Abstract
Objective: Chronic renal insufficiency may be a relative contraindication to endovascular aneurysm repair (EVAR) for the use of contrast enhanced mediums. It is thought that more contrast enhanced media are needed in patients who are not anatomically suitable for EVAR, because of procedural difficulties. We reviewed a 2 year EVAR experience at our institution to determine whether the procedure and use of contrast enhanced mediums has any deleterious effect on renal function in patients with pre-existing chronic renal insufficiency.
Materials and Methods: EVAR was performed in 46 patients with pre-existing chronic renal insufficiency without hemodialysis. Patients were retrospectively assigned to two groups on the basis of their preoperative creatinine clearance levels. Furthermore, patients were assigned to two other groups on the basis of anatomical suitability for EVAR. The absolute change in the serum creatinine (Cr) level was reviewed in the each renal insufficiency group between the preoperative and post-operative time periods.
Results: No increase in the serum Cr level was noted, and no patient required temporary or permanent hemodialysis, in any of the groups.
Conclusions: EVAR with contrast agents can be accomplished in patients with chronic renal insufficiency without hemodialysis; therefore,elevated Cr levels maynot be a contraindication in EVAR.
Keywords: endovascular, aneurysm, renal insufficiency, contrast enhanced medium
Introduction
Over the past decade, endovascular aneurysm repair (EVAR) with intra-arterial contrast enhanced mediums has become the predominant method in the treatment of infrarenal abdominal aortic aneurysms (AAA). Despite recent, prospective studies that have demonstrated better outcomes after EVAR in the perioperative and intermediate time periods, concerns remain about the long-term durability and safety of EVAR.1–6)
EVAR requires intra-arterial administration of contrast enhanced mediums, which can cause impaired renal function, leading to end-stage renal disease. The incidence of contrast-induced acute kidney injury is low (2%) in the general population, but it is high in serum creatinine (Cr) at-risk groups of patients.7) One patient population at increased risk for perioperative complications after open or endovascular repair is the group with baseline renal insufficiency. Renal insufficiency is a well-recognized complication after open aortoiliac aneurysm repair. However, the effects of EVAR on renal function in these patients remain uncertain. Chronic renal insufficiency is a relative contraindication in the use of intra-arterial contrast enhanced mediums and is thought to further increase the risks of EVAR. Although recent studies have shown that progressive renal dysfunction may develop in patients after EVAR, data are conflicting with regard to the effects of EVAR on renal function compared with standard open repair.8–10)
In addition, EVAR is usually possible if patients have the appropriate anatomical morphology. It is thought that more contrast enhanced media is needed for patients who were not suitable for EVAR, because their procedures are more difficult and additional devices are used.
The purpose of this study was to compare the effects of EVAR on renal function in patients with pre-existing renal insufficiency. We reviewed a 2 year experience with EVAR at our institution to determine whether the procedure, when performed with intra-arterial contrast enhanced mediums, has any deleterious effects on the renal function in patients with pre-existing chronic renal insufficiency.
Materials and Methods
From April 2009 to March 2011, patients with an AAA larger than 5 cm in diameter, with and without iliac involvement, were eligible for EVAR. Patients at high risk, with severe cardiopulmonary disease, hostile abdomen, or other major comorbid conditions, were offered treatment with a variety of commercial endografts, including the Excluder (W. L. Gore & Associates, Flagstaff, Ariz), Zenith (Cook, Bloomington, Ind) and Powerlink System (Endologix, Irvine, Calif).
We performed EVAR using the almost same procedure that has been described previously.11) Distal fixation sites were usually the common iliac artery. MReye embolization coils (Cook) were mostly used to exclude flow to the hypogastric arteries intraoperatively when the distal fixation site was the external iliac artery.
In patients with chronic renal insufficiency, perioperative intravenous hydration with 0.5–1L of normal saline solution plays a well-established role in reducing the risk of nephropathy induced by contrast medium. In addition, the administration of all nephrotoxic drugs was discontinued. Non-ionic contrast medium Iohexol (Omnipaque 300), thought to be the more suitable contrast medium, was used.7) Furthermore, to minimize the cumulative nephrotoxic effects of intravascular contrast agents, intraoperative imaging, including arteriography and intravascular ultrasounds (Volcano s5 Imaging System), were used for target site identification, landing zone measurement, neck quality analysis, preprocedural quality assessment, and trouble-shooting during EVAR.12)
A retrospective review was performed from the charts, surgical reports, and laboratory data of these patients. In the initial post-operative period, the serum Cr was measured on post-operative days 1, 2 and 7. Patients with worsening renal function, illustrated by an increase in serum Cr, were observed for daily serum Cr measurement until acute tubular necrosis resolved or improved. Creatinine clearance (CCr) indicated the measure of glomerular filtration rate (GFR), and was calculated with the Cockroft-Gault formula: CCr = (140 − Age) × Weight (kg) (0.85 if female)/ (72 × Serum Cr [mg/dl]).13)
Patients were retrospectively assigned to two groups based on their preoperative CCr: group I (n = 31) who had CCr 30 to 50 mL/min and group II (n = 15) who had CCr less than 30 mL/min. None of the patients had a history of hemodialysis. Exclusion criteria included chronic renal insufficiency requiring hemodialysis, and symptomatic high-grade renal artery stenosis requiring renal angioplasty and stenting. And the patients were excluded if there was coiling of hypogastric arteries the day before operation.
EVAR is usually possible if patients have appropriate anatomical morphology. Patients who were suitable for EVAR have been thought to have a reduced procedural length, fewer transfusion requirements, and less morbidity.14) In a different viewpoint, we then further divided the patients into two groups according to the anatomical suitability criteria, which included an aortic neck diameter of 18–28 mm, neck length more than 15 mm, neck angulation less than 60 degrees, iliac diameter of 7.5–20 mm, and a length of the iliac sealing zone more than 10 mm. Relative contraindications to endovascular repair, such as the thrombus load within the aortic neck were not considered.14) Patients who have appropriate anatomical morphology for EVAR were included in group A (n = 25), the other patients were included in group B (n = 21).
Statistical analyses were performed with the t-test and Fisher’s exact test, and statistical significance was defined when the P value <0.05.
Results
Forty-six patients with renal insufficiency who were not receiving hemodialysis underwent EVAR using an intra-arterial contrast enhanced medium over the 2 year period. The mean follow-up was 6.3 months.
All patients had pre-existing chronic renal insufficiency with baseline CCr of either 30 to 50 mL/min (group I) or less than 30 mL/min (group II). The average patient age was 79.4 years. Co-morbid conditions included diabetes mellitus (8 of all 46 cases; 17.4%). The mean diameter of the AAA was 55.4 mm. A total of 25 cases were treated with Excluder (W. L. Gore & Associates, Flagstaff, Ariz); 19 cases, with Zenith (Cook, Bloomington, Ind); and 2 cases, with the Powerlink System (Endologix, Irvine, Calif). Six patients had an aortoiliac aneurysm that extended to the iliac bifurcation, so coil embolization of the hypogastric artery was performed with MReye embolization coils (Cook), intraoperatively.
The mean intraoperative contrast agent administration was 97.7 mL during EVAR. The preoperative aneurysm diameter, duration of surgery, prevalence of co-morbid conditions, and amount of intraoperative contrast agent administration are summarized in Table 1.
Changes in renal function were evaluated by comparing preoperative with the latest available postoperative serum Cr. Patients with a 20% or greater increase in serum Cr were not recognized. Five patients with a 10% or greater increase in serum Cr were defined to have impaired renal function.
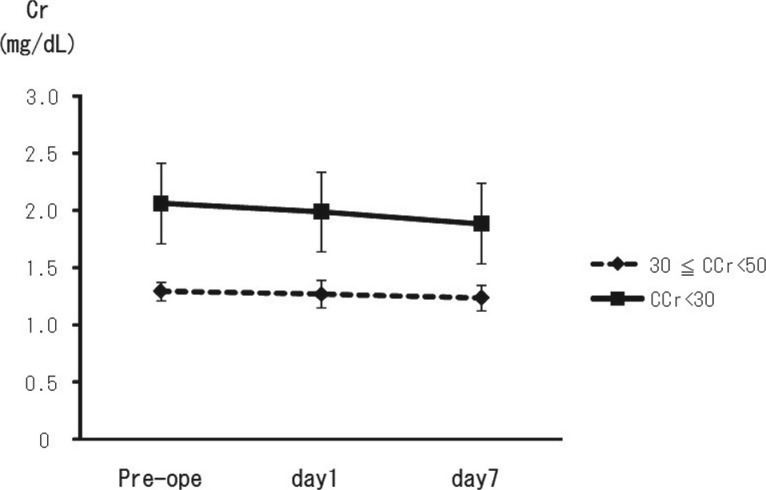
The absolute change in serum Cr in the each renal insufficiency group between the post-operative and preoperative time periods was similar (Table 1 and Fig. 1).
Fig. 1.
All patients had pre-existing chronic renal insufficiency with baseline CCr of 30 to 50 mL/min (group I) or less than 30 mL/min (group II). The absolute change in the serum creatinine level in each renal insufficiency group between the post-operative and preoperative time periods was similar between groups.
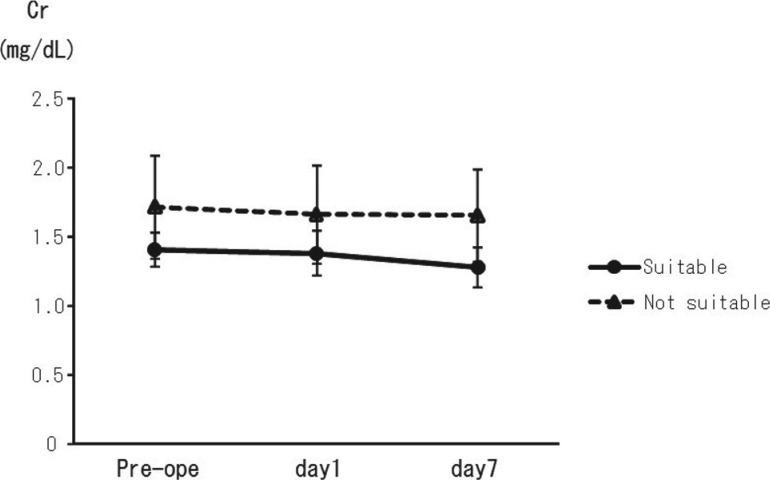
We hypothesized that patients who were identified as being unsuitable for EVAR (group B, n = 21), based on anatomical criteria, needed a larger volume of intraoperative contrast agent compared with patients having suitability for EVAR (group A, n = 25). Contrary to our hypothesis, differences in the volume of contrast needed did not reach statistical significance among the groups. Significantly fewer additional devices, such as an aortic extension graft and a bare stent, were needed for patients with anatomical suitability for EVAR (group A) (0.7 versus 1.5: P = 0.02). In patients who were anatomically suitable for EVAR (group A), the CCr tended to be higher in the preoperative period (38.1 mL/min) compared with the group of patients who were deemed anatomically unsuitable for the procedure (group B) (33.8 mL/min, though this difference was not significant; Table 2) however, there were no statistically significant differences in serum Cr levels during the preoperative, post-operative, or the follow-up periods (Table 2 and Fig. 2) in these patients.
Fig. 2.
In group A, which were anatomically suitable for EVAR, the CCr tended to be higher in the preoperative period compared with the unsuitable group (group B), however, there were no statistically significant differences in serum creatinine levels observed during the preoperative, postoperative, or the follow-up periods.
To reduce the cumulative nephrotoxic effects of intravascular contrast agents in patients with severe, chronic renal insufficiency, intravascular ultrasounds (Volcano s5 Imaging System) were used for 4 cases (of 46 cases: 8.7%), performing arteriography using only a minimal volume of contrast enhanced medium after the device is in place, near the level of the renal arteries; and not performing contrast-enhanced imaging studies after the operation. We have also performed color flow duplex ultrasound as the surveillance method post-EVAR to reduce the amount of contrast enhanced medium used in these patients.
None of the patients in our study required temporary or permanent hemodialysis during the follow-up period.
Discussion
Over the past decade, EVAR, which provides a less invasive alternative to open AAA repair, has become an established method for aortoiliac aneurysm repair.1–6) EVAR requires intra-arterial administration of radiographic contrast enhanced mediums, which can cause impaired renal function, thus leading to end-stage renal disease. Chronic renal insufficiency is a relative contraindication to the use of intra-arterial contrast enhanced mediums and is thought to further increase the risks of EVAR.
Renal insufficiency is a well-recognized complication after open AAA repair; its incidence after EVAR remains poorly documented. The incidence of worsening renal function in patients undergoing open surgical AAA repair with normal preoperative renal function was found to be 5.4%, and increases two- to three-fold in patients with pre-existing chronic renal insufficiency.15,16) Mortality with associated renal failure in patients undergoing EVAR with and without pre-existing chronic renal insufficiency has been reported to be 47% and 3%, respectively. 17) Acute renal failure requiring hemodialysis has been reported to complicate the course of 0.5% to 2% of patients undergoing elective AAA repair, with associated in-hospital mortality ranging from 25% to 66%.15,18–26) Contrary to that report, our findings demonstrated that at 7 day after the operation, the incidence of a 10% or greater increase in serum Cr over the preoperative level was 10.9%, which was significantly lower in comparison to the previous report.
It was previously reported that chronic renal insufficiency increases the incidence of contrast-induced nephrotoxicity. High osmolar contrast agents, dehydration, nephrotoxic drugs, and diabetes mellitus further increase the risk for worsening renal failure. Nephropathy induced by contrast enhanced media is defined as an impairment in renal function that occurs within 48 to 72 hours of giving contrast enhanced medium. This impairment is characterized by an increase in serum Cr of at least 0.5 mg/dL, or 25% above the baseline. Cr typically peaks three to five days after contrast administration and returns to baseline values within two weeks. We focused a great deal of interest on the serum Cr at post-operative days 1, 2 and 7.
However, serum Cr is a poor indicator of renal function. The best measure of renal function—and, therefore, the risk of contrast nephropathy—is the glomerular filtration rate. The rate can be estimated from serum Cr using the Cockroft-Gault formula or by modification of diet in the renal disease formula. It is especially useful to estimate the GFR in patients with borderline serum Cr, a value of less than 60 mL/min predicts increased risk of contrast nephropathy.7,27)
EVAR is usually possible if patients have appropriate anatomical morphology. Patients who are suitable for EVAR had been thought to have a reduced procedural length, decreased transfusion requirements and require lower amounts of contrast enhanced mediums. In our study, it was a most notable point that no statistically significant differences were found in the duration of surgery or the amount of contrast agents needed between the groups, despite additional devices needed for patients with inappropriate anatomical morphology for EVAR. Patients who were suitable for EVAR tended to indicate higher CCr in the preoperative period compared with patients who were not suitable for EVAR (not statistically significant), however, there were no statistically significant differences in serum Cr levels during the preoperative, post-operative, or the follow-up periods.
Our findings demonstrate that with perioperative precautions, including adequate intravenous hydration, use of low osmolar contrast agents, and avoidance of nephrotoxic drugs, the risk for worsening renal failure is low and not significantly increased in patients with pre-existing chronic renal insufficiency compared with patients with normal renal function. The incidence of post-operative complications between the study groups was not significantly different. In patients with chronic renal insufficiency who do not require dialysis, EVAR with intra-arterial contrast enhanced media can be performed with a limited and acceptable morbidity and mortality. With appropriate precautions, elevated Cr levels need not be a contraindication to the use of intra-arterial contrast enhanced mediums in EVAR. In addition, we have preferentially used duplex ultrasound imaging to evaluate maximum aneurysm diameter and presence of an endoleak in the follow-up period after EVAR.
Considering our results, the mean intraoperative contrast agent administration was 97.7 mL during EVAR, acceptable amounts of contrast enhanced mediums was about 100 mL for patients with pre-existing chronic renal insufficiency. However, in order to recommend precise tolerance level of contrast media in EVAR, further investigation in a larger number of patients is needed to assess the short-term and long-term results.
Conclusion
Endovascular repair of abdominal aortic aneurysms in patients with pre-existing renal insufficiency can be performed safely with preservation of renal function, however, patients with pre-existing renal insufficiency continue to be at risk for progressive renal dysfunction, and protective measures should be taken to preserve renal function in this patient population. With appropriate precautions, including limiting the volume of nonionic contrast agents, chronic renal insufficiency, therefore, need not be a contraindication for EVAR with intraarterial contrast agents, even if the patients did not have anatomical suitability for EVAR.
Disclosure Statement
Atsushi Guntani and co-authors have no conflicts of interest.
References
- Greenhalgh RM, Brown LC, Kwong GP, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004; 364: 843-8 [DOI] [PubMed] [Google Scholar]
- Prinssen M, Verhoeven EL, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004; 351: 1607-18 [DOI] [PubMed] [Google Scholar]
- Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg 2000; 32: 739-49 [DOI] [PubMed] [Google Scholar]
- Zarins CK. The US AneuRx Clinical Trial: 6 year clinical update 2002. J Vasc Surg 2003; 37: 904-8 [DOI] [PubMed] [Google Scholar]
- EVAR Trial Participants Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomized controlled trial. Lancet 2005; 365: 2179-86 [DOI] [PubMed] [Google Scholar]
- EVAR Trial Participants Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomized controlled trial. Lancet 2005; 365: 2187-92 [DOI] [PubMed] [Google Scholar]
- Goldfarb S, Mccullough PA, Mcdermott J, et al. Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc 2009; 84: 170-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alric P, Hinchliffe RJ, Picot MC, et al. Long-term renal function following endovascular aneurysm repair with infrarenal and suprarenal aortic stent-grafts. J Endovasc Ther 2003; 10: 397-405 [DOI] [PubMed] [Google Scholar]
- Alsac JM, Zarins CK, Heikkinen MA, et al. The impact of aortic endografts on renal function. J Vasc Surg 2005; 41: 926-30 [DOI] [PubMed] [Google Scholar]
- Parmer SS, Carpenter JP. Endovascular aneurysm repair with suprarenal vs infrarenal fixation: a study of renal effects. J Vasc Surg 2006; 43: 19-19 e9 [DOI] [PubMed] [Google Scholar]
- Ohki T, Veith FJ, Sanchez LA, et al. Varying strategies and devices for endovascular repair of abdominal aortic aneurysms. Semin Vasc Surg 1997; 10: 242-56 [PubMed] [Google Scholar]
- von Segesser LK, Marty B, Ruchat P, et al. Routine Use of Intravascular Ultrasound for Endovascular Aneurysm Repair: Angiography is not Necessary. Eur J Vasc Endovasc Surg 2002; 23: 537-42 [DOI] [PubMed] [Google Scholar]
- Mehta M, Veith FJ, Lipsitz EC, et al. Is elevated creatinine level a contraindication to endovascular aneurysm repair? J Vasc Surg 2004; 39: 118-23 [DOI] [PubMed] [Google Scholar]
- Perrott S, Puckridge PJ, Foreman RK, et al. Anatomical suitability for endovascular AAA repair may affect outcomes following rupture. Eur J Vasc Endovasc Surg 2010; 40: 186-190 [DOI] [PubMed] [Google Scholar]
- Johnston KW. Multicenter prospective study of non-ruptured abdominal aortic aneurysms. II: Variables predicting morbidity and mortality. J Vasc Surg 1989; 9: 437-47 [DOI] [PubMed] [Google Scholar]
- Joseph MG, McCollum PT, Lusby RJ. Abnormal pre-operative creatinine levels and renal failure following abdominal aortic aneurysm repair. Aust N Z J Surg 1989; 59: 539-41 [DOI] [PubMed] [Google Scholar]
- Walker SR, Yusuf SW, Wenham PW, et al. Renal complications following endovascular repair of abdominal aortic aneurysms. J Endovasc Surg 1998; 5: 318-22 [DOI] [PubMed] [Google Scholar]
- Chen JC, Hildebrand HD, Salvian AJ, et al. Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. J Vasc Surg 1996; 24: 614-20, discussion 621-3. [DOI] [PubMed] [Google Scholar]
- Hertzer NR, Mascha EJ, Karafa MT, et al. Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg 2002; 35: 1145-54 [DOI] [PubMed] [Google Scholar]
- Hua HT, Cambria RP, Chuang SK, et al. Early outcomes of endovascular versus open abdominal aortic aneurysm repair in the National Surgical Quality Improvement Program-Private Sector (NSQIP-PS). J Vasc Surg 2005; 41: 382-9 [DOI] [PubMed] [Google Scholar]
- Kazmers A, Jacobs L, Perkins A. The impact of complications after vascular surgery in Veterans Affairs Medical Centers. J Surg Res 1997; 67: 62-6 [DOI] [PubMed] [Google Scholar]
- Hallin A, Bergqvist D, Holmberg L. Literature review of surgical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2001; 22: 197-204 [DOI] [PubMed] [Google Scholar]
- Braams R, Vossen V, Lisman BA, et al. Outcome in patients requiring renal replacement therapy after surgery for ruptured and non-ruptured aneurysm of the abdominal aorta. Eur J Vasc Endovasc Surg 1999; 18: 323-7 [DOI] [PubMed] [Google Scholar]
- Olsen PS, Schroeder T, Perko M, et al. Renal failure after operation for abdominal aortic aneurysm. Ann Vasc Surg 1990; 4: 580-3 [DOI] [PubMed] [Google Scholar]
- Parmer SS, Fairman RM, Karmacharya J, et al. A comparison of renal function between open and endovascular aneurysm repair in patients with baseline chronic renal insufficiency. J Vasc Surg 2006; 44: 706-11 [DOI] [PubMed] [Google Scholar]
- Brown LC, Brown EA, Greenhalgh RM, et al. UK EVAR Trial Participants Renal function and abdominal aortic aneurysm (AAA): the impact of different management strategies on long-term renal function in the UK EndoVascular Aneurysm Repair (EVAR) Trials. Ann Surg 2010; 251: 966-75 [DOI] [PubMed] [Google Scholar]
- Mathew R, Haque K, Woothipoom W. Acute renal failure induced by contrast medium: steps towards prevention. BMJ 2006; 333: 539-40 [DOI] [PMC free article] [PubMed] [Google Scholar]