Abstract
Circadian systems regulate the immune system by various molecular and physiological pathways. Disruption to the circadian temporality of these pathways is associated with disease formation and progression. Circadian clock genes have been shown to regulate pathways involved in cellular proliferation, apoptosis, and DNA damage response, as aberrant rhythms in these genes are associated with various diseases. However, there is growing evidence that specific circadian genes differentially regulate functional pathways of immunocompetent cells. To extend our previous findings of the role of Period 2 in regulating splenocyte rhythms, we report that mice carrying a mutation in the Period 1 gene (Per1−/− mice), involved in the negative limb of the molecular clock, display significantly altered rhythms of cytokine (eg, interferon-γ) and cytolytic factors (eg, perforin and granzyme B) in splenic natural killer (NK) cells. Altered rhythms of NK cell immune factors were accompanied by changes in circadian expression of circadian clock genes, Bmal1 and Per2. In addition, Per1−/− circadian running-wheel activity rhythms remained rhythmic during constant darkness, although with a shortened free-running circadian period, suggesting primary involvement of peripheral molecular clocks. These findings indicate that the Per1 gene through NK cellular clocks modulates immune pathways.
Introduction
In mammals, circadian rhythms are ubiquitous, governing many cellular and behavioral functions that are essential to maintaining homeostasis. Circadian coordination among multiple organ systems appears to be critical for health, as circadian disruption or desynchrony is associated with negative health consequences. One of the primary connections between circadian disruption and disease is the involvement of circadian clocks in regulating the immune system (Logan and Sarkar 2012). Many immune parameters, including lymphocyte proliferation, antigen presentation, cytokine expression, macrophage, and natural killer (NK) cell activity, vary in a daily pattern (Keller and others 2009; Castanon-Cervantes and others 2010; Logan and others 2011; Silver and others 2012a, 2012b). The circadian system coordinates both the innate and adaptive immune system through autonomic and neuroendocrine pathways originating from the suprachiasmatic nucleus (SCN) (Arjona and Sarkar 2008), and at the cellular level by molecular clock mechanisms (Logan and Sarkar 2012).
Molecular clocks are important modulators of cell-specific physiology. Self-sustained autoregulatory transcriptional and translational feedback loops are maintained by near 24 h expression of clock genes and their protein products (Reppert and Weaver 2002). Briefly, clock genes Period (Per1, 2, 3) and Cryptochromes (Cry1, 2) are transcriptionally activated by CLOCK and BMAL1 heterodimers, which are then suppressed by their own transcription. Other factors are intimately involved in regulating the molecular clock provide intracellular cyclicity (Albrecht 2012). We and others have shown that clock genes are rhythmically expressed in immunocompetent cells, including NK cells, macrophages, and other lymphocytes (Arjona and Sarkar 2005, 2006a, 2006b; Bollinger and others 2011; Richards and Gumz 2012; Silver and others 2012a, 2012b). Particularly in NK cells, alterations in clock gene rhythms lead to disruptions in rhythms of cytolytic factors (perforin and granzyme B) and cytokine [interferon-γ (IFN-γ)], which is associated with decreased cytotoxic activity and increased tumor growth (Logan and others 2012). These immune factors are primarily involved in cell-mediated killing of tumor cells (Shankaran and others 2001; Vivier and others 2008; Cullen and others 2010). However, there is very little evidence characterizing the role of specific clock genes in NK cell rhythms.
Immune studies on mice with circadian clock gene mutations provide initial evidence supporting particular functional roles of specific clock genes in immunocompetent cells. For example, Bmal1 mutants have poor B-cell development, an essential component of adaptive immune response (Sun and others 2006), and Clock mutants exhibit arrhythmic expression of immunoregulatory genes in the liver (Oishi and others 2003). In Per2 mutants, only IFN-γ expression in splenocytes is altered across the day (Arjona and Sarkar 2006a). In addition, knockdown of either Per2 or Bmal1 in splenocytes selectively alters gene expression of cytokine and cytolytic factors (Arjona and Sarkar 2006a, 2006b), suggesting that specific circadian clock genes mediate different pathways of immune cell physiology. This notion is supported by studies reporting that circadian transcription of Bmal1 in the SCN and peripheral tissues, such as liver, heart, and kidney, and of Clock mutant mice is regulated by different molecular mechanisms depending on cell and tissue types (Oishi and others 2000). Further, Per1 gene expression in Per1 null mice remains rhythmic in the SCN, while displaying a delay in the heart, kidney, and muscle, suggesting that Per1 could be more involved with circadian regulation in peripheral tissues (Cermakian and others 2001).
In the present study, we examined the role of the clock gene Per1 in regulating rhythms in cytokines and cytolytic factors in splenic NK cells. Enriched NK cells were collected across the circadian cycle during constant darkness (DD) in wild-type (WT) and Per1−/− mice. Exploring the effects of a clock gene mutation on the circadian profile of immune factors in DD allows for interpretation of peripheral clock involvement irrespective of potential masking effects of light. Per1−/− mice retain rhythmic gene expression in the SCN and locomotor activity in DD (Zheng and others 2001; Bode and others 2011), and thus provide an informative model for beginning to separate the role of SCN and intracellular clocks in NK cell function. Circadian rhythms of gene and protein expression of Per2 and Bmal1 were altered in NK cells of Per1−/− mice. As expected, changes in clock gene expression were paralleled by significant alterations in expression rhythms of IFN-γ, perforin, and granzyme B in NK cells.
Methods
Animals
Initial breeding pairs of homozygous Per1tm1Drw mutant mice (Per1−/−) were backcrossed for 10 generations with male and female C57BL/6J mice (The Jackson Laboratory; stock No. 010491). Male WT and Per1−/− mice were bred and maintained at the animal facility by an experienced breeder following institutional guidelines. The 2 breeding pairs were expanded over multiple generations in order to produce male offspring of a similar age. Experimental groups were comprised of mice from multiple litters to mitigate litter effects. All animals were given free access to food and water for duration of the experiment. Experimental procedures, animal handling, and breeding protocols were approved by the Rutgers Animal Care and Facilities Committee and also complied with policies of the National Institutes of Health.
Circadian locomotor activity
At 14–16 weeks, male WT and Per1−/− mice (n=30 per strain) were acclimated to individual housing conditions with access to a running wheel (wheel diameter: 23 cm; Mini Mitter Co.), for 2–3 weeks. Mice were maintained under a standard 12:12 light dark with lights on and off at 600 and 1,800 h, respectively, in sound-attenuating climate controlled cubicles. Following an acclimation period, mice were kept under constant DD for 10–12 days to avoid the potential masking effects of light on circadian rhythms. Running-wheel activity was continuously recorded by a computer and stored for later data analysis using ClockLab software (Actimetrics Co.). To determine the circadian phase of each mouse, actograms were inspected and circadian time (CT) 12 was defined as the onset of activity. At 10–12 days in DD, mice (n=5 per time point) were sacrificed by cervical dislocation and rapid decapitation at 6 times across the circadian cycle, which was respective to each WT and Per1−/− mouse, and corresponded to CTs 3, 7, 11, 15, 19, and 23. Calculating CTs for each mouse accounts for differences in circadian period, which allowed for direct comparison at specific circadian time points for gene and protein levels between WT and Per1−/− mice. Spleen was collected and NK cells were separated for gene and protein assays.
Enrichment of splenic NK cells in mice
Spleens were stored in RPMI (Invitrogen) upon sacrifice until further processing. Spleens were prepared using standard methods, as described previously (Logan and others 2012). Briefly, samples were gently homogenized in RPMI, and debris and cell clumps were removed using pre-separation filters (30 μm; Miltenyi Biotec). RBCs and granulocytes were removed from splenocyte suspensions by density centrifugation using Histopaque 1083 (Sigma-Aldrich). Splenocytes were extracted from the middle layer, washed with RPMI, and resuspended in buffer (phosphate-buffered saline and 0.5% bovine serum albumin). Splenocytes were incubated with primary antibodies followed by microbeads conjugated to monoclonal antibiotin antibody, as per manufacturer's instructions for the mouse NK cell isolation kit (Miltenyi Biotec). This particular enrichment method takes ∼ 40–60 min leaving untouched NK cells by depleting non-target cells. The enriched fraction is purported to have a consistent purity of 95% (Miltenyi Biotec). Following enrichment, NK cells were divided and lysed in appropriate buffers for RNA and protein analyses.
RNA extraction, reverse transcriptase–polymerase chain reaction, and real-time reverse transcriptase–polymerase chain reaction
Total RNA was isolated from NK cells using the RNeasy Mini Kit (Qiagen). Using Superscript III First-Strand Synthesis Super Mix (Invitrogen), 100 ng of total RNA was reverse transcribed. mRNA was quantified by real-time reverse transcriptase–polymerase chain reaction (ABI Prism 7700 Sequence Detector) using SYBR green and TaqMan gene expression assays (Applied Biosystems). Primer sequences are stated elsewhere (Karolczak and others 2004; Arjona and Sarkar 2006a, 2006b). Analyses were performed using the relative standard curve method with GAPDH probe (Applied Biosystems) as the normalizing endogenous control.
Western blot
Total protein was extracted from NK cells in Pierce IP lysis buffer containing Halt protease inhibitor cocktail and phosphatase inhibitors (Thermo Fisher Scientific). The protein concentration of each sample was analyzed by Bradford protein assay (BioRad Laboratories). Twenty micrograms of protein was size-fractionated in 12% sodium dodecyl sulfate–polyacrylamide gel and electrotransferred to polyvinyl difluoride membranes. Blocking was for 2 h in 5% nonfat dry milk–TBS–0.1% Tween 20 at room temperature and incubated overnight with the appropriate primary antibody at 4°C. The primary antibodies were as follows: anti-p38 MAPK 1:1,000 and anti-phospho-p38 MAPK 1:2,000. Membranes were incubated for 1 h at room temperature with the corresponding peroxidase conjugated secondary antibody. Immunoreactivity was detected by enhanced chemiluminescence, and membranes were developed on X-ray films. Densitometry was performed using Image J analysis software (National Institutes of Health). Each protein was normalized to β-actin (1:7,500; Calbiochem).
Statistical analyses
Relative gene and protein expression levels were calculated as the percentage of the maximum value over the entire 24-h period. Two-way analysis of variance (ANOVA) tested the main effects of mouse strain (WT vs. Per1−/−) and CT, and strain×time interactions. Bonferroni post hoc tests were used when a significant interaction was revealed to determine difference in expression levels of genes and proteins between WT and Per1−/− mice at each circadian phase. CircWave v1.4 software fits linear harmonic regression curves with an assumed period of 24 h (α=0.05) to determine whether gene and protein expression data displayed significant circadian rhythmicity (Keller and others 2009; Logan and others 2012). Center of gravity of fitted curves was used as the circadian acrophase, and estimation errors were used as standard deviations (Hut and others 2000; Logan and others 2012). One-way ANOVA with Newman–Keuls multiple comparison test compared gene and protein acrophases between WT and Per1−/− mice. Circadian period of running-wheel activity during DD was analyzed with Lomb–Scargle periodograms (ClockLab). Student's t-test was used to compare free-running period between WT and Per1−/− mice.
Results
Circadian running-wheel activity in WT and Per1−/− mice
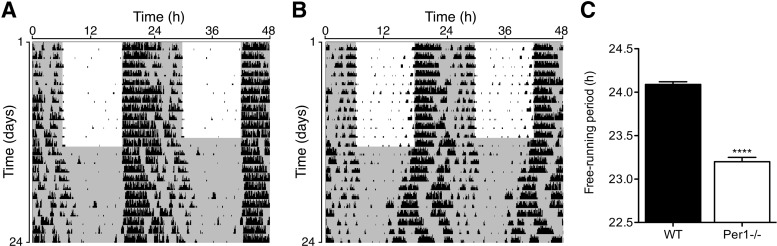
Under standard LD conditions, both WT and Per1−/− mice displayed typical circadian entrainment with an approximate period of 24 h (Fig. 1A, B). To avoid potential masking effects of light to discern circadian rhythms of NK cells, mice were placed under constant DD. During constant DD, Per1−/− mice displayed rhythmic circadian wheel-running activity with a significantly shorter free-running period compared with WT mice (WT, 24.09±0.03 vs. Per1−/−, 23.2±0.02, P<0.0001; Fig. 1B, C), consistent with previous results (Zheng and others 2001, Bode and others 2011).
FIG. 1.
Circadian wheel-running activity under entrained and free-running conditions. Both wild-type (WT) (A) and Per1−/− mice (B) display typical entrainment under a standard 12:12 LD cycle. Per1−/− mice remain rhythmic under free-running darkness (DD) conditions (B), while displaying a shortened circadian period (B, C). ****P<0.0001, significant difference between groups.
Circadian expression of clock genes in enriched NK cells is altered in Per1−/− mice
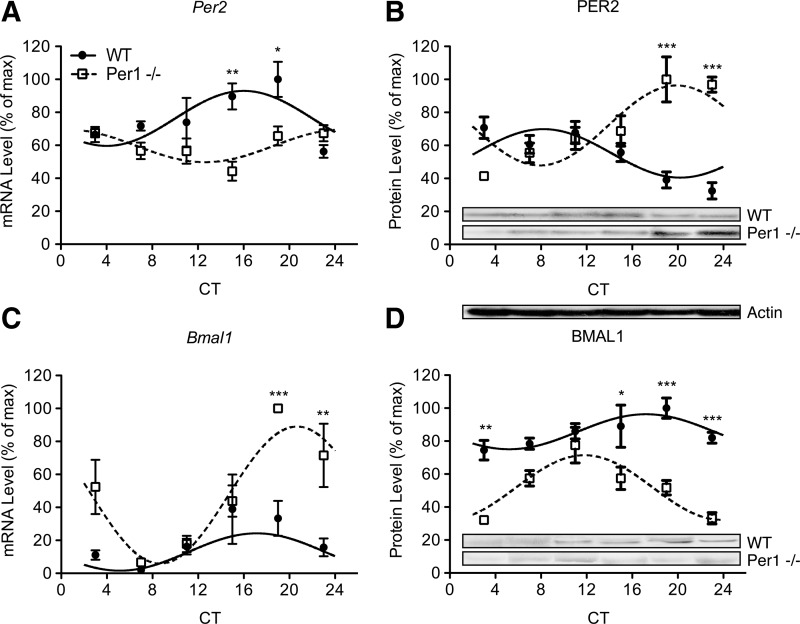
To investigate the effects of Per1 mutation on circadian expression of core clock genes in splenic NK cells, NK cells were enriched at 4-h intervals in DD. Core clock genes display robust circadian rhythmicity in NK cells of WT mice (Fig. 2; fitted curves, P's<0.05), with alternating phases of peak protein levels of Per2 and Bmal1 (Table 1; Fig. 2B, D). Circadian rhythmicity of core clock genes was altered in NK cells from Per1−/− mice (Fig. 2). In Per1−/− mice, Per2 gene (Fig. 2A) and protein (Fig. 2B) rhythms were reversed relative to WT mice, evident by differences in acrophase (Table 1) and decreased expression at CTs 16 and 20 (P<0.01 and P<0.05, respectively). Also, Bmal1 expression rhythms were altered in Per1−/− mice. Bmal1 gene expression was increased later in the circadian cycle (Fig. 2C; P's<0.01) and, conversely, decreased protein levels were observed at CTs 3, 15, 19, and 23 (P's<0.05). As expected, robust clock gene rhythmicity in NK cells is altered in Per1−/− mice.
FIG. 2.
A mutation in the Per1 gene alters circadian rhythm gene and protein expression of core circadian genes Per2 (A, B) and Bmal1 (C, D) in natural killer (NK) cells. Male WT and Per1−/− mice were sacrificed at 4-h intervals across the circadian day in DD. Protein levels were quantified using densitometric analyses by Image J software. Representative immunoblots are shown for each protein across each circadian time (CT). Sine wave fits using linear harmonic regression assumed a 24 h period for both WT and mutant mice (solid and dotted lines, respectively). Lines are superimposed on group means±standard error of the means (SEMs) for each CT. All curve fits are significant (P<0.01). Significant differences determined by Bonferroni post hoc tests between WT and mutant mice at given CT: *P<0.05, **P<0.01, and ***P<0.001.
Table 1.
Acrophases Were Determined from Curves Fitted to Gene and Protein Expression Levels in Natural-Killer Cells over the Circadian Cycle
| |
Gene |
Protein |
||
|---|---|---|---|---|
| WT | Per1−/− | WT | Per1−/− | |
| Per2 | 15.7±3.1 | 1.0±3.5a | 9.9±2.9 | 18.1±2.6b |
| Bmal1 | 17.1±2.1 | 20.7±2.2 | 13.0±3.3 | 14.2±2.6 |
| IFN-γ | 21.2±3.4 | 24.0±3.2 | 12.1±3.2 | 20.8±3.4c |
| Perforin | 20.7±1.8 | 5.0±3.2a | 13.2±3.2 | 15.8±3.2 |
| Granzyme B | 18.4±3.4 | 1.0±3.2a | 8.3±2.7 | 14.6±2.9b |
Acrophases (±standard deviation) represent estimations of circadian phase corresponding to rhythm peaks. One-way analysis of variance with Newman–Keuls multiple comparison tests was used to compare gene and protein acrophases between control and Per1−/− mice (n=6 per lighting regimen).
P<0.001, bP<0.05, and cP<0.01: significant differences between groups.
WT, wild type.
Circadian expression of cytokine IFN-γ and cytolytic factors perforin and granzyme B in enriched NK cells is altered in Per1−/− mice
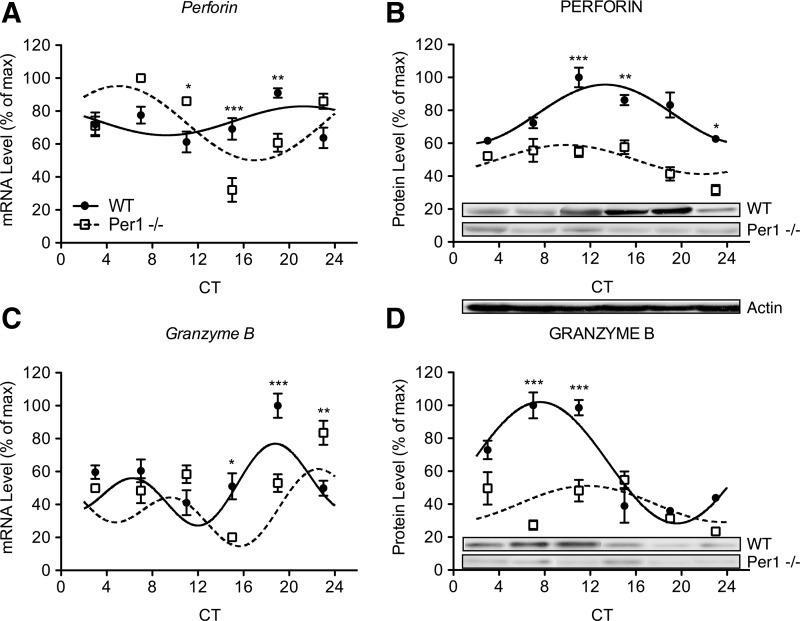
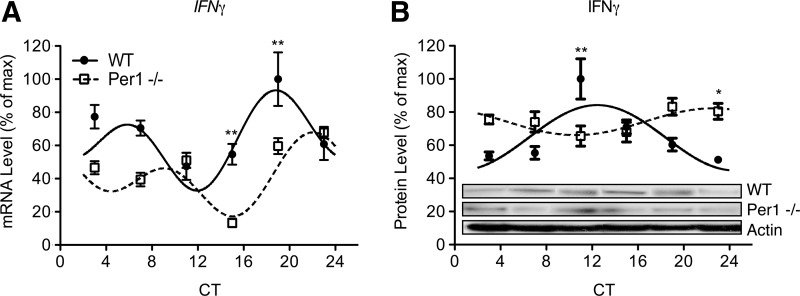
Circadian alterations of circadian clock genes were paralleled by changes in expression rhythms of cytokines and cytolytic factors in enriched NK cells of Per1−/− mice (Figs. 2–4). Consistent with previous results (Arjona and Sarkar 2006a, 2006b; Logan and others 2012), perforin, granzyme B, and IFN-γ expression rhythms were robust in enriched NK cells of WT mice (Figs. 3 and 4; fitted curves, P's<0.05). Although gene and protein expression rhythms were intact for immune factors in Per1−/− mice, these rhythms were significantly altered compared with WT mice (Figs. 3 and 4; fitted curves, P's<0.05). IFN-γ gene rhythms were shifted later in the circadian cycle, which is indicated by a delayed acrophase (Table 1), and lower expression at CTs 15 and 19 (Fig. 2A; P's<0.01). IFN-γ protein rhythms appeared to be reversed (Fig. 3B; Table 1). Rhythms for cytolytic factors perforin and granzyme B displayed similar alterations. Perforin gene expression rhythms were reversed in Per1−/− mice with an advance in acrophase (Table 1), and increased levels at CT 11 (P<0.05) followed by decreased levels at CTs 15 and 19 (P<0.001 and P<0.01, respectively) (Fig. 4A). Protein levels of perforin were decreased at CT 11 (P<0.001), CT 15 (P<0.01), and CT 23 (P<0.05), suggesting a dampening of rhythm later in the circadian cycle in Per1−/− mice (Fig. 4B). Gene rhythms of granzyme B were delayed in Per1−/− mice (Table 1), with decreases at CTs 15 and 19 (P<0.05 and P<0.001), followed by an increase at CT 23 (P<0.01) (Fig. 4C). Granzyme B protein rhythms were completely dampened in Per1−/− mice (Fig. 4D), indicated by decreased levels across phases of peak expression observed in WT mice (CTs 7 and 11, P's<0.001). In NK cells, a mutation in the Per1 gene seems to differentially alter gene and protein rhythms of cytokines and cytolytic factors. In Per1−/− mice, circadian rhythms of immune factors were reversed for the perforin gene and IFN-γ protein, advanced or delayed for the granzyme B and IFN-γ genes, and dampened for perforin and granzyme B proteins.
FIG. 4.
Circadian rhythms of perforin and granzyme B gene and protein expression are altered in Per1−/− mice. Gene rhythms of perforin appear reversed (A) and perforin protein rhythms are dampened (B) in NK cells due to Per1 mutation. Granzyme B gene rhythms appear phase delayed (C) while protein rhythms are attenuated (D) in NK cells of Per1−/− mice. Protein levels were quantified using densitometric analyses by Image J software. Representative immunoblots are shown for each protein across each CT. Sine wave fits using linear harmonic regression assumed a 24 h period for WT and mutant mice (solid and dotted lines, respectively). Lines are superimposed on group means±SEMs for each CT. All curve fits are significant (P<0.01). Significant differences determined by Bonferroni post hoc tests between WT and mutant mice at given CT: *P<0.05, **P<0.01, and ***P<0.001.
FIG. 3.
Circadian rhythms of interferon-γ (IFN-γ) gene and protein expression are altered in Per1−/− mice. Gene rhythms of IFN-γ appear phase delayed (A), whereas protein rhythms are reversed (B) in NK cells due to the Per1 mutation. Protein levels were quantified using densitometric analyses by Image J software. Representative immunoblots are shown for each protein across each CT. Sine wave fits using linear harmonic regression assumed a 24 h period for WT and mutant mice (solid and dotted lines, respectively). Lines are superimposed on group means±SEMs for each CT. All curve fits are significant (P<0.01). Significant differences determined by Bonferroni post hoc tests between WT and mutant mice at given CT: *P<0.05 and **P<0.01.
Discussion
Many studies are reporting involvement of the circadian system in immune functioning and possible disease manifestation in animal models (eg, Castanon-Cervantes and others 2010; Bedrosian and others 2011; Bollinger and others 2011; Fortier and others 2011; Gibbs and others 2012; Logan and others 2012; Silver and others 2012b) and humans (eg, Copertaro and others 2011; Mazzoccoli and others 2011, 2012a, 2012b). We have recently demonstrated extensive evidence for circadian oscillations of gene and protein expression of cytolytic factors and cytokines in heterogeneous splenocytes and splenic NK cells in rats and mice (Arjona and others 2004; Arjona and Sarkar 2005, 2006a, 2006b; Logan and others 2011, 2012). In addition, we reported that Per2 and Bmal1 could have differential roles in regulating NK cell function (Arjona and Sarkar 2006a, 2006b). In the present study, circadian rhythms of gene and protein levels of IFN-γ, perforin, and granzyme B, and clock genes Per2 and Bmal1, were altered in Per1−/− mice. Together, these data with previous evidence indicate that Per1 mediates circadian expression of IFN-γ, perforin, and granzyme B, which implicates this circadian gene as having a primary role in NK cell rhythms.
To expand on our previous results reporting that Per2 selectively regulates circadian expression of IFN-γ, but not perforin or granzyme B, in splenocytes (Arjona and Sarkar 2006a, 2006b), we characterized circadian expression of these immune factors in NK cells of Per1−/− mice. In contrast to Per2, gene and protein expression rhythms of perforin, granzyme B, and IFN-γ were altered in NK cells, posing the possibility of distinct regulatory pathways that are partially dependent on specific isoforms of Period genes. It is worth noting that both of our studies (Arjona and Sarkar 2006a; present results) utilized Per1 and Per2 mutant mice that were originally congenic with a 129/sv background and backcrossed for at least 10 generations to a C57BL6/J background. Both of these mutant strains, regardless of the nature of the mutation (ie, functional exon deletion or complete gene knockout), retain behavioral rhythmicity under DD if maintained on a C57BL6 background (eg, Cermakian and others, 2001; Xu and others 2007; Pendergast and others 2010; Bode and others 2011). However, when maintained on the original 129/sv background, Per1 and Per2 mutations lead to severe disruptions in behavioral rhythms (eg, Zheng and others 1999; Bae and others 2001). It appears that there is a degree of genetic redundancy when these mutations are placed on a C57BL6/J background. Thus, the comparison of Per1 and Per2 involvement in NK cell rhythms between our studies seems reasonable because both mutants retain behavioral rhythms under DD, yet display obvious differences in temporal expression of cytokines and cytolytic factors explored here. Using Per mutants maintained on the 129/sv background could reveal dramatically different conclusions on the role of SCN and peripheral clock integrity in NK cell rhythms.
In Per1−/− mice, gene and protein expression of immune factors in NK cells displayed significant rhythm changes compared with WT mice. Alterations in acrophases of the immune factor gene and protein expression rhythms indicated that IFN-γ and granzyme B genes were phase delayed, whereas perforin rhythms were phase advanced. Both perforin and granzyme B protein rhythms were dampened throughout the circadian cycle, as evidenced by significantly reduced expression levels relative to controls. Notably, the IFN-γ protein rhythm was completely reversed, although the gene rhythm was phase delayed. Although different from our other study in mice under LD conditions, circadian expression of perforin, granzyme B, and IFN-γ gene in control animals resembled, for the most part, those observed in rat NK cells under similar DD conditions (Logan and others 2012). Further studies examining the role of clock genes, or the integrity of the molecular clock, to influence transcription, translational, and post-translational regulatory pathways of cytokines and cytolytic factors in NK cells would prove useful in deciphering the temporal relationship between gene and protein expression under basal and immune-stimulated states.
Alterations in expression rhythms of perforin, granzyme B, and IFN-γ were accompanied by changes in clock gene rhythms in Per1−/− mice. Both gene and protein rhythms for Per2 were reversed, while Bmal1 gene and protein levels were significantly increased and decreased, respectively, across the entire circadian cycle. In mutant mice, Bmal1 gene and protein overall expression levels did not coincide with one another, such that pronounced increases in gene expression were accompanied by decreased protein expression. Interestingly, PER2 protein expression was also increased, suggesting an enhanced downregulation of BMAL1 in NK cells of Per1−/− mice. PER2 directly interacts with BMAL1 to repress protein activity (Langmesser and others 2008), and other alterations in post-translational mechanisms may also be factors here (Gallego and Virshup 2007), although it is speculative. In general, acrophases of gene and protein rhythms of Per2 and Bmal1 were shifted toward later in the circadian cycle, suggesting these rhythms were phase delayed. Similar phase delays of clock gene expression have been observed in other peripheral tissues, including heart, kidney, and muscle, in Per1 null mice (Cermakian and others 2001). In this study, phase delays in clock genes are associated with phase delays in both IFN-γ and granzyme B, but not perforin (phase advanced). Interestingly, clock gene expression is rhythmic in the SCN of Per1-deficient mice (Bae and others 2001; Cermakian and others 2001; Bode and others 2011), possibly indicating a prominent role of Per1 in peripheral clocks. In DD, running-wheel activity also remains rhythmic (present results; Cermakian and others 2001; Bode and others 2011), leading to the possibility that temporal inputs to splenic NK cells are unaltered, and although dampened SCN rhythms may also affect temporal organization in peripheral tissues (Pendergast and others 2009), our results suggest that Per1 is integral in NK cell clocks and cytokine production.
Much research attention has been placed on exploring the consequences of aberrant clock gene expression in tumor cells, which has revealed their roles in pathways controlling cell cycle checkpoints, cell proliferation, and apoptosis, as well as DNA repair (Fu and Lee 2003; Filipski and others 2006; Gery and Koeffler 2007; Miyazaki and others 2010; Sukumaran and others 2010). Altered Per1 expression and mutations in the gene have been found in human gliomas (Xia and others 2010) and other types of tumors (Wood and others 2009). However, the role of circadian systems and clock genes in immune functioning is important to understand disease formation and progression. Our study reports novel evidence of Per1 to modulate circadian expression of cytokines and cytolytic factors in splenic NK cells. Attenuated expression of perforin and granzyme B accompanied by changes in IFN-γ rhythms could detrimentally affect the ability of NK cells to kill tumor cells (Logan and others 2012). The mechanistic link between Per1, or other clock genes, and cytokine and cytolytic factor transcription is currently unknown, but may involve known transcription factors involved in immune response, such as NF-κB (Hayashi and others 2007; Gibbs and others 2012; Narasimamurthy and others 2012). In-depth molecular studies are required to tease apart differential roles of clock genes in immunocompetent cells, and how disruptions to cellular clocks within the diseased cell and immune cell may create particular vulnerabilities to disease.
Author Disclosure Statement
No competing financial interests exist.
References
- Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74(2):246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Arjona A. Boyadjieva N. Sarkar DK. Circadian rhythms of granzyme B, perforin, IFNgamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J Immunol. 2004;172(5):2811–2817. doi: 10.4049/jimmunol.172.5.2811. [DOI] [PubMed] [Google Scholar]
- Arjona A. Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol. 2005;174(12):7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- Arjona A. Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFNgamma. J Interferon Cytokine Res. 2006a;26(9):645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- Arjona A. Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006b;20(5):469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Arjona A. Sarkar DK. Are circadian rhythms the code of hypothalamic-immune communication? Insights from natural killer cells. Neurochem Res. 2008;33(4):708–718. doi: 10.1007/s11064-007-9501-z. [DOI] [PubMed] [Google Scholar]
- Bae K. Jin X. Maywood ES. Hastings MH. Reppert SM. Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30(2):525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA. Fonken LK. Walton JC. Nelson RJ. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol Lett. 2011;7(3):468–471. doi: 10.1098/rsbl.2010.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode B. Shahmoradi A. Taneja R. Rossner MJ. Oster H. Genetic interaction of Per1 and Dec1/2 in the regulation of circadian locomotor activity. J Biol Rhythms. 2011;26(6):530–540. doi: 10.1177/0748730411419782. [DOI] [PubMed] [Google Scholar]
- Bollinger T. Leutz A. Leliavski A. Skrum L. Kovac J. Bonacina L. Benedict C. Lange T. Westermann J. Oster H. Solbach W. Circadian clocks in mouse and human CD4+ T cells. PLoS One. 2011;6(12):e29801. doi: 10.1371/journal.pone.0029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon-Cervantes O. Wu M. Ehlen JC. Paul K. Gamble KL. Johnson RL. Besing RC. Menaker M. Gewirtz AT. Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185(10):5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N. Monaco L. Pando MP. Dierich A. Sassone-Corsi P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 2001;20(15):3967–3974. doi: 10.1093/emboj/20.15.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copertaro A. Bracci M. Gesuita R. Carle F. Amati M. Baldassari M. Mocchegiani E. Santarelli L. Influence of shift-work on selected immune variables in nurses. Ind Health. 2011;49(5):597–604. doi: 10.2486/indhealth.ms1210. [DOI] [PubMed] [Google Scholar]
- Cullen SP. Brunet M. Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17(4):616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- Filipski E. Li XM. Levi F. Disruption of circadian coordination and malignant growth. Cancer Causes Control. 2006;17(4):509–514. doi: 10.1007/s10552-005-9007-4. [DOI] [PubMed] [Google Scholar]
- Fortier EE. Rooney J. Dardente H. Hardy MP. Labrecque N. Cermakian N. Circadian variation of the response of T cells to antigen. J Immunol. 2011;187(12):6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- Fu L. Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3(5):350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- Gallego M. Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8(2):139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Gery S. Koeffler HP. The role of circadian regulation in cancer. Cold Spring Harb Symp Quant Biol. 2007;72:459–464. doi: 10.1101/sqb.2007.72.004. [DOI] [PubMed] [Google Scholar]
- Gibbs JE. Blaikley J. Beesley S. Matthews L. Simpson KD. Boyce SH. Farrow SN. Else KJ. Singh D. Ray DW. Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(2):582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. Shimba S. Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30(4):621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- Hut RA. Scheper A. Daan S. Can the circadian system of a diurnal and a nocturnal rodent entrain to ultraviolet light? J Comp Physiol A. 2000;186(7–8):707–715. doi: 10.1007/s003590000124. [DOI] [PubMed] [Google Scholar]
- Karolczak M. Burbach GJ. Sties G. Korf HW. Stehle JH. Clock gene mRNA and protein rhythms in the pineal gland of mice. Eur J Neurosci. 2004;19(12):3382–3388. doi: 10.1111/j.0953-816X.2004.03444.x. [DOI] [PubMed] [Google Scholar]
- Keller M. Mazuch J. Abraham U. Eom GD. Herzog ED. Volk HD. Kramer A. Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106(50):21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmesser S. Tallone T. Bordon A. Rusconi S. Albrecht U. Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol Biol. 2008;9:41. doi: 10.1186/1471-2199-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW. Arjona A. Sarkar DK. Role of sympathetic nervous system in the entrainment of circadian natural-killer cell function. Brain Behav Immun. 2011;25(1):101–109. doi: 10.1016/j.bbi.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW. Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349(1):82–90. doi: 10.1016/j.mce.2011.06.039. [DOI] [PubMed] [Google Scholar]
- Logan RW. Zhang C. Murugan S. O'Connell S. Levitt D. Rosenwasser AM. Sarkar DK. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol. 2012;188(6):2583–2591. doi: 10.4049/jimmunol.1102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoccoli G. Pazienza V. Panza A. Valvano MR. Benegiamo G. Vinciguerra M. Andriulli A. Piepoli A. ARNTL2 and SERPINE1: potential biomarkers for tumor aggressiveness in colorectal cancer. J Cancer Res Clin Oncol. 2012a;138(3):501–511. doi: 10.1007/s00432-011-1126-6. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G. Piepoli A. Carella M. Panza A. Pazienza V. Benegiamo G. Palumbo O. Ranieri E. Altered expression of the clock gene machinery in kidney cancer patients. Biomed Pharmacother. 2012b;66(3):175–179. doi: 10.1016/j.biopha.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G. Sothern RB. Greco A. Pazienza V. Vinciguerra M. Liu S. Cai Y. Time-related dynamics of variation in core clock gene expression levels in tissues relevant to the immune system. Int J Immunopathol Pharmacol. 2011;24(4):869–879. doi: 10.1177/039463201102400406. [DOI] [PubMed] [Google Scholar]
- Miyazaki K. Wakabayashi M. Hara Y. Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells. 2010;15(4):351–358. doi: 10.1111/j.1365-2443.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R. Hatori M. Nayak SK. Liu F. Panda S. Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(31):12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K. Fukui H. Ishida N. Rhythmic expression of BMAL1 mRNA is altered in clock mutant mice: differential regulation in the suprachiasmatic nucleus and peripheral tissues. Biochem Biophys Res Commun. 2000;268(1):164–171. doi: 10.1006/bbrc.1999.2054. [DOI] [PubMed] [Google Scholar]
- Oishi K. Miyazaki K. Kadota K. Kikuno R. Nagase T. Atsumi G. Ohkura N. Azama T. Mesaki M. Yukimasa S. Kobayashi H. Iitaka C. Umehara T. Horikoshi M. Kudo T. Shimizu Y. Yano M. Monden M. Machida K. Matsuda J. Horie S. Todo T. Ishida N. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003;278(42):41519–41527. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- Pendergast JS. Friday RC. Yamazaki S. Endogenous rhythms in Period1 mutant suprachiasmatic nuclei in vitro do not represent circadian behavior. J Neurosci. 2009;29(46):14681–14686. doi: 10.1523/JNEUROSCI.3261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS. Friday RC. Yamazaki S. Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS One. 2010;5(1):e8552. doi: 10.1371/journal.pone.0008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM. Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Richards J. Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J. 2012;26(9):3602–3613. doi: 10.1096/fj.12-203554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V. Ikeda H. Bruce AT. White JM. Swanson PE. Old LJ. Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Silver AC. Arjona A. Hughes ME. Nitabach MN. Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav Immun. 2012a;26(3):407–413. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AC. Arjona A. Walker WE. Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012b;36(2):251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S. Almon RR. DuBois DC. Jusko WJ. Circadian rhythms in gene expression: relationship to physiology, disease, drug disposition and drug action. Adv Drug Deliv Rev. 2010;62(9–10):904–917. doi: 10.1016/j.addr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Yang Z. Niu Z. Peng J. Li Q. Xiong W. Langnas AN. Ma MY. Zhao Y. MOP3, a component of the molecular clock, regulates the development of B cells. Immunology. 2006;119(4):451–460. doi: 10.1111/j.1365-2567.2006.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E. Tomasello E. Baratin M. Walzer T. Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Wood PA. Yang X. Hrushesky WJ. Clock genes and cancer. Integr Cancer Ther. 2009;8(4):303–308. doi: 10.1177/1534735409355292. [DOI] [PubMed] [Google Scholar]
- Xia HC. Niu ZF. Ma H. Cao SZ. Hao SC. Liu ZT. Wang F. Deregulated expression of the Per1 and Per2 in human gliomas. Can J Neurol Sci. 2010;37(3):365–370. doi: 10.1017/s031716710001026x. [DOI] [PubMed] [Google Scholar]
- Xu Y. Toh KL. Jones CR. Shin JY. Fu YH. Ptacek LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128(1):59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B. Albrecht U. Kaasik K. Sage M. Lu W. Vaishnav S. Li Q. Sun ZS. Eichele G. Bradley A. Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105(5):683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zheng B. Larkin DW. Albrecht U. Sun ZS. Sage M. Eichele G. Lee CC. Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]