Abstract
Background: Endovascular aneurysm repair has gained widespread acceptance, and there has been a significant increase in the number of aneurysms treated with stent grafts. However, the endovascular technique alone is often not appropriate for anatomically complex aneurysms involving the neck branches. We used the TAG stent for thoracic aortic aneurysms (TAA), and report our initial results.
Patients and Results: We deployed 80 TAG stents in 65 patients electively treated with TAA between June 2006 and June 2008. Thoracic endovascular aneurysm repair (TEVAR) was performed in 45 cases of descending aortic aneurysm with no morbidity or mortality. A combination of open surgery and TEVAR was performed in 11 out of 20 cases with aneurysms of the aortic arch. The prior total arch replacement and elephant trunk procedure was performed in 3 cases with dilated ascending aorta, total debranching from ascending aorta with sternotomy in 5, and carotid-carotid artery crossover bypass in 3 cases. Meanwhile, TEVAR with coverage of the left subclavian artery was performed in the remaining 9 distal arch cases. In 3 cases with extremely short necks, a 0.018” guide wire was inserted percutaneously in a retrograde manner through the common carotid artery (CCA) into the ascending aorta to place the stent graft in close proximity to the CCA (wire protection). In 1 of these 3 cases, the TAG stent was deployed through the CCA, and the 0.018” guide wire was used to deliver a balloon-expandable stent in order to restore the patency of the CCA. In arch and distal arch aneurysm cases, perioperative mortality and the incidence of stroke were both 5.0%; dissection of the ascending aorta was seen in one case (5.0%).
Conclusion: As treatment for descending aortic aneurysms, TEVAR can replace conventional open repair. However, TEVAR for arch aneurysms has some problems, and further improvement is necessary. (English Translation of Jpn J Vasc Surg 2010; 19: 547-555.)
Keywords: Keywordsthoracic aortic aneurysm, endovascular surgery, stent graft
Introduction
The TAG thoracic aortic stent graft system (TAG) (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) was approved from the Ministry of Health, Labor and Welfare (MHLW) as a stent graft for thoracic aortic aneurysms (TAA) in Japan on July 2008, which may further promote the application of thoracic endovascular aneurysm repair (TEVAR). The advantages of TEVAR in this field are applicability without thoracotomy, which may be performed under local anesthesia in some cases, and a low incidence of paraplegia because aortic clamp is not necessary. However, TEVAR alone is insufficient in many cases. Herein, we report the current outcomes and problems of ourTEVAR and measures taken to overcome the problems.
Subjects and Methods
1. Subjects
Seventy-three patients underwent TEVAR at our hospital during a 2-year period between June 2006 and the approval of TAG as a device for TAA. Emergency surgery for rupture was performed in 8 of them. Excluding the rupture cases, 65 cases were investigated (Table 1). Since the device for TAA was not approved by MHWL in Japan during this period, it was purchased by private import after approval by the Internal Review Board (IRB). For the device, TAG was used. TAA was a thoracic descending aortic aneurysm in 45 cases and arch and distal arch aneurysms in 20. There were 49 males and 16 females aged 22–94 years (mean: 71.1 ± 13.5 years). TEVAR was specified as the first choice for thoracic descending aortic aneurysms when it was anatomically applicable, and it was selected for 45 of 46 cases (97.8%). Accordingly, TEVAR was applied even for 22- and 40-year-old patients with traumatic TAA. For arch and distal arch aneurysms, open repair (aortic arch replacement) were specified as the first choice, and TEVAR was selected in 20 of 34 cases (58.8%). When the patients were divided into those with a thoracic descending aortic aneurysm and those with arch and distal arch aneurysms, the age was significantly higher, aneurysm size was greater, and more concomitant diseases were present in patients with arch and distal arch aneurysms (Table 1).
Table 1. Patients’ profile.
2. Surgical method
In all patients, the access route and aneurysm were evaluated by contrast CT before surgery to decide on a therapeutic indication and device size, and a treatment strategywas prepared. Basically, a treatment plan was designed to prepare an at least 20-mm-long neck on both the proximal and distal sides. Particularly, a 20-mm length of the neck on the proximal side was ensured as much as possible in arch aneurysm cases because of a disadvantage of placement in the curve region. When this was not possible, the left subclavian artery was covered to deploy a stent graft, for which communication of the bilateral vertebral arteries was confirmed by preoperative CT. When no communication was noted, bypass to the left subclavian artery was considered. When a 20-mm length of the proximal neck could not be obtained by covering the left subclavian artery, carotid-carotid artery bypass and bypass from the ascending aorta to the arch branch were considered. In surgery, one side of the common femoral artery was exposed, and the other side was punctured, through which a 5-F sheath was inserted. In cases with extremely tortuous and arch aneurysms, guide wire descent from the right upper limb was caught with a snare catheter inserted through the cut-down side and pulled through. A pigtail catheter was inserted through the 5-F sheath for angiography, a 20–24-F sheath was inserted through the cut-down side to deploy a stent graft, and the stent was expanded with a Tri-Lobe balloon. After confirmation byangiography, the sheath was removed and exchanged with a 9-F sheath, followed by confirmation of the absence of access route injury by angiography and intravascular ultrasonography (IVUS).
Results
1. Thoracic descending aortic aneurysm (Table 2)
Table 2. Results of TEVAR.
There were 45 patients with thoracic descending aortic aneurysms. All patients were discharged without any complication. The mean operation time was about 2.5 hours, the blood loss was about 300 ml, and about 150 ml of contrast medium was used. An approach through the common iliac artery was necessary in 3 of the 45 cases (6.7%), and pull-through wire was necessary due to tortuousity in 3 (6.7%) (Table 2). The duration of hospital stay after treatment was about 5 days. No endoleak was noted after TEVAR in any patient.
2. Arch and distal arch aneurysms
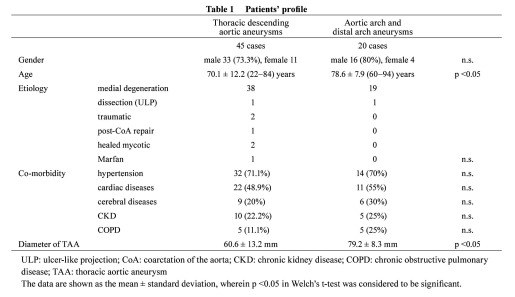
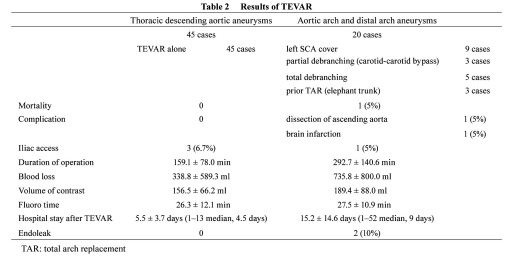
Arch replacement was performed in 14 patients with arch and distal arch aneurysms because the systemic condition was favorable and indicated open repair. The remaining 20 patients were treated with TEVAR, and the left subclavian artery was covered to deploy a stent graft in 9 of the 20 cases, in which communication of the bilateral vertebral arteries was confirmed by preoperative CT, and intraoperative selective angiography was performed to confirm that the bilateral vertebral arteries were connected to the basilar arteries, respectively. Surgery was combined because of the lack of a proximal landing zone in 11 of 31 cases (Table 2). Carotid−carotid artery (−subclavian artery) bypass was applied in 3 (Fig. 1), total debranching from the ascending aorta was applied in 5 (Figs. 2 and 3), and 2-stage TEVAR with prior ascending arch replacement with an elephant trunk in 3 (Fig. 4, Table 2). To deploy a stent graft in the closest proximity to the left common carotid artery, the carotid artery was percutaneously punctured and 0.018” guide wire was inserted and advanced to the ascending aorta, followed by stent graft deployment (carotid puncture wire protection method) in 3 cases (Fig. 5). In one of these, an Express Stent (Boston Scientific) was retrogradely deployed in the left common carotid artery because this artery was covered.
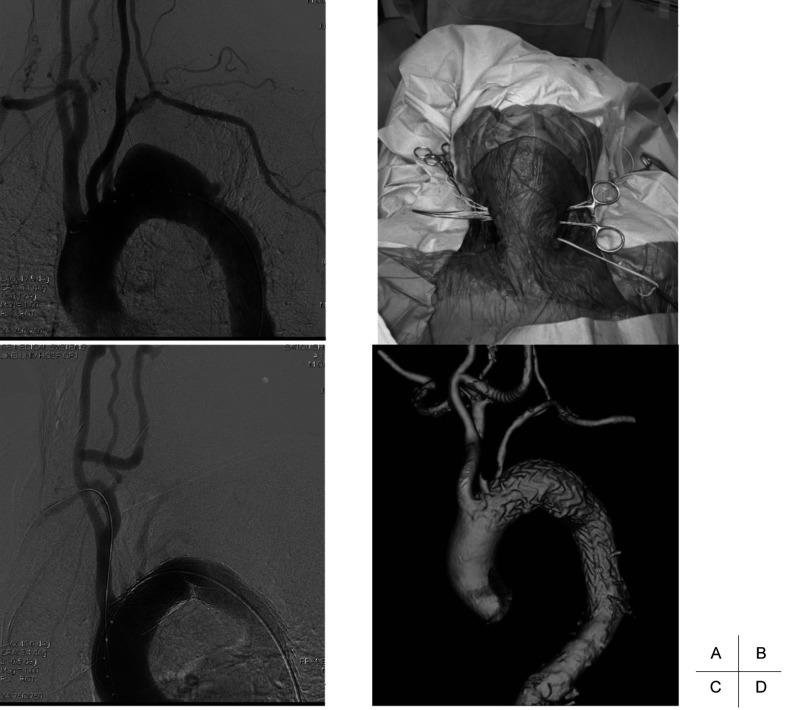
Fig. 1.
TEVAR with partial debranching (carotid-carotid artery cross-over bypass).
A: Preoperative angiography shows a distal arch aneurysm located just distal to the left subclavian artery.
B: The graft is passed between the esophagus and cervical vertebra.
C: Postoperative angiography shows that the aneurysm is excluded from the systemic circulation after partial debranching and TEVAR.
D: Postoperative CT angiography reveals that the aneurysm is completely excluded from the systemic circulation.
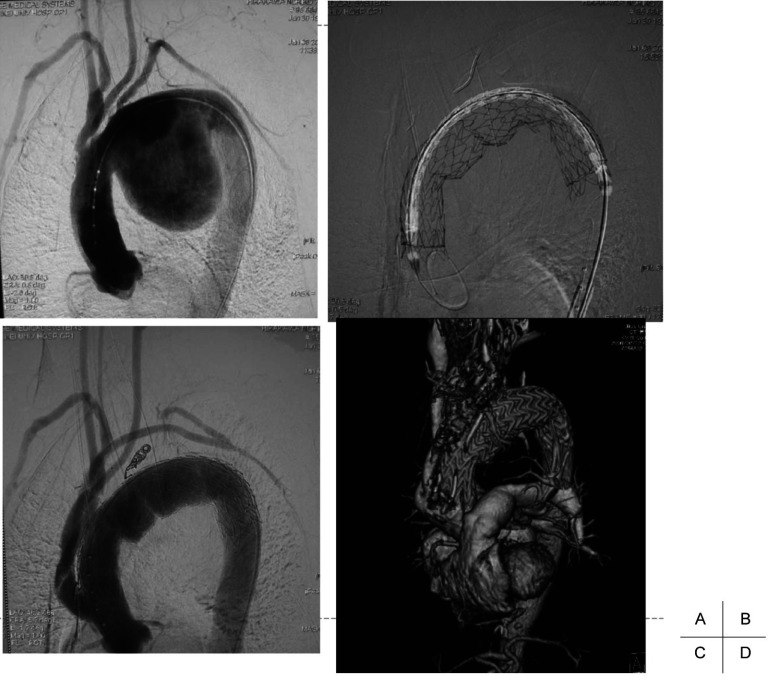
Fig. 2.
TEVAR with total debranching.
A: Preoperative angiography shows that neck branches are involved with a huge arch aneurysm.
B: The TAG stent graft is deployed from the ascending aorta after total debranching of the neck vessels with a median sternotomy.
C: Postoperative angiography shows that the aneurysm is excluded from the systemic circulation after total debranching and TEVAR.
D: Postoperative CT angiography reveals that the aneurysm is completely excluded from the systemic circulation.
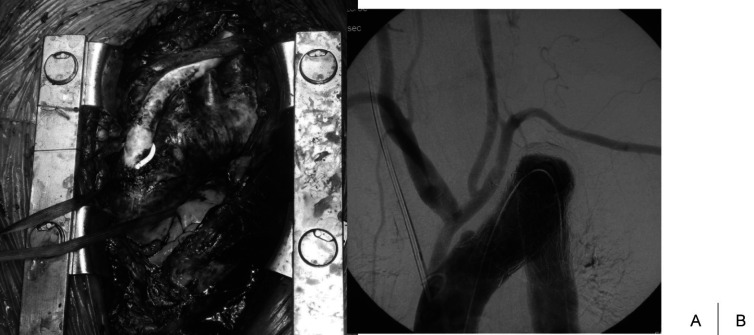
Fig. 3.
Operative view of total debranching + TEVAR.
We usually use the tube graft for debranching the neck vessels.
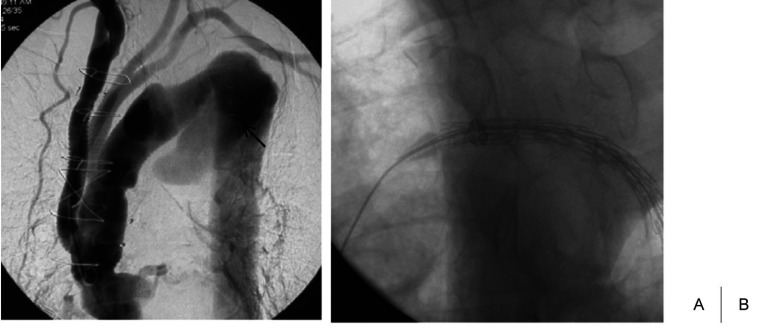
Fig. 4.
Prior total arch replacement with an elephant trunk and TEVAR.
Initial angiography shows a residual thoracic aneurysm after total arch replacement with an elephant trunk. Arrows show the vascular clip to identify the distal end of the elephant trunk.
Fig. 5.
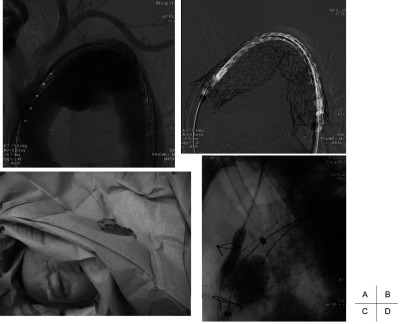
TEVAR with carotid puncture wire protection.
A 0.018” guide wire was inserted percutaneously in a retrograde manner through the common carotid artery (CCA) into the ascending aorta with the objective of deploying the stent graft in close proximity to the CCA. When the stent graft is deployed across the CCA, this 0.018” guide wire is used to deliver a balloon-expandable stent in order to restore the patency of the CCA.
Approaches through the common iliac artery and ascending aorta were necessary for 1 of the 20 cases (5%) and 2 debranching cases (10%), respectively. One case in which the left subclavian artery was covered to apply TEVAR for a distal arch aneurysm was lost due to sudden death from myocardial infarction. Regarding complications, dissection of the ascending aorta occurred in one, and right hemiplegia associated with cerebral infarction occurred in one treated with debranching. In addition, endoleak was noted in 2: a distal arch aneurysm case treated with TEVAR and an arch aneurysm case treated with debranching, and additional stent grafting was necessary after one year.
Discussion
The TAG stent graft system (W. L. Gore & Associates, Inc.) approved by MHWL as a thoracic stent graft on July 12, 2008 and was made available in Japan (Fig. 6). It was approved by the FDA in 2005 and has been widely used in the U.S., and it will also become widely used in Japan. We have been using TAG since 2006, 2 years earlier than the approval by MHWL in Japan. TAG is relatively flexible and bends, readily fitting the curve of the arch, as its characteristics. In addition, it can be accurately placed and is less likely to be moved by blood pressure toward the distally because it is released from the center. Furthermore, the Tri-lobe balloon for expansion does not completely occupy the aortic lumen when it is inflated, which also avoids moving the stent toward the distally.
Fig. 6.
Treatment strategies for aortic arch and distal arch aneurysms.
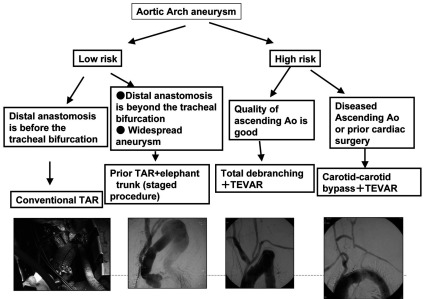
TAR: total arch replacement
The indication is, briefly, aneurysms with a long neck (20 mm or longer) and a diameter of 23–37 mm. According to the clinical trial results of TEVAR using TAG and open repair of thoracic aneurysms in the U.S., the surgical mortality rates were 2.1 and 11.7%, respectively, showing a significantly lower rate in TEVAR.1) In addition, the incidence of postoperative complications was also significantly lower in the TEVAR group: spinal ischemia, 14% in open repair vs. 3% in TEVAR; respiratory insufficiency, 20 vs. 4%; renal failure, 13 vs. 1%.1) Our outcomes regarding the thoracic descending aortic aneurysms were also favorable. These early outcomes suggested that our treatment plan specifying TEVAR as the first-choice for thoracic aneurysms meeting the anatomical conditions is appropriate.
The delivery sheath size is 20–24 F, which frequently causes problems in peripheral blood vessels including the access route even in western people, and it should be more carefully applied in Japanese with a smaller physique. An unfavorable access route can be dealt with by an iliac arterial approach in many cases.2–4) We also investigated the presence or absence of an injury by angiography of the access route while leaving the guide wire after sheath removal, and noted iliac arterial dissection in 8 (12.3%), for which a stent was placed.
The usefulness and low-invasiveness of TEVAR for thoracic descending aneurysms are apparent, but problems of TEVAR are concentrated in arch aneurysms5,6): placement in the curve and maintenance of branch blood flow. To deploy a stent graft in the arch, it is necessary to secure a landing zone as long as possible, for which we devised various methods.
Covering of the left subclavian artery and wire protection (carotid puncture wire protection)
For distal arch aneurysms, covering of the left subclavian artery was necessary to deploy a stent graft in many cases, for which communication between the bilateral vertebral arteries was confirmed on preoperative CT, and the communication was ensured by intraoperative angiography of the vertebral arteries. When the communication is absent, reconstruction of the subclavian artery is considered in cases using the left internal thoracic artery as a graft for coronary artery bypass grafting (CABG). Wire protection is useful to deploy a stent graft directly below the left common carotid artery to elongate the neck, in which the carotid artery is percutaneously punctured using a micropuncture kit, and a 0.018” guide wire is inserted and advanced to the ascending aorta, followed by stent graft deployment (Fig. 5).7,8) This method enables stent graft deployment at the closest proximity of the carotid artery using this wire as a marker, as well as securing antegrade blood flow of the carotid artery by retrograde deployment of a balloon-expandable stent using this wire, as needed. This is a recovery method for the covered carotid artery, giving a sense of security, and it is also useful because the closest proximity of the carotid artery can be targeted. We deployed a stent at the closest site to the left carotid artery in 3 cases, and a stent was placed in the common carotid artery because the left common carotid artery was covered.
Hybrid surgery
1. Carotid-carotid artery bypass (partial debranching)
This is effective when the aneurysm can be excluded by placing a stent graft at the closest site to the brachiocephalic artery. We make an about 3-cm incision along the anterior margin of the bilateral sternocleidomastoid muscles to expose the common carotid artery, and, normally, an 8-mm PTFE graft is passed along the posterior surface of the esophagus for the shortest distance (Fig. 1). In a case using the left internal thoracic artery for CABG and a case with poor communication between the bilateral vertebral arteries, TEVAR was performed after applying bypass to the left subclavian artery. The longest proximal landing zone can be prepared by deploying a stent graft at the closest site to the brachiocephalic artery on combining the wire protection described above, i.e., the following method is useful: 0.018” wire or a 4-F sheath is inserted into the right brachial artery through the exposed right carotid artery, and 0.035” wire is placed in the ascending aorta to deploy a stent graft. Carotid−carotid artery bypass can be safely applied even after heart surgery because no median sternotomy is necessary. On the other hand, a wide landing zone cannot be prepared in cases in which the brachiocephalic and left common carotid arteries are not sufficiently apart or the arteries share a common vessel. Moreover, the proximal side of the stent may be positioned at the arch peak in some cases, to which attention should be paid because the small curvature side rises like a bird beak and may cause endoleak.
2. Debranching of all arch branches (total debranching)
When an aneurysm cannot be excluded unless it reaches t ascending aorta, debranching of all neck branches is useful9,10) on conditions that the ascending aortic properties are favorable (without calcification and thrombus) and the diameter is 37 mm or smaller. The properties of the ascending aorta are confirmed on preoperative CT, and when the properties are favorable, the ascending aorta is clamped with partial aortic clamps, and the proximal side of a prosthetic graft is end-to-side sutured. We normally end-to-side anastomose the proximal side of an 8-10-mm single prosthetic graft with the ascending aorta, followed by exposure of the brachiocephalic artery and side-to-end anastomosis with the prosthetic graft. The left common carotid artery is similarly side-to-end and the left subclavian artery is end-to-end anastomosed. The proximal sides of the neck branches were closed by suture (Fig. 2). The bifurcated prosthetic graft may be used, but, since proximal-side anastomosis was performed under partial clamp, fewer problems occur when the graft diameter is small. Since aneurysms do not disappear, unlike those after arch replacement, a smaller diameter of an additional prosthetic graft is advantageous because the prosthetic graft can be entirely covered with the thymus and fat tissue on closing the sternum. Thus, we use a tube prosthetic graft for reconstruction (Fig. 3). After debranching neck branches of an aortic aneurysm, a stent graft is deployed through the femoral artery. We temporary block the prosthetic graftl when TAG is passed through the arch to prevent cerebral infarction. When the access route from the femoral artery is unfavorable, it is useful to deploy a stent graft through one leg of a bifurcated prosthetic graft sutured to the ascending aorta. When the proximal side landing zone is short, we apply banding of the proximal-side neck from outside the aorta or fixation of the stent graft with polypropylene suture applied outside the aorta.
3. Two-stage surgery with prior arch replacement (elephant trunk)
Debranching is not indicated for cases with a dilated ascending aorta. When an aneurysm extends widely, ascending arch replacement precedes to prepare an elephant trunk on the distal side, followed by TEVAR. Setting the proximal-side landing zone in the elephant trunk, a stent graft is deployed in the region ranging to the normal distal region (Fig. 4).11,12) Extended aneurysms can be treated, and this method is also applicable for dissection cases. It is convenient to apply 3-5 vascular clips to the distal end of the elephant trunk during arch replacement as markers for TEVAR.
The open stent method,13) which has been performed in Japan, is now also used as the frozen elephant trunk technique14) in western countries. Reportedly, the systemic circulatory arrest can be shortened because distal-side anastomosis in arch replacement can be applied at a more proximal site. This method is indicated for arch replacement with poor properties of the ascending aorta, arch replacement requiring heart surgery, and aortic dissection requiring total arch replacement. However, employment of this method should be carefully decided because the occurrence of paraplegia has been pointed out,15) and we do not adopt this method.
The indication of TEVAR was expanded by combining surgery for arch and distal arch aneurysms in which a sufficient landing zone cannot be prepared because of the curve and neck branches. When debranching is employed, it is advantageous to perform TEVAR in one stage because temporary clamp of the right common carotid or artery or prosthetic graft during passing a stent graft through the arch may prevent cerebral embolus. However, in the current situation, there is still room for improvement based on the short-term outcomes including the incidence of cerebral infarction; while arch replacement can be performed safely, TEVAR cannot be specified as the first-choice for arch aneurysms. The incidence of endoleak was also higher than that after TEVAR for thoracic descending aortic aneurysms, for which careful follow-up is necessary. At present, we decide on a therapeutic strategy based on the systemic condition and anatomical characteristics of patients (Fig. 6).
Conclusion
TEVAR was investigated. The outcome of TEVAR for thoracic descending aortic aneurysms was satisfactory, suggesting that TEVAR is appropriate as a first-choice treatment. In arch and distal arch aneurysms, preparation of a sufficient landing zone is difficult in some cases due to the curve and to maintain blood flow in the neck branches. For these cases, the indication of TEVAR was expanded by combining surgery, but there is still room to improve based on the short-term outcomes. At present, the first-choice treatment for aneurysms involving the arch is arch replacement, and TEVAR should be selected corresponding to cases.
Footnotes
*This article is English Translation of Jpn J Vasc Surg 2010; 19: 547-555.
*The abstract of this report was presented at the 36th Annual Meeting of the Japanese Society for Vascular Surgery (April 2008, Tokyo).
References
- 1.Bavaria JE, Appoo JJ, Makaroun MS.Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007; 133: 369-77 10.1016/j.jtcvs.2006.07.040 [DOI] [PubMed] [Google Scholar]
- 2.Ohki T, Veith FJ. Technical adjuncts to facilitate endovascular repair of various thoracic pathology. J Card Surg 2003; 18: 351-8 10.1046/j.1540-8191.2003.02075.x [DOI] [PubMed] [Google Scholar]
- 3.)Ohki T. Techniques to facilitate thoracic stenting. Endovascular today 2006: 33-5. [Google Scholar]
- 4.Criado FJ. Iliac arterial conduits for endovascular access: technical considerations. J Endovasc Ther 2007; 14: 347-51 10.1583/06-2059.1 [DOI] [PubMed] [Google Scholar]
- 5.Criado FJ, Abul-Khoudoud OR, Domer GS.Endovascular repair of the thoracic aorta: lessons learned. Ann Thorac Surg 2005; 80: 857-63 10.1016/j.athoracsur.2005.03.110 [DOI] [PubMed] [Google Scholar]
- 6.Steinbauer MG, Stehr A, Pfister K.Endovascular repair of proximal endograft collapse after treatment for thoracic aortic disease. J Vasc Surg 2006; 43: 609-12 10.1016/j.jvs.2005.11.045 [DOI] [PubMed] [Google Scholar]
- 7.Criado FJ. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): retrograde catheterization and stenting. J Endovasc Ther 2007; 14: 54-8 10.1583/06-2010.1 [DOI] [PubMed] [Google Scholar]
- 8.Criado FJ. Chimney grafts and bare stents: aortic branch preservation revisited. J Endovasc Ther 2007; 14: 823-4 10.1583/07-2247.1 [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Kaneko M, Kuratani T.New operative method for distal aortic arch aneurysm: combined cervical branch bypass and endovascular stent-graft implantation. J Thorac Cardiovasc Surg 1999; 117: 832-4 10.1016/S0022-5223(99)70311-9 [DOI] [PubMed] [Google Scholar]
- 10.Melissano G, Civilini E, Bertoglio L.Results of endografting of the aortic arch in different landing zones. Eur J Vasc Endovasc Surg 2007; 33: 561-6 10.1016/j.ejvs.2006.11.019 [DOI] [PubMed] [Google Scholar]
- 11.Svensson LG, Kim KH, Blackstone EH.Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78: 109-16 10.1016/j.athoracsur.2004.02.098 [DOI] [PubMed] [Google Scholar]
- 12.Greenberg RK, Haddad F, Svensson L.Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112: 2619-26 10.1161/CIRCULATIONAHA.105.552398 [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Ohnishi K, Kaneko M.New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996; 94: II188-93 [PubMed] [Google Scholar]
- 14.Karck M, Chavan A, Hagl C.The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003; 125: 1550-3 10.1016/S0022-5223(03)00045-X [DOI] [PubMed] [Google Scholar]
- 15.Usui A, Fujimoto K, Ishiguchi T.Cerebrospinal dysfunction after endovascular stent-grafting via a median sternotomy: the frozen elephant trunk procedure. Ann Thorac Surg 2002; 74: S1821-4 10.1016/S0003-4975(02)04131-0 [DOI] [PubMed] [Google Scholar]