psoas abscess or osteomyelitis of vertebra or diaphragmatic abscess
There are several theories to explain the pathology of aortic infection. Infected microemboli may lodge in the vasa vasorum causing occlusion and damage to the aortic wall, which leads to degeneration and aneurysm formation. Emboli can also lodge in the irregular intima or the thrombus in the aneurysm during bacteremia.6–9) Aortic wall infection can occur due to direct inoculation into the arterial wall. Organisms may colonize the intact vascular wall through the vasa vasorum, where the arterial wall is weakened by a local suppurative process, which results in Microbial arteritis with aneurysm formation.3,10,11)
With the introduction of antibiotics during the past 50 years, the incidence of infective endocarditis decreased gradually, and infected aortic aneurysms associated with endocarditis became rare.11,12) In nonendocarditis bacteremia or secondary to septic emboli, the most commonly reported site of infected aneurysms was the abdominal aorta due to infection of existing atherosclerotic plaque and aneurysms with a larger vasa vasorum, where infected emboli may dislodge.10) Salmonella species in particular have a strong predilection to infect damaged aortic intima and abnormal arterial intima, especially arteries harboring atherosclerotic plaque.13,14) Characterization of the different bacteria is important, since gram-negative sepsis results in higher rupture rates than infection with gram-positive bacteria.15) The virulence species, Salmonella typhimurium and Salmonella chloeraesuis, account for over 50% of the reported cases of infected aneurysms.13,14) Other common organisms are Streptococcus, Bacteroides, Escherichia coli, and Staphylococcus aureus.16–19) In our report, Salmonella sp. was responsible in 37.5% (n = 6) of patients (Table 1). E. coli 31.2% (n = 5), Burkholderia pseudomallei 18.7% (n = 3) and Staph. aureus 12.5% (n = 2). Gram-negative organisms were by far the most common isolates from aortoiliac wall cultures. Salmonella sp. responded in 66.66% (4/6) of patients and is thought to exhibit a more virulent course because of its ability to invade the normal intima and cause early aneurysm rupture.19)
The complicated early outcome in patients with infected aortoiliac aneurysms probably reflects the combination of an aggressive presentation, high rupture rate, and complex aneurysm location in hosts with chronic comorbid conditions. This study shows that infected aneurysms are 17.2% of all aortoiliac aneurysms with 37.5% (6/16) incidence of aneurysm rupture. All of our patients had at least one chronic comorbid condition— 6 of the 16 patients (37.5%) had a history of hypertension, 37.5% (6/16) had chronic renal disease with diabetes mellitus present in 31.2% (5/16). However, late mortality seems to be caused by the initial infected aneurysm per se, but rather by the natural history of associated comorbidities and underlying atherosclerotic disease.
Despite the fact that infected aneurysms occur in all age groups, the elderly comprised the largest group in our series. All of the 16 patients; 14 were men and 2 were women of mean age 66 years (range, 45–79 years).
In our series, all patients were symptomatic, with symptoms having lasted for more than 7 days, and even up to 2 months. Classic manifestations include abdominal pain, fever, and a pulsatile abdominal mass. The presence of leukocytosis-elevated sedimentation rate and positive blood cultures strengthen the surgeon's suspicion of the diagnosis of infected aneurysm. Symptoms of sepsis may be discrete and may easily go unrecognized, especially in the early stages. Broad-spectrum antibiotics should be started preoperatively after taking blood cultures. If blood cultures are positive, they are helpful to signal the need for the specific antibiotic therapy. However, blood cultures are negative in up to 50% of cases; negative blood cultures do not rule out the presence of an infected aneurysm. Gram stains and cultures obtained from the aneurysm wall at the time of surgery are also not uniformly positive. CT scan is the cornerstone in establishing the diagnosis of infected aortic aneurysm.20,21)
Although a period of antimicrobial therapy before surgery is advised, immediate surgery is indicated, irrespective of bacteriologic status, when there are signs of rupture. Emergency surgery was performed in 37.5% (n = 6) of patients due to rupture. Four patients had focal aortic pseudoaneurysms with contained ruptures (25%) and 2 free ruptures (12.5%); all these were confirmed at operation. All 4 of the 6 rupture of the infected aortoiliac aneurysms which were infected by Salmonella species died in 4, 11, 14, 17 days after an operation. After rupture of infected aortoiliac aneurysms, surgery is often too late, and septic emboli from the aneurysm may spread to peripheral tissues. Therefore, surgical intervention, as soon as possible after confirmation of the diagnosis, is mandatory.1,2,4,22)
The surgical management of infected aortoiliac aneurysms includes eradication of the source of infection and reconstruction of the arterial flow. Adequate drainage, administration of organism-specific parenteral antibiotics, and surgical debridement are the strategies used to resolve infection.1,2,22) The virulence of the organism and severity of the arterial infection are more important determinants than any single operative procedure or method of arterial reconstruction.11,15) When there is gross contamination from infected aneurysm, excision and extra-anatomical bypass is the treatment of choice. The associated mortality was still high, ranging from 25% to 30%.2–4,23) Death is usually related to persistent sepsis with multi-organ failure. The magnitude and long duration of the operation, especially in shocked and unstable patients, are major contributing factors to the perioperative mortality. The argument in favor of extra-anatomic bypass is the theoretic advantage of reducing the risk of graft infection, because revascularization is generally performed in a location remote from the site of infection. Furthermore, axillobifemoral bypass followed by aneurysm excision is a prolonged operation, which may account for the increased mortality rate in some series. Therefore, we should re-evaluate the classic doctrine that extra-anatomic reconstruction is the treatment of choice for primary aortic sepsis. Follow-up angiography for the 1 patient who received an extra-anatomic bypass showed good graft patency.
When contamination is less severe, the aorta may be replaced in situ. Extra-anatomical reconstruction is not suitable for cases of suprarenal or supraceliac aneurysms with visceral artery involvement; in situ aortic replacement is necessary.3,5,6,22) The choice of the ideal conduit is still controversial. Rifampin soaked grafts may prevent graft infection by reducing early graft seeding.24) Antibiotic-coated Dacron grafts presented an attractive adjunct. Prolonged anti-staphylococcal activity of rifampin-bonded, gelatin impregnated Dacron grafts has been demonstrated after implantation in the arterial circulation, in experimental and human studies. Infection rates, as well as mortality and morbidity rates, are much lower for rifampin-treated grafts than for plain in situ graft replacement.24) Encouraging results have also been reported with the use of cryopreserved human allograft and silver coated polyester grafts.25,26)
In this series, for the 5 patients who had infected aortoiliac aneurysms, we applied an omentum patch to cover the silver coated polyester grafts. We had no reinfection when using silver coated polyester grafts. Other safe alternatives include autogenous superficial femoral vein grafts27,28) and cryopreserved arterial allografts.29–31) For patients with fulminant sepsis, extensive aortic infection, and aortic-enteric fistula, there is no ideal surgical option, but in situ prosthetic grafting may represent the most expeditious method of arterial reconstruction, with acceptable early and late morbidity rates. Regarding long- term follow-up, all 5 patients had a good clinical condition, without any recurrent aneurysm formation.
Endovascular treatment with stent-grafts has been introduced as an alternative, with the anticipation that lesser surgical trauma, especially in the septic patient with considerable co-morbidity, will reduce the risk of cardiopulmonary, neurological and renal complications. This could be a temporizing treatment prior to definitive open surgical repair, or a therapeutic alternative in critically ill patients who may not survive open surgery.32–35) An additional drainage procedure is usually required either via CT guided drainage or by open surgical approach.33) Endovascular exclusion was performed in 1 patient with hostile abdomen and unfit for major operation.
Antibiotic administration according to postoperative sensitivity testing is crucial: although the optimum duration of postoperative antibiotic therapy remains unclear, recommendations have ranged from 6 weeks to lifelong.5,12–15) An interesting feature is that Salmonella spp., rather than Staphylococcus spp., seem to cause most infected aortic aneurysms in Eastern countries, but no definitive reason for this can be offered at present. It may be plausible that people in Eastern countries eat contaminated foods.15) The duration of antibiotic therapy is not well established, but most authors recommend a minimum of 6 weeks intravenously and orally for another 6 weeks.5,36) Longer durations and even life-long antibiotic-therapy have been recommended by others.3,15,36–38) Some authors believe that patients with a prosthetic reconstruction should continue on low-dose antibiotics for life. However, the advantage of a more prolonged therapy has not been confirmed.
Disclosure Statement
I have nothing to disclose.
Footnotes
The abstract was presented at the Annual Meeting of Asian Society for Vascular Surgery in Taiwan, 2011.
References
- Reddy DJ, Shepard AD, Evans JR, et al. Management of infected aortoiliac aneurysms. Arch Surg 1991; 126: 873-8, discussion 878-9 [DOI] [PubMed] [Google Scholar]
- Hsu RB, Tsay YG, Wang SS, et al. Surgical treatment for primary infected aneurysm of the descending thoracic aorta, abdominal aorta, and iliac arteries. J Vasc Surg 2002; 36: 746-50 [DOI] [PubMed] [Google Scholar]
- Müller BT, Wegener OR, Grabitz K, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg 2001; 33: 106-13 [DOI] [PubMed] [Google Scholar]
- Kyriakides C, Kan Y, Kerle M, et al. 11 year experience with anatomical and extra-anatomical repair of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg 2004; 27: 585-9 [DOI] [PubMed] [Google Scholar]
- Hsu RB, Chen RJ, Wang SS, et al. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg 2004; 40: 30-5 [DOI] [PubMed] [Google Scholar]
- Sekar N. Primary aortic infections and infected aneurysms. Ann Vasc Dis 2010; 3: 24-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready RA, Bryant MA, Divelbiss JL, et al. Arterial infections in the new millenium: an old problem revisited. Ann Vasc Surg 2006; 20: 590-5 [DOI] [PubMed] [Google Scholar]
- Naktin J, DeSimone J. Lumbar vertebral osteomyelitis with mycotic abdominal aortic aneurysm caused by highly penicillin-resistant Streptococcus pneumoniae. J Clin Microbiol 1999; 37: 4198-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery PT, Smith MD, Cammisa FP, et al. Mycotic aortic aneurysm in patients who have lumbar vertebral osteomyelitis. J Bone Joint Surg Am 1995; 77:1729-32 [DOI] [PubMed] [Google Scholar]
- Stengel A, Wolferth CC. Mycotic bacterial aneurysms of intravascular origin. Arch Int Med 1923; 31: 527-54 [Google Scholar]
- Brown SL, Busuttil RW, Baker JD, et al. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg 1984; 1: 541-7 [PubMed] [Google Scholar]
- Osler W. The Gulstonian Lectures, on Malignant Endocarditis. Br Med J 1885; 1: 467-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MN, Choyke PL, Wallace RB. Infected aortic aneurysms. A changing entity. Ann Surg 1992; 215: 435-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Guerrero ML, Aguado JM, Arribas A, et al. The spectrum of cardiovascular infections due to Salmonella enterica: a review of clinical features and factors determining outcome. Medicine (Baltimore) 2004; 83: 123-38 [DOI] [PubMed] [Google Scholar]
- Hsu PJ, Lee CH, Lee FY, et al. Clinical and microbiological characteristics of mycotic aneurysms in a medical center in southern Taiwan. J Microbiol Immunol Infect 2008; 41: 318-24 [PubMed] [Google Scholar]
- Cohen PS, O'Brien TF, Schoenbaum SC, et al. The risk of endothelial infection in adults with salmonella bacteremia. Ann Intern Med 1978; 89: 931-2 [DOI] [PubMed] [Google Scholar]
- Zak FG, Strauss L, Saphra I. Rupture of diseased large arteries in the course of enterobacterial (Salmonella) infections. N Engl J Med 1958; 258: 824-8 [DOI] [PubMed] [Google Scholar]
- Soravia-Dunand VA, Loo VG, Salit IE. Aortitis due to Salmonella: report of 10 cases and comprehensive review of the literature. Clin Infect Dis 1999; 29: 862-8 [DOI] [PubMed] [Google Scholar]
- Salzberger LA, Cavuoti D, Barnard J. Fatal salmonella aortitis with mycotic aneurysm rupture. Am J Forensic Med Pathol 2002; 23: 382-5 [DOI] [PubMed] [Google Scholar]
- Blair RH, Resnik MD, Polga JP. CT appearance of mycotic abdominal aortic aneurysms. J Comput Assist Tomogr 1989; 13: 101-4 [DOI] [PubMed] [Google Scholar]
- Shih CC, Lai ST, Chang Y. Computer tomography in the determination of surgical emergency for symptomatic abdominal aortic aneurysm. Zhonghua Yixue Zazhi (Taipei) 1998; 61: 210-5 [PubMed] [Google Scholar]
- Moneta GL, Taylor LM, Yeager RA, et al. Surgical treatment of infected aortic aneurysm. Am J Surg 1998; 175: 396-9 [DOI] [PubMed] [Google Scholar]
- Bacourt F, Koskas F. Axillobifemoral bypass and aortic exclusion for vascular septic lesions: a multicenter retrospective study of 98 cases. French University Association for Research in Surgery. Ann Vasc Surg 1992; 6: 119-26 [DOI] [PubMed] [Google Scholar]
- Chervu A, Moore WS, Gelabert HA, et al. Prevention of graft infection by use of prostheses bonded with a rifampin/collagen release system. J Vasc Surg 1991; 14: 521-4, discussion 524-5 [PubMed] [Google Scholar]
- Kim YW. Infected aneurysm: Current management. Ann Vasc Dis 2010; 3: 7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt M, Magne JL, Alric P, et al. In situ revascularization with silver-coated polyester grafts to treat aortic infection: early and midterm results. J Vasc Surg 2003; 38: 983-9 [DOI] [PubMed] [Google Scholar]
- Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg 1997; 25: 255-66, discussion 267-70 [DOI] [PubMed] [Google Scholar]
- Wells JK, Hagino RT, Bargmann KM, et al. Venous morbidity after superficial femoral-popliteal vein harvest. J Vasc Surg 1999; 29: 282-9, discussion 289-91 [DOI] [PubMed] [Google Scholar]
- Kieffer E, Gomes D, Chiche L, et al. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg 2004; 39: 1009-17 [DOI] [PubMed] [Google Scholar]
- Noel AA, Gloviczki P, Cherry KJ, et al. Abdominal aortic reconstruction in infected fields: early results of the United States cryopreserved aortic allograft registry. J Vasc Surg 2002; 35: 847-52 [DOI] [PubMed] [Google Scholar]
- Lesèche G, Castier Y, Petit MD, et al. Long-term results of cryopreserved arterial allograft reconstruction in infected prosthetic grafts and mycotic aneurysms of the abdominal aorta. J Vasc Surg 2001; 34: 616-22 [DOI] [PubMed] [Google Scholar]
- Berchtold C, Eibl C, Seelig MH, et al. Endovascular treatment and complete regression of an infected abdominal aortic aneurysm. J Endovasc Ther 2002; 9: 543-8 [DOI] [PubMed] [Google Scholar]
- Katz BH, Black RA, Colley DP. CT-guided fine needle aspiration of a periaortic collection. J Vasc Surg 1987; 5: 762-4 [PubMed] [Google Scholar]
- Jones KG, Bell RE, Sabharwal T, et al. Treatment of mycotic aortic aneurysms with endoluminal grafts. Eur J Vasc Endovasc Surg 2005; 29: 139-44 [DOI] [PubMed] [Google Scholar]
- Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg 2007; 46: 906-12 [DOI] [PubMed] [Google Scholar]
- Oz MC, Brener BJ, Buda JA, et al. A ten-year experience with bacterial aortitis. J Vasc Surg 1989; 10: 439-49 [DOI] [PubMed] [Google Scholar]
- Oderich GS, Panneton JM, Bower TC, et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg 2001; 34: 900-8 [DOI] [PubMed] [Google Scholar]
- Laohapensang K, Rutherford R, Arworn S. Infected aneurysm. Ann Vasc Dis 2010; 3: 16-23 [DOI] [PMC free article] [PubMed] [Google Scholar]