Abstract
Between 1998 and 2008, feline fecal specimens were submitted to provincial veterinary diagnostic laboratories in Regina and Saskatoon, Saskatchewan, for sucrose centrifugation-flotation (n = 635), parasite identification (n = 17), and/or Giardia (n = 283) or Cryptosporidium (n = 266) commercial direct immunofluorescence assay (IFA). The most commonly detected parasites on flotation were Toxocara cati (4.7%), Isospora (3.8%), and taeniid eggs (Echinococcus or Taenia) (1.3%). Cats less than 2 years of age were twice as likely to have a positive parasite test as cats older than 2 years. Using IFA, Giardia was detected in 9.9% of samples, and Cryptosporidium in 2.3% of samples. Relative to IFA, flotation had sensitivity values of 39% and 50% for detection of Giardia and Cryptosporidium, respectively. Giardia and Isospora were detected in a higher proportion of samples in our study population than reported in the general cat population in western Canada. This study highlights the importance of sensitivity when interpreting diagnostic tests and provides information to guide region-specific recommendations for helminth parasite prevention and treatment.
Résumé
Enquête rétrospective des parasites gastro-intestinaux félins dans l’Ouest canadien. Entre 1998 et 2008, des échantillons fécaux félins ont été soumis aux laboratoires de diagnostic vétérinaires provinciaux à Regina et à Saskatoon, en Saskatchewan, pour une centrifugation-flottaison par saccharose (n = 635), l’identification des parasites (n = 17), et/ou d’un essai par immunofluorescence direct commercial pour Giardia (n = 283) ou Cryptosporidium (n = 266). Les parasites les plus couramment détectés à la flottaison étaient Toxocara cati (4,7 %), Isospora (3,8 %) des œufs de ténia (Echinococcus ou Taenia) (1,3 %). Il était deux fois plus probable que les chats âgés de moins de 2 ans aient un test positif pour les parasites que les chats âgés de plus de 2 ans. À l’aide d’un essai par immunofluoresnce, Giardia a été détecté dans 9,9 % des échantillons et Cryptosporidium dans 2,3 % des échantillons. En rapport avec l’essai par immunofluorescence, la flottaison avait des valeurs de sensibilité de 39 % et de 50 % pour la détection de Giardia et de Cryptosporidium, respectivement. Giardia et Isospora ont été détectés dans une proportion supérieure d’échantillons dans notre population étudiée comparativement à celle des résultats signalés dans la population générale de chats de l’Ouest canadien. Cette étude souligne l’importance de la sensibilité lors de l’interprétation des tests diagnostiques et des renseignements pour guider des recommandations spécifiques à des régions pour la prévention et le traitement du parasite helminthe.
(Traduit par Isabelle Vallières)
Introduction
An understanding of local parasite diversity is essential for optimal recommendations for parasite prevention and treatment. For western Canada, current recommendations are outlined in the “Canadian Guidelines for Treatment of Parasites in Dogs and Cats” developed by the Canadian Parasitology Expert Panel (CPEP) and published in March 2009 (1). As there are limited reports of the parasite fauna of cats in western Canada, these recommendations are based primarily on expert opinion. The diversity and prevalence of gastrointestinal parasites in cats in Saskatchewan and Alberta appear to be low (2,3). In particular, protozoan parasites (e.g., Giardia, Cryptosporidium, and Isospora) are thought to be rare in cats in western Canada (1,3). However, this may reflect the lower sensitivity of flotation of a single fecal sample for protozoan cysts and oocysts relative to more recently adopted immunofluorescence assays. The objectives of this study were, therefore, to determine the diversity and abundance of gastrointestinal parasites in cats in Saskatchewan based on retrospective analysis of diagnostic test results from 1998 through 2008, and to compare the relative sensitivity of fecal flotation and a commercially available immunofluorescence assay (IFA) for detection of Giardia and Cryptosporidium in diagnostic submissions.
Materials and methods
Between 1 January, 1998 and 31 December, 2008, fecal samples and parasite specimens from cats were submitted to the Prairie Diagnostic Services (PDS) laboratories in Regina and Saskatoon for tests for endoparasites. Results for samples submitted for fecal sucrose centrifugation-flotation (4), parasite identification, and/or commercial IFA for Giardia cysts and Cryptosporidium oocysts (Cyst-A-GloTM, FL, Comprehensive Kit, Waterborne, New Orleans, Louisiana, USA) were accessed in the PDS database (Table 1). Information regarding patient history and individual patient characteristics (age, gender, and breed) was collected when available. The percentage of positive samples for each parasite species for each test (flotation or IFA) was calculated. The sensitivity [true positives/(true positives + false negatives)] and negative predictive value [true negative/(true negative + false negative)] of routine fecal flotation (relative to IFA) for detection of Giardia and Cryptosporidium were determined for those samples for which both tests were performed (261 and 247 samples, respectively). Age (< or ≥ 2 years of age) and infection status were entered into a 2 × 2 contingency table. The strength of association between variable (age) and outcome (parasite status) was reported as a Taylor series odds ratio (OR) with 95% confidence intervals (CI) (OpenEpi version 2.3.1, Atlanta, Georgia, USA).
Table 1.
Positive tests in feline fecal samples submitted (1998–2008) to the PDS laboratories in Saskatchewan for centrifugation-flotation, adult helminth identification, and immunofluorescence assay (IFA) for protozoan parasites
| Number of tests | Number of positive tests (%) | |
|---|---|---|
| Centrifugation-flotation | 635 | 73 (11.5%) |
| Adult helminth identification | 17 | 17 |
| Giardia IFA | 283 | 28 (9.9%) |
| Cryptosporidium IFA | 266 | 6 (2.3%) |
Results
The diversity of parasites detected and the proportion of samples containing each parasite on flotation are reported in Table 2. Eggs of Toxocara cati, taeniid cestode eggs, and oocysts of Isospora were the 3 parasites most commonly detected on flotation. There were 17 adult helminth parasites submitted for identification: Taenia (n = 8), T. cati (n = 6), Toxascaris leonina (n = 1), Spirometra (n = 1), and an unidentified cyclophyllid tapeworm (n = 1).
Table 2.
Parasites detected in feline fecal samples submitted (1998–2008) to the PDS laboratories in Saskatchewan for sucrose centrifugation-flotation (n = 635)
| Parasite | Number positive | Percent |
|---|---|---|
| Toxocara cati | 30 | 4.7 |
| Taeniid (Taenia or Echinococcus spp.) | 8 | 1.3 |
| Toxascaris leonina | 1 | 0.2 |
| Ancyclostoma | 1 | 0.2 |
| Physaloptera | 1 | 0.2 |
| Unidentified helminth | 3 | 0.5 |
| Isospora | 24 | 3.8 |
| Giardia | 13 | 2.0 |
| Cryptosporidium | 3 | 0.5 |
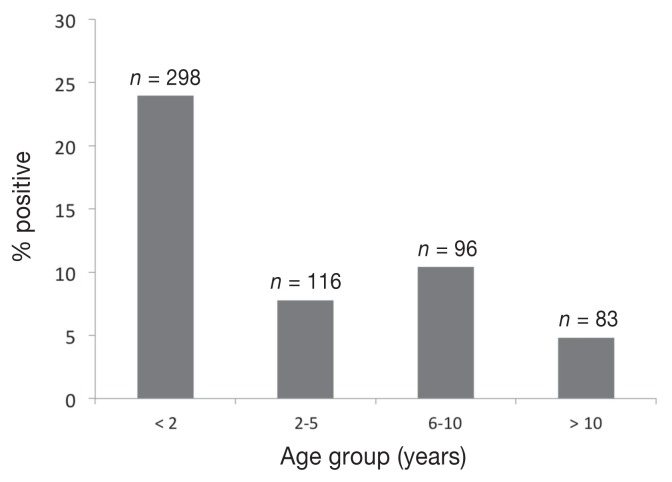
Of those submissions in which age (n = 583) and gender (n= 621) were reported, the mean age and standard deviation of the cats sampled was 4.2 ± 4.9 years, and there were 267 (43%) female and 354 (57%) males. Cats younger than 2 y of age were twice as likely to have positive fecal samples as those ≥ 2 y of age [odds ratio (OR): 2.07, 95% CI: 1.34 to 3.19]. The proportions of samples that were positive for any parasite among 4 age groups of cats are reported in Figure 1. Of the cats positive for at least 1 endoparasite species, sample submission forms for 96 (78.5%) included a reason for submission: diarrhea (70.2%), parasite noted (14.3%), previous parasitic infection (11.9%), vomiting (8.3%), blood or mucus in the feces (9.5%), and sudden death or near-fatal illness of unknown cause (10.7%).
Figure 1.
Percentage of fecal samples positive for at least 1 parasite in 4 age groups of cats. Samples were submitted to the PDS laboratories in Saskatchewan (1998–2008) for parasitological testing.
On IFA, 9.9% of 283 samples were positive for Giardia, and 2.3% of 266 were positive for Cryptosporidium (Table 1). On routine fecal flotation, Giardia was detected in only 2% of 635 samples, and Cryptosporidium in 0.5% of 635 samples (Table 2). Of the 261 samples submitted for both flotation and Giardia IFA, 4% of samples were positive for Giardia on flotation compared to 10.0% on IFA. Compared to IFA, the sensitivity of fecal flotation for detecting cysts of Giardia was 38.5%, with a negative predictive value of 93.6%. Of the 247 samples submitted for both flotation and Cryptosporidium IFA, 1.2% were positive on flotation compared to 2.4% on IFA. Compared to IFA, the sensitivity of fecal flotation for detecting oocysts of Cryptosporidium was 50%, with a negative predictive value of 98.8%.
Discussion
The diversity of helminth parasites in cat fecal samples submitted to the provincial veterinary diagnostic laboratories in Saskatchewan over an 11-year period was comparable to that reported in earlier studies in Canada. Toxocara cati and taeniid cestodes were the most commonly detected helminth parasites, similar to findings in a previous study in Saskatchewan (2) and elsewhere in Canada (1,3,5). Toxascaris leonina, Ancyclostoma, Spirometra, and Physaloptera were rare (each detected in < 1% of samples). Parasites were more commonly detected in cats < 2 y of age, as observed elsewhere (3,6). Although reported in cats in Canada, we did not find evidence of Aelurostrongylus, Aoncotheca, Capillaria, Diphyllobothrium, Eucoleus aerophila, Ollulanus tricuspis, Paragonimus, or Strongyloides(2,5,7–9). This reflects the rarity as well as the unique life cycles of these parasites (some of which, such as O. tricuspis, may not be detected on routine fecal flotation). As well, this was not a true prevalence study in a representative feline population, as samples were submitted for diagnostic purposes in client-owned animals with an underlying reason for submission, compared with cats sourced from shelters in most previous studies.
Meaningful comparisons of helminth diversity and abundance with other studies are difficult because of differences in laboratory techniques and anthelmintic use since the earlier studies. Although the sample population in our study was biased towards clinically ill animals, perhaps more likely to be parasitized than the general population, the proportions of samples positive for helminths were lower than those found in previous surveys of pet cats in eastern and central Canada. Therefore, our finding supports previous reports that gastrointestinal helminths seem to be uncommon in cats in western Canada (3,5,6,8,10). Such geographic variation in relative parasite abundance might be attributable to differences in climate, landscape, relative abundance of alternate host species, and the demographics of the sample population.
For protozoan parasites, we found a higher proportion of samples positive for Isospora (3.8%) in diagnostic submissions from cats in Saskatchewan than reported in the general population of cats in a recent study from Calgary, Alberta, in which only 1 of 85 shelter cats was positive for a coccidian parasite, and none of 68 client-owned animals were shedding oocysts (3). Prevalence of Isospora in cats elsewhere in Canada ranged from 4.0% to 10.0% (1,10). Giardia was the most commonly detected parasite in the current study. The proportion of samples positive for Giardia (10%) using IFA was considerably higher than the 1% to 2% previously reported in healthy cat populations in Canada (6), and on par with findings of 4% to 8% of 389 cats with gastrointestinal signs (11). These findings suggest an association between these protozoan parasites and clinical illness (primarily diarrhea) in cats; however, limitations of the current study include its retrospective nature, with inconsistent reporting of clinical signs and incomplete histories (such as parasiticide treatment and diet). As such, further research is needed to determine links between parasitism (mono- or poly-parasitism) and clinical manifestations of disease in client-owned cat populations.
Our study indicated that the sucrose-centrifugation flotation method is relatively insensitive for the detection of Giardia and Cryptosporidium in feline fecal samples, relative to commercial IFA. Generally, fecal smears and flotations are less sensitive for these protozoans compared with fecal antigen testing such as the IFA or other commercially available tests (e.g., enzyme-linked immunoassay snap test) (6,11,12). As well, the intermittent nature of excretion of Giardia cysts and low sensitivity of conventional testing methods make it difficult to interpret negative results. For example, using a zinc sulphate centrifugation-flotation, Giardia and Cryptosporidium were not detected in a recent study in cats in the Calgary area (3). Our findings serve as a reminder for veterinary practitioners to request IFA or other immunological tests for Giardia, which is difficult to detect on routine fecal flotation, particularly in cats presenting with chronic or intermittent diarrhea unresponsive to conventional treatment. Further research with repeated sampling of animals would help address false negatives due to inconsistent fecal shedding.
Several of the parasites reported in this study have zoonotic potential, including Toxocara cati, the hookworm Ancylostoma, taeniid eggs (potentially Echinococcus multilocularis), and Giardia and Cryptosporidum(13–18). Human infection from Toxocara has been associated with the development of visceral and ocular larval migrans (13,14). Ancyclostoma has been associated with the development of cutaneous larval migrans in humans (15). The cestode E. multilocularis is endemic to prairie Canada and has been reported in cats in Saskatoon (16); however, cats are not thought to be a primary source of human exposure and only 1 human case of alveolar hydatid disease has been reported in Canada (17). Species of Giardia and Cryptosporidium most commonly present in cats are G. cati (previously G. duodenalis Assemblage F) and C. felis, both of which are believed to be uncommon in humans, other than those infected with HIV (18–20). Zoonotic species of these protozoans found rarely in cats include G. duodenalis (previously G. duodenalis Assemblage A), G. enteritica (previously G. duodenalis Assemblage B), C. parvum, and C. muris. For G. duodenalis, it is not yet known whether the predominant genotype in cats is AI (present in animals and humans), AIII or AIV (present mainly in animals), or AII (present mainly in humans) (21). In a recent study in Ontario (22), fecal samples from 12 of 13 cats contained Assemblage A (G. duodenalis); the samples from 1 cat contained Assemblage B (G. enteritica). Both these assemblages are considered zoonotic. Further molecular genotyping is required to determine the role of zoonotic transmission in the epidemiology of enteric protozoan disease in humans.
A recent survey of veterinarians in western Canada indicated that Toxocara canis or T. cati, Giardia lamblia, T. gondii, and Echinococcus spp. were associated with the highest perceived zoonotic risk (23). Despite the potential for transmission of feline enteric parasites to their owners, only about half of veterinarians in western Canada followed established guidelines for recommending anthelmintic treatment in kittens, which include treatment at 2, 4, 6, and 8 wk of age, and then monthly until 6 mo of age (1,23). A possible reason for this is that the guidelines recommend that treatment should start when the kittens are < 1 mo old, when most have no contact with a veterinarian. Our results suggest that routine anthelmintic protocols for cats in western Canada should continue to focus on nematodes such as T. cati, and also consider enhanced testing and treatment for cestodes and protozoans in high-risk animals (i.e., outdoor cats and clinically ill cats, especially those with diarrhea). Given our findings of lower prevalence in cats > 2 y of age, diagnostic testing is warranted (as opposed to blanket therapy) for parasites in adult feline veterinary patients. Finally, as this study was retrospective and does not describe prevalence in the general “healthy” cat population, a current cross-sectional study of free-ranging and client-owned cats in Saskatchewan (using powerful molecular and immunological approaches to identification of parasite stages shed in feces), and comparative studies from cats across all of the Canadian provinces are warranted.
Acknowledgments
We gratefully acknowledge Prairie Diagnostic Services for access to their database, J. Schurer for assistance with statistical analyses, reviewers, and the Western College of Veterinary Medicine Interprovincial Summer Undergraduate Student Research Scholarship for financial support for J. Hoopes. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Beck K, Conboy G, Gilleard J, et al. Canadian Guidelines for the Treatment of Parasites in Dogs and Cats. Report of the Canadian Parasitology Expert Panel (CPEP) 2009. [Last accessed February 11, 2013]. p. 21. Available from http://www.wormsandgermsblog.com/uploads/file/CPEP%20guidelines%20ENGLISH.pdf.
- 2.Pomroy WE. A study of helminth parasites of cats from Saskatoon. Can Vet J. 1999;40:339–340. [PMC free article] [PubMed] [Google Scholar]
- 3.Joffe D, van Niekerk D, Gagné F, Gilleard J, Kutz S, Lobingier R. The prevalence of intestinal parasites in dogs and cats in Calgary, Alberta. Can Vet J. 2011;52:1323–1328. [PMC free article] [PubMed] [Google Scholar]
- 4.Foreyt WJ. Veterinary Parasitology Reference Manual. 5th ed. Ames, Iowa: Iowa State University Press; 2001. p. 235. [Google Scholar]
- 5.Malloy WF, Embil JA. Prevalence of Toxocara spp. and other parasites in dogs and cats in Halifax, Nova Scotia. Can J Comp Med. 1978;42:29–31. [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla R, Giraldo P, Kraliz A, Finnigan M, Sanchez AL. Cryptosporidium spp. and other zoonotic enteric parasites in a sample of domestic dogs and cats in the Niagara region of Ontario. Can Vet J. 2006;47:1179–1184. [PMC free article] [PubMed] [Google Scholar]
- 7.Threlfall W. Further records of helminths from Newfoundland mammals. Can J Zool. 1969;47:197–201. doi: 10.1139/z69-043. [DOI] [PubMed] [Google Scholar]
- 8.Slocombe JO. Parasitisms in domesticated animals in Ontario. I. Ontario Veterinary College Records 1965–1970. Can Vet J. 1973;14:36–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Blagburn BL, Schenker R, Gagné F, Drake J. Prevalence of intestinal parasites in companion animals in Ontario and Quebec, Canada, during the winter months. Vet Ther. 2008;9:169–175. [PubMed] [Google Scholar]
- 10.Stojanovic V, Foley P. Infectious disease prevalence in a feral cat population on Prince Edward Island, Canada. Can Vet J. 2011;52:979–982. [PMC free article] [PubMed] [Google Scholar]
- 11.Olson MR, Leonard NJ, Strout J. Prevalence and diagnosis of Giardia infection in dogs and cats using a fecal antigen test and fecal smear. Can Vet J. 2010;51:640–642. [PMC free article] [PubMed] [Google Scholar]
- 12.Mekaru SR, Marks SL, Felley AJ, Chouicha N, Kass PH. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 northern California animal shelters. J Vet Intern Med. 2007;21:959–956. doi: 10.1892/0891-6640(2007)21[959:codiia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Lee ACY, Schantz PM, Kazacos KR, Montgomery SP, Bowman DD. Epidemiologic and zoonotic aspects of ascarid infections in dogs and cats. Trends Parasitol. 2010;26:155–161. doi: 10.1016/j.pt.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Fisher M. Toxocara cati: An underestimated zoonotic agent. Trends Parasitol. 2003;19:167–170. doi: 10.1016/s1471-4922(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 15.Kwon I, Kim H, Lee J, et al. A serologically diagnosed human case of cutaneous larval migrans caused by Ancyclostoma caninum. Korean J Parasitol. 2003;41:233–237. doi: 10.3347/kjp.2003.41.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wobeser G. The occurrence of Echinococcus multilocularis (Leukart, 1863) in cats near Saskatoon, Saskatchewan. Can Vet J. 1971;47:197–201. [PMC free article] [PubMed] [Google Scholar]
- 17.James E, Boyd W. Echinococcus alveolaris (with the report of a case) Can Med Assoc J. 1937;36:354–356. [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson RCA, Smith A. Zoonotic enteric protozoa. Vet Parasitol. 2011;182:70–78. doi: 10.1016/j.vetpar.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Lucio-Forster A, Griffiths JK, Cama VA, et al. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010;26:174–179. doi: 10.1016/j.pt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Cacciò S, Pinter E, Fantini R, Mezzaroma I, Pozio E. Human infection with Cryptosporidium felis: Case report and literature review. Emerg Infect Dis. 2002;8:85–85. doi: 10.3201/eid0801.010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballweber LR, Xiao LH, Bowman DD, et al. Giardiasis in dogs and cats: Update on epidemiology and public health significance. Trends Parasitol. 2010;26:180–189. doi: 10.1016/j.pt.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.McDowall RM, Peregrine AS, Leonard EK, et al. Evaluation of the zoonotic potential of Giardia duodenalis in fecal samples from dogs and cats in Ontario. Can Vet J. 2011;52:1329–1333. [PMC free article] [PubMed] [Google Scholar]
- 23.Stull JW, Carr AP, Chomel BB, Berghaus RD, Hird DW. Small animal deworming protocols, client education and veterinary perception of zoonotic parasites in western Canada. Can Vet J. 2007;48:269–276. doi: 10.4141/cjas68-037. [DOI] [PMC free article] [PubMed] [Google Scholar]