Abstract
Background: Peripheral arterial disease (PAD) has been recognized as an independent risk factor for vascular events and contributes to an adverse prognosis. Long-term administration of clopidogrel is recommended to prevent atherothrombotic events for patients with established PAD. We investigated the benefits of clopidogrel treatment in Japanese patients with PAD.
Materials and Methods: COOPER (Clopidogrel for atherOthrombOtic event management in patients with PERipheral arterial disease) was a multicenter, randomized, double-blind study to evaluate the safety and efficacy of clopidogrel (75 mg/day) compared to ticlopidine (200 mg/day) in Japanese patients with PAD. The primary endpoint was the cumulative incidence of “safety events of interest” comprising clinically significant bleeding, blood disorders, hepatic dysfunction and other serious adverse events up to 12 weeks. The other safety events and vascular events were also assessed. Patients were followed up to 52 weeks.
Results: A total of 431 patients with PAD were randomly assigned to receive either clopidogrel or ticlopidine. The cumulative incidences of “safety events of interest” at 12 weeks were 2.4% and 13.6% of patients who received clopidogrel and ticlopidine, respectively (adjusted hazard ratio, 0.161; 95% confidence interval, 0.062 to 0.416; p <0.0001). Bleeding and vascular events were similar in both groups.
Conclusion: Clopidogrel demonstrated a favorable benefit/risk profile than ticlopidine in Japanese patients with PAD.
(Trial registration: ClinicalTrials.gov, Identifier: NCT00862420)
Keywords: clopidogrel, peripheral arterial disease, antiplatelets, vascular event, clinical study
Introduction
Atherothrombosis is a systemic disease caused by thrombosis based on atherosclerotic vascular plaques and considered as the leading cause of death in the Western world,1) causing a huge economic and social burden. According to a Japanese statistical report,2) the second and third leading causes of death are heart disease and cerebrovascular disease (stroke), respectively, and the major part of those diseases are included in atherothrombosis. Therefore, it is critical to prevent the occurrence of vascular events in high risk patients with atherothrombosis. Peripheral arterial disease (PAD) is considered to be a clinical manifestation of systemic atherothrombosis: the presence of atherosclerosis in the peripheral vasculature, such as the iliac arteries and the arteries of the lower extremities, is often indicative of other atherothrombotic diseases in other vascular beds such as the coronary and cerebral arteries. Consequently, PAD is associated with a high risk of myocardial infarction (MI), stroke and vascular death,3) in addition to representative symptoms of the lower extremities such as peripheral coldness, intermittent claudication, pain, necrosis, and ulcer leading to amputation of the leg.4)
According to the results of the one-year follow-up investigation of the REACH (REduction of Atherothrombosis for Continued Health) registry, the combined endpoint comprising cardiovascular (CV) death, MI and stroke was observed in 6.47% of patients with ischemic cerebrovascular disease (CVD), in 4.52% of patients with coronary artery disease (CAD) and 5.35% of patients with PAD.5) Approximately 60% of PAD patients in the REACH registry had multiple CV risk factors, and the risk of vascular events was correlated with the number of these risk factors. For patients with PAD, prevention of vascular events is essential for improving their morbidity-mortality prognosis.6)
The ankle brachial index (ABI) is particularly important for the diagnosis in asymptomatic patients; an ABI score of <0.9 is defined as PAD. Indeed, in the German Epidemiological Trial on the Ankle Brachial Index (getABI), conducted in patients ≥65 years (8.7% had symptomatic and 12.3% had asymptomatic PAD), the risk of all-cause death or vascular events in patients with PAD was higher than in those without PAD, regardless of the presence or absence of symptoms.7)
In Japan, PAD is increasing mainly due to the increase of aging and westernized dietary habit.8) The risk of vascular events in patients with PAD has been underestimated for a long time and treatment was prescribed for improvement of symptoms rather than for prevention of vascular events, which is essential for the improvement of prognosis.
Clopidogrel is a thienopyridine derivative which selectively inhibits the binding of adenosine 5′-diphosphate to the P2Y12 receptors on platelets, thereby inhibiting platelet aggregation.9,10) In many countries, use of clopidogrel has been approved to prevent atherothrombotic events in patients with recent strokes, recent MI or established PAD, based on the results of the large-scale CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events) clinical study.11) CAPRIE showed a statistically significant decrease in the incidence of the combined endpoint of vascular events (MI, ischemic stroke and vascular death) with the administration of clopidogrel compared to aspirin. Moreover, a statistically significant decrease in the incidence of vascular events was observed in the clopidogrel group with PAD, according to a subgroup analysis.
The Trans-Atlantic Inter-Society Consensus II (TASCII),12) which is recognized as an international guideline for the management of PAD, recommends long-term administration of antiplatelet drugs in all patients with symptomatic PAD (Grade A). In TASC-II, clopidogrel is mentioned as a drug expected to prevent atherothrombotic vascular events regardless of complications with CV diseases.
However, indications of clopidogrel in Japan are limited for the reduction of recurrence after ischemic cerebrovascular disorder, and ischemic heart disease to which percutaneous coronary intervention is being planned so far. Therefore, the Clopidogrel for atherOthrombOtic event management in patients with PERipheral arterial disease (COOPER) study was conducted to evaluate the safety and efficacy of clopidogrel (75 mg/day), compared to ticlopidine (200 mg/day), up to 12 weeks in Japanese patients with PAD. Additionally, the safety and efficacy of long-term administration of clopidogrel was assessed.
Materials and Methods
This was a multicenter, randomized, double-blind, double-dummy, parallel-group clinical study at 52 medical centers across Japan from February 2009 to May 2011. The study was approved by the institutional review boards of participating medical institutions and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization (ICH) and Japanese guidelines for Good Clinical Practice. Written informed consent was obtained from all patients prior to their participation in the study.
Men and women aged ≥20 years with established PAD who fulfilled at least one of the following criteria were eligible for inclusion: current intermittent claudication associated with an ABI <0.90 or a history of intermittent claudication in association with previous intervention in a leg, such as angioplasty, atherectomy, bypass graft, or other vascular intervention, including amputation. Exclusion criteria included: acute atherothrombotic events or any invasive therapies within 30 days prior to randomization, or any planned invasive therapies within 12 weeks after randomization; a previous disabling condition with a severe cerebral deficit; bleeding diathesis, coagulopathy or active bleeding diseases; previous intracranial bleeding or hemorrhagic stroke (excluding micro-bleeding detected with MRI); present diabetic retinopathy; uncontrolled hypertension; presence or history of leucopenia, neutropenia or thrombocytopenia within 1 year prior to randomization; or severe renal or hepatic insufficiency.
Study design
Eligible patients were randomized in a 1:1 ratio to either a clopidogrel group or a ticlopidine group in double-blind fashion at an independent registration center (Fig. 1). Concomitant use of other antiplatelet therapy, past or recent MI or ischemic cerebrovascular disorder, and concomitant diabetes mellitus were used as stratification variables. In the first 12 weeks of the study (double-blind period), patients in the clopidogrel group received 75 mg of clopidogrel once daily and patients in the ticlopidine group received 200 mg of ticlopidine once daily, for 12 weeks. For the study, the dosage of clopidogrel was set to 75 mg/day according to approved package inserts in overseas and the results obtained from previous studies. The dosage of ticlopidine was set to 200 mg/day based on Japanese real clinical practice.13) In the double-blind period, matching placebos, with appearances identical to that of investigational products, were taken in the same manner as investigational products. In the following 40 weeks of the study (open treatment period), all of the patients who had completed 12 weeks of treatment received 75 mg of clopidogrel once daily up to 52 weeks, regardless of the treatment group in the first 12 weeks. Concomitant administration of antiplatelets other than clopidogrel, ticlopidine, aspirin, beraprost sodium, limaprost alfadex, alprostadil, and alprostadil alfadex was permitted as background antiplatelet therapies for the improvement of symptoms at the investigator's discretion. However, during the first 12 weeks, the dosage was not permitted to be changed, to avoid confounding the evaluation. Hematologic and biochemical tests were scheduled at baseline, every 2 weeks for the first 8 weeks and 12 weeks after randomization in the double-blind period, and thereafter every 8 weeks up to the end of the study. ABI was measured at baseline, every 4 weeks in the double-blind period, then every 8 weeks up to the end of the study using each institution's procedure.
Fig. 1.
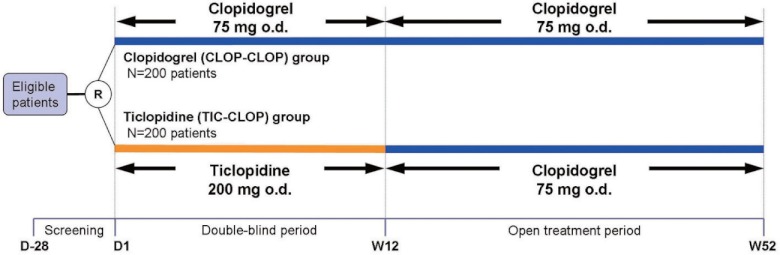
Study design.
R: randomization; o.d.: once daily
The primary objective of the study was to compare the risk of “safety events of interest” between clopidogrel and ticlopidine up to 12 weeks. “Safety events of interest” is defined as the following adverse events for which a causal relationship to the investigational products could not be ruled out: 1) clinically significant bleeding (non-traumatic hemorrhage that results in death, or requires inpatient hospitalization or prolongation of existing hospitalization); 2) blood disorders (leukopenia, neutropenia or thrombocytopenia); 3) hepatic dysfunction (an elevated value of AST, ALT, γ-GTP, ALP, total bilirubin or detection of jaundice); or 4) other serious adverse events.
Secondary objectives were to compare the risk of bleeding adverse events, serious adverse events, adverse events, adverse drug reactions and vascular events, for clopidogrel and ticlopidine up to 12 weeks. Any adverse event related to bleeding was defined as a bleeding adverse event. Any adverse event that results in death; is life-threatening; requires inpatient hospitalization or prolongation of existing hospitalization; results in persistent or significant disability/incapacity, a congenital anomaly/birth defect or a medically important event were defined as serious adverse events. For vascular events, the following two composites of an event cluster were evaluated: 1) cerebral infarction, MI or other CV death and 2) cerebral infarction, MI, other CV death or hospitalization due to an ischemic event (except for events that did not accompany exacerbation of disease or symptoms). Moreover, the safety and efficacy of administering clopidogrel for a total of 52 weeks were documented.
Statistical analysis
The sample size was calculated, based on the clinical study for Japanese patients with noncardioembolic cerebral infarction.14) Based on the assumption that 3.1% of the clopidogrel group and 12.3% of the ticlopidine group would reach the primary endpoint at week 12, 182 patients per group were required to detect a significant difference between treatments, using a log-rank test with a 5% two sided significance level and 90% statistical power. In consideration of the possibility of dropouts, 200 patients per group were set as the sample size.
Safety and efficacy were analyzed, based on the all randomized population. The first occurrence of the “safety events of interest” up to 12 weeks in each group was compared using a log-rank test stratified by the randomization stratification variables. Cumulative incidences with 95% confidence interval (CI) were estimated using the Kaplan-Meier technique and Greenwood's formula. Hazard ratios (HR) with 95% CIs were estimated using the Cox model with the treatment group and the allocation variables. Other secondary endpoints were analyzed in the same manner as the primary endpoint. All statistical tests were two-tailed, and the level of statistical significance was set at 5% for validity testing.
This study has been registered at www.ClinicalTrials.gov, with Identifier: NCT00862420.
Results
Patient characteristics
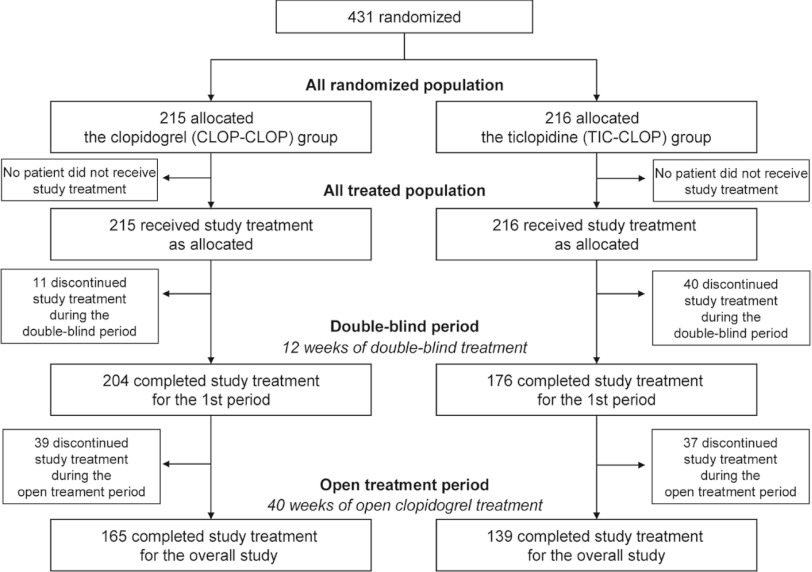
A total of 431 patients with PAD were randomly assigned to receive either clopidogrel (75 mg/day) or ticlopidine (200 mg/day) in the first 12 weeks (Fig. 2). During the first 12 weeks treatment, 11 patients (5.1%) in the clopidogrel group and 40 patients (18.5%) in the ticlopidine group discontinued the administration of investigational product prematurely: of these, 6 patients in the clopidogrel group, and 35 patients in the ticlopidine group discontinued the study treatment due to adverse events. The mean (standard deviation [SD]) duration of exposure was 82.8 (14.2) days for the clopidogrel group and 77.3 (20.3) days for the ticlopidine group. In the subsequent open clopidogrel treatment period, 39 patients (18.1%) in the group treated with clopidogrel for the entire 52 weeks (CLOP-CLOP group), and 37 patients (17.1%) in the group treated with ticlopidine for the first 12 weeks followed by clopidogrel for the remaining 40 weeks (TIC-CLOP group), discontinued the study treatment. The main reason was also an adverse event.
Fig. 2.
Patient flow diagram.
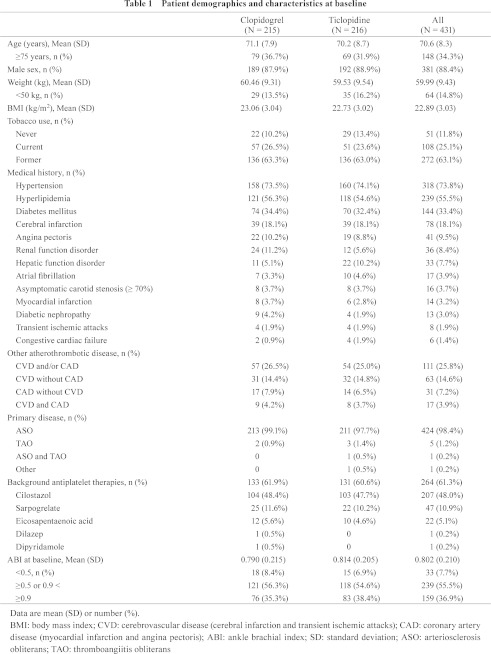
Baseline demographics and clinical characteristics were well balanced between the treatment groups (Table 1). Overall, 34.3% of patients were 75 years or older, and 88.4% were male. 63.1% of the patients were former tobacco users, and 25.1% were still smoking. The most frequently observed medical histories were hypertension (73.8%), hyperlipidemia (55.5%), diabetes mellitus (33.4%), and cerebral infarction (18.1%). A medical history of CVD (cerebral infarction and transient ischemic attacks) and/or CAD (MI and angina pectoris) was reported in 25.8% of the patients. Many more cases of hepatic function disorder were observed for medical history in the ticlopidine group than in the clopidogrel group, and the proportion of cases of hepatic dysfunction that is one of the components of the primary endpoint, “safety events of interest”, at the first 12 weeks was no higher in the population with hepatic function disorder in the medical history. As a primary disease of PAD, although a few patients with thromboangiitis obliterans (TAO) were reported, arteriosclerosis obliterans (ASO) was the most reported primary disease, accounting for 98.6% of patients. At randomization, a total of 207 patients (48.0%) used cilostazol, and 47 (10.9%) used sarpogrelate for background antiplatelet therapy. ABI at baseline was 0.802 ± 0.210 (mean ± SD), and 7.7% of patients had an ABI lower than 0.5.
Safety assessment
The risk of “safety events of interest” (primary endpoint) up to 12 weeks was statistically significantly lower in the clopidogrel group compared with the ticlopidine group (adjusted HR, 0.161 [95% CI, 0.062 to 0.416]; p <0.0001, stratified log-rank test) (Table 2). The cumulative incidences of first “safety events of interest” at 12 weeks for the clopidogrel and ticlopidine groups were 2.4% and 13.6%, respectively (Fig. 3A). In both groups, the most frequently observed first events were hepatic dysfunction. The risk of hepatic dysfunction was also statistically significantly lower in the clopidogrel group than in the ticlopidine group (adjusted HR, 0.160; 95% CI, 0.056 to 0.462; p = 0.0001, stratified log-rank test) (Table 2).
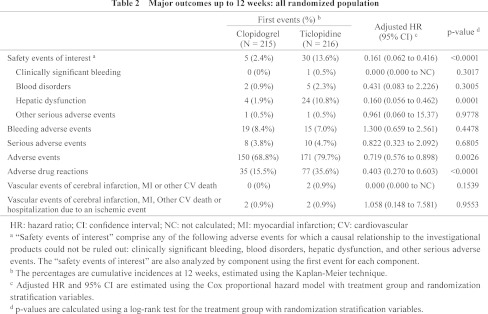
Fig. 3.
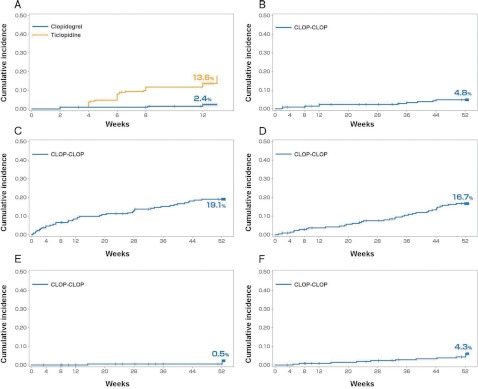
Kaplan-Meier curves of first events up to 12 weeks and 52 weeks: all randomized population.
A: The cumulative incidence of “safety events of interest” in the clopidogrel group was significantly lower than that of the ticlopidine group (2.4% [95% CI, 0.3% to 4.4%] vs. 13.6% [95% CI, 9.0% to 18.2%] at 12 weeks; adjusted HR, 0.161; 95% CI, 0.062 to 0.416; p <0.0001). B: The cumulative incidence of “safety events of interest” for the CLOP-CLOP group up to 52 weeks (4.8% [95% CI, 1.9% to 7.8%]). C: The cumulative incidence of bleeding adverse events for the CLOP-CLOP group up to 52 weeks (19.1% [95% CI, 13.8% to 24.4%]). D: The cumulative incidence of serious adverse events for the CLOP-CLOP group up to 52 weeks (16.7% [95% CI, 11.7% to 21.8%]). E: The cumulative incidence of vascular events of cerebral infarction, MI or other CV death for the CLOP-CLOP group up to 52 weeks (0.5% [95% CI, 0.0% to 1.4%]). F: The cumulative incidence of vascular events of cerebral infarction, MI, other CV death or hospitalization due to an ischemic event for the CLOP-CLOP group up to 52 weeks (4.3% [95% CI, 1.6% to 7.1%]).
The cumulative incidences of first bleeding adverse events and serious adverse events up to 12 weeks showed no statistically significant differences between the two groups (p = 0.4478 and p = 0.6805, respectively, stratified log-rank test), although treatment with clopidogrel was associated with a significantly lower risk of adverse events and adverse drug reactions compared to treatment with ticlopidine (p = 0.0026 and p <0.0001, respectively, stratified log-rank test) (Table 2).
With regards to long-term safety, the cumulative incidences of first “safety events of interest”, bleeding adverse events, and serious adverse events at 52 weeks for the CLOP-CLOP group were 4.8%, 19.1% and 16.7%, respectively (Fig. 3B–3D). The occurrences of the first “safety events of interest” and bleeding adverse events were constant from 12 weeks to 52 weeks, but the cumulative incidence curves were more gradual after the first 12 weeks. Meanwhile, the occurrence of first serious adverse events was constant throughout the 52 weeks of treatment.
Efficacy assessment
The cumulative incidence of first vascular events of cerebral infarction, MI or other CV death at 12 weeks was 0% in the clopidogrel group and 0.9% in the ticlopidine group, which was not different between the two treatment groups (p = 0.1539, stratified log-rank test) (Table 2). The two events reported in the ticlopidine group were fatal cerebral infarction and nonfatal MI. The cumulative incidence of first vascular events of cerebral infarction, MI, other CV death or hospitalization due to an ischemic event at 12 weeks for the clopidogrel and ticlopidine groups was 0.9% for both groups. The two hospitalizations due to an ischemic event reported in the clopidogrel group were due to exacerbation of intermittent claudication and transient ischemic attack. In this study, the incidence of PAD-related ischemic symptoms in lower extremities, which is associated with an indication for ticlopidine and changes in ABI value which is an indicator of PAD were evaluated exploratively, and the results showed a similar tendency between the ticlopidine and clopidogrel groups.
Regarding long-term efficacy, the cumulative incidence of first vascular events of cerebral infarction, MI or other CV death at 52 weeks for the CLOP-CLOP group was 0.5% (Fig. 3E). The occurrence rates were constant and remained low throughout the 52 weeks of treatment. The cumulative incidence of first vascular events of cerebral infarction, MI, other CV death or hospitalization due to an ischemic event was 4.3% for the CLOP-CLOP group at 52 weeks. The occurrence rates of vascular events were constant throughout the long-term exposure (Fig. 3F).
Discussion
Antiplatelet therapy is recommended by the international guideline, TASC II, to reduce the risk of CV complications and mortality in patients with symptomatic PAD and aspirin or clopidogrel is recommended as a standard treatment.12) In contrast to TASC II, Japanese guidelines recommend aspirin or ticlopidine.15,16) However, aspirin does not have an indication for PAD in Japan, although it has been reported to reduce the risk of atherothrombotic events in meta-analysis.17) Ticlopidine has been used in patients with PAD with the intention of preventing atherothrombotic events, because it has been reported to reduce the risk of MI, stroke, and vascular death in patients with PAD in Swedish study.18) However, the use of ticlopidine has decreased because clinically significant adverse reactions such as thrombotic thrombocytopenic purpura, agranulocytosis, and severe liver dysfunction have been reported.19,20) Therefore, an alternate antiplatelet drug that may be effective for preventing vascular events in patients with PAD is needed to fulfill unmet clinical needs in Japan.
Clopidogrel has been shown to have superior efficacy to aspirin, in terms of reducing the risk of vascular events in patients with PAD but with similar safety in the CAPRIE study. However, the safety and efficacy of clopidogrel in Japanese patients with PAD is unknown. Therefore, this study evaluated the safety and efficacy of clopidogrel compared to ticlopidine in Japanese patients with PAD. Ticlopidine, a powerful antiplatelet drug, was selected as a comparator of the same thienopyridine class as clopidogrel, and a two-step study design (a comparative double-blind treatment of clopidogrel and ticlopidine for the first 12 weeks, followed by an open treatment of clopidogrel up to 52 weeks) was devised in order to avoid safety concerns with the long-term use of ticlopidine (Fig. 1).
The cumulative incidence of the first “safety events of interest” at 12 weeks for the clopidogrel group was significantly lower compared to the ticlopidine group (2.4% and 13.6%, respectively). This difference was mainly due to a greater incidence of hepatic dysfunction and blood disorders in the ticlopidine group (1.9% and 0.9% in clopidogrel, 10.8% and 2.3% in ticlopidine, respectively), even blood disorders did not show a statistically significant difference (Table 2). The tendency of greater incidence of “safety events of interest” (especially, hepatic dysfunction and blood disorders) in the ticlopidine group than in the clopidogrel group had been observed in the clinical study in Japanese patients with noncardioembolic cerebral infarction, too.14)
Long-term administration of antiplatelets is recommended in TASC II, and thus, an assessment of the long-term safety of an antiplatelet drug is essential. Although a direct comparison study between clopidogrel to ticlopidine in long-term safety was not performed in Japanese patients with PAD, it can be said that favorable, long-term safety of clopidogrel was suggested, taking into consideration the results of bleeding adverse events, “safety events of interest”, serious adverse events, adverse events and adverse drug reactions of this study, which showed that the incidence of these events did not notably increase with the prolongation of the administration of clopidogrel, up to 52 weeks.
Bleeding adverse events are supposed to be the most concerned events during administration of antiplatelet drugs. Therefore, the bleeding adverse events occurred from the start of study treatment up to 14 days after discontinuation of study treatment (hereafter on-treatment bleeding adverse events) were evaluated. The incidence of on-treatment bleeding adverse events in the CLOP-CLOP group for the 52 weeks of treatment with clopidogrel was 18.1% (39/215). Among them, the bleeding adverse events leading to treatment discontinuation were observed in 5 patients (2.3%), including 2 subdural hematomas and 1 gastrointestinal hemorrhage (Table 3). No intracranial hemorrhage except the subdural hematomas was occurred. A patient who showed gastrointestinal hemorrhage suffered from gastric cancer and large intestine carcinoma.
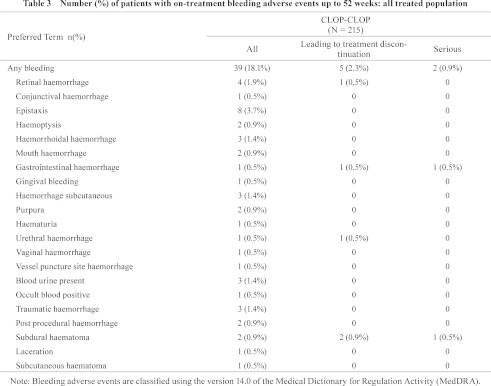
It was reported that the bleeding risk under the combination antiplatelet therapy was higher than single antiplatelet therapy for minor and major bleeding events; however, there was no significant difference in fatal or intracranial hemorrhage in the evaluation by meta-analysis.21) In this study, although some antiplatelet drugs, such as aspirin and beraprost, were prohibited from being used concomitantly, other antiplatelet drugs were allowed to be used concomitantly. The rate of concomitant use of other antiplatelet drugs (background antiplatelet therapy) was 61.9% (133/215) in the clopidogrel group. Among background antiplatelet therapies, the one most frequently used was cilostazol at 48.4% (104/215), followed by sarpogrelate at 11.6% (25/215) and eicosapentaenoic acid at 5.6% (12/215). The incidence of on-treatment bleeding adverse events was relatively higher in patients using other antiplatelet drugs concomitantly, but the difference was not statistically significant. Indeed, there was no fatal or cerebral hemorrhaging during 52 weeks of treatment with clopidogrel in this study, regardless of use with other antiplatelet agents concomitantly, which is consistent with the findings of the meta-analysis.
Subanalysis of the CAPRIE study, a comparative study between clopidogrel and aspirin in patients who had experienced a recent ischemic stroke, a recent MI, or symptomatically established PAD, suggests greater efficacy of clopidogrel in preventing recurrent vascular events, including cerebral infarction, MI or other CV deaths, in patients with symptomatically established PAD, as compared with aspirin up to 3 years.11) In our study, clopidogrel did not show a significantly greater reduction in either vascular events as the composite of cerebral infarction, MI or other CV death (p = 0.1539) or as the composite of cerebral infarction, MI, other CV death or hospitalization due to an ischemic event (p = 0.9553) compared to ticlopidine at 12 weeks (Table 2). It should be noted, however, that there was no cerebral infarction, MI, nor other CV death in the clopidogrel group but one fatal cerebral infarction and one nonfatal MI did occur in the ticlopidine group.
In Japan, ticlopidine is indicated for the improvement of ischemic symptoms such as ulcer, pain, and feeling of coldness associated with chronic arterial occlusion in the lower extremities, which are also considered as the main symptoms of PAD. The incidence of ischemic events of the lower extremities as described above and changes in ABI were similar in both groups. This result suggests that clopidogrel may provide a certain level of protection of PAD-related ischemic events, which is an indication for ticlopidine, although further studies are warranted.
Regarding long-term prevention, the cumulative incidence of first vascular events of cerebral infarction, MI or other CV death at 52 weeks was sufficiently low (0.5%) in the CLOP-CLOP group, and the occurrence rate of events was constant during long-term exposure. The results are consistent with the findings of the CAPRIE study, indicating that clopidogrel provides substantial benefits in preventing vascular events in patients with PAD up to 3 years.11)
Coexistence of PAD with symptomatic coronary or cerebrovascular diseases is common, and patients with those concomitant diseases have a higher risk of experiencing a vascular event.22,23) Subanalysis of the CAPRIE study demonstrated that clopidogrel was more effective at reducing the risk of vascular events, as compared to aspirin in patient populations that were either with or without a history of other atherothrombotic diseases.11) Therefore, it is likely that clopidogrel can reduce the risk of vascular events in Japanese patients with PAD, regardless of concomitant atherothrombotic diseases.
Limitations on the interpretation of the results of this study should be noted. Because the last 40 weeks of this study employed open administration of clopidogrel, in the absence of a control group, objective assessment of the superiority of clopidogrel over ticlopidine in terms of both safety and efficacy is limited. Ideally, the double-blinded, parallel comparison design should have been expanded into the long-term assessment, but the long-term administration of ticlopidine should be avoided, due to safety concerns. In addition, the number of patients included in this study was not large enough to detect a statistically significant difference in the incidence of vascular events between the two groups.
Conclusion
Clopidogrel demonstrated a significantly lower risk of “safety events of interest” comprising clinically significant bleeding, blood disorders, hepatic dysfunction and other serious adverse events than ticlopidine during 12 weeks of treatment in Japanese patients with PAD, without increasing risk of bleeding. The risk of vascular events were similar in both groups. In addition, bleeding and vascular event occurrence was constant throughout the long-term exposure of clopidogrel.
In summary, clopidogrel demonstrated a favorable benefit/risk profile than ticlopidine in Japanese patients with PAD.
Disclosure Statement
HS, KK, KT and MN have received honoraria and/or research funding from Sanofi K. K. and/or served as consultants to Sanofi K. K. YH is an employee of Sanofi K.K.
Acknowledgment
The authors wish to thank all the investigators, staffs and patients who participated in this study for their valuable contributions. This study was supported by Sanofi K.K., an affiliate of Sanofi group.
Appendix. List of Medical Centers Participated in the Cooper Study
Jikei University Hospital
Japanese Red Cross Society Matsuyama Red Cross Hospital
Saitama Cardiovascular and Respiratory Center
Shizuoka Red Cross Hospital
JR Sendai Hospital
Toyonaka Municipal Hospital
Mito Red Cross Hospital
Hyogo Brain and Heart Center
Aichi Medical University Hospital
National Hospital Organization Kyushu Medical Center
Ehime Prefectural Central Hospital
Fukushima Kouseikai Fukushima Daiichi Hospital
Southern Tohoku Medical Clinic
Tokyo Women's Medical University Yachiyo Medical Center
Aichi Cardiovascular and Respiratory Center
Kasugai Municipal Hospital
Saiseikai Wakayama Hospital
National Hospital Organization Okayama Medical Center
Oita Prefectural Hospital
Sapporo City General Hospital
Aishin Memorial Hospital
Nikko Memorial Hospital
Tokushima Prefectural Central Hospital
Fukui Prefectural Hospital
Asahikawa Medical College Hospital
Isesaki Municipal Hospital
Suwa Red Cross Hospital
Shizuoka Medical Center
Kanazawa Medical Center
Okayama Rosai Hospital
Shinsuma General Hospital
Kumamoto City Hospital
Chiba Cardiovascular Center
The University of Tokyo Hospital
Kanazawa Medical University Himi Municipal Hospital
Kanamori Clinic
Kanazawa Cardiovascular Hospital
Hiroshima City Asa Hospital
Hokkaido Junkanki Hospital
Maebashi Red Cross Hospital
Japanese Red Cross Kumamoto Hospital
Fukuoka City Medical Association Hospital
Fukuoka University Chikushi Hospital
Saga Prefectural Hospital Koseikan
Kokura Memorial Hospital
Ohta Nishinouchi Hospital
Cardiovascular Hospital of Central Japan
Shinkoga Hospital
Tokyo Medical University Hospital
Nagoya University Hospital
Kawasaki Medical School Hospital
Toho University Ohashi Medical Center
References
- Viles-gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J 2004; 25: 1197-207 [DOI] [PubMed] [Google Scholar]
- Abridged life tables for Japan 2009, Japanese Statistics and Information Department. Available on web <http://www.mhlw.go.jp/english/database/db-hw/lifetb09/dl/contents.pdf>. [Google Scholar]
- Hiatt WR. Pharmacologic therapy for peripheral arterial disease and claudication. J Vasc Surg 2002; 36: 1283-91 [DOI] [PubMed] [Google Scholar]
- Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes 2010; 3: 642-51 [DOI] [PubMed] [Google Scholar]
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297: 1197-206 [DOI] [PubMed] [Google Scholar]
- Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326: 381-6 [DOI] [PubMed] [Google Scholar]
- Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009; 120: 2053-61 [DOI] [PubMed] [Google Scholar]
- Shigematsu H. Medical care for vascular diseases. Treatment and diagnosis for chronic arterial obliteration. J Jpn Med Assoc 2009; 115: 317-23 (in Japanese). [Google Scholar]
- Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 2001; 107: 1591-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001; 409: 202-7 [DOI] [PubMed] [Google Scholar]
- A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) CAPRIE Steering Committee. Lancet 1996; 348: 1329-39 [DOI] [PubMed] [Google Scholar]
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007; 33(Suppl 1): S1-75 [DOI] [PubMed] [Google Scholar]
- Matsubara J, Shiiya N, Ishida A, et al. Current status of medication therapy for arteriosclerosis obliterans (ASO) in university hospitals in Japan. J Jpn Coll Angiol 2007; 47: 153-62 (in Japanese) [Google Scholar]
- Fukuuchi Y, Tohgi H, Okudera T, et al. A randomized, double-blind study comparing the safety and efficacy of clopidogrel versus ticlopidine in Japanese patients with noncardioembolic cerebral infarction. Cerebrovasc Dis 2008; 25: 40-9 [DOI] [PubMed] [Google Scholar]
- Guidelines for management of anticoagulant and anti-platelet therapy in cardiovascular disease (JCS 2009). Circ J 2009; 73Suppl III (in Japanese) [PubMed] [Google Scholar]
- Guidelines for management of peripheral arterial occlusive diseases (JCS 2009). Circ J 2009; 73, Suppl III (in Japanese) [Google Scholar]
- Antithrombotic Trialists' (ATT) Collaboration. Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L, Bergqvist D, Boberg J, et al. Prevention of myocardial infarction and stroke in patients with intermittent claudication: effects of ticlopidine. Results from STIMS, the Swedish Ticlopidine Multicentre Study. J Intern Med 1990; 227: 301-8 [DOI] [PubMed] [Google Scholar]
- Hirata K, Takagi H, Yamamoto M, et al. Ticlopidineinduced hepatotoxicity is associated with specific human leukocyte antigen genomic subtypes in Japanese patients: a preliminary case-control study. Pharmacogenomics J 2008; 8: 29-33 [DOI] [PubMed] [Google Scholar]
- Bennett CL, Davidson CJ, Raisch DW, et al. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Arch Intern Med 1999; 159: 2524-8 [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Malinin AI, Ferguson JJ, et al. Bleeding risks of combination vs. single antiplatelet therapy: a meta-analysis of 18 randomized trials comprising 129,314 patients. Fundam Clin Pharmacol 2008; 22: 315-21 [DOI] [PubMed] [Google Scholar]
- Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline). J Am Coll Cardiol 2011; 58:2020-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Stroke Organisation; Authors/Task Force Members. Tendera M, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extra-cranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2851-906 [DOI] [PubMed] [Google Scholar]