Abstract
Besides stimulating angiogenesis or cell survival, basic fibroblast growth factor (bFGF) has the potential for protecting neurons in the injured spinal cord.
Objective
To investigate the effects of a sustained-release system of bFGF from gelatin hydrogel (GH) in a rat spinal cord contusion model.
Methods
Adult female Sprague–Dawley rats were subjected to a spinal cord contusion injury at the T10 vertebral level using an IH impactor (200 kdyn). One week after contusion, GH containing bFGF (20 µg) was injected into the lesion epicenter (bFGF – GH group). The GH-only group was designated as the control. Locomotor recovery was assessed over 9 weeks by Basso, Beattie, Bresnahan rating scale, along with inclined plane and Rota-rod testing. Sensory abnormalities in the hind paws of all the rats were evaluated at 5, 7, and 9 weeks.
Results
There were no significant differences in any of the motor assessments at any time point between the bFGF – GH group and the control GH group. The control GH group showed significantly more mechanical allodynia than did the group prior to injury. In contrast, the bFGF – GH group showed no statistically significant changes of mechanical withdrawal thresholds compared with pre-injury.
Conclusion
Our findings suggest that bFGF-incorporated GH could have therapeutic potential for alleviating mechanical allodynia following spinal cord injury.
Keywords: Allodynia, Basic fibroblast growth factor, Scaffold, Spinal cord injuries, Motor deficits, Neuroprotection, Locomotor recovery, Paraplegia
Introduction
Spinal cord injury (SCI) is the most devastating type of trauma for patients due to the long-lasting disability and limited responses to acute drug administration and efforts at rehabilitation. Previously, we reported on combinational therapy, bone marrow stromal cell (BMSC) transplantation, and Rho-kinase inhibitor administration for spinal cord contusion.1 Combination therapy showed better recovery than controls, but we detected no synergy between the components of the combination. We counted lower numbers of remaining BMSCs and saw a gradual decrease in the number of BMSCs over the observation period. We hypothesized that we might have observed more locomotor recovery had the remaining cells been more abundant.
Gelatin hydrogel (GH) incorporating basic fibroblast growth factor (bFGF) is one of the more promising tools for treating SCI. bFGF-incorporated GH has enhanced angiogenesis in several experimental models,2–4 and it has already found some clinical usage, including a phase I/IIa study in humans in the hope of enhancing angiogenesis.5 Multiple studies have also identified various functions for bFGF itself in damaged central nerve system tissue, including the following: attenuating neurotoxicity and increasing antioxidant enzyme activities in hippocampal neurons;6 protecting against excitotoxicity and chemical hypoxia in both neonatal and adult rat neurons;7 preventing the death of lesioned cholinergic neurons in vivo;8 and protecting spinal motor neurons after experimental SCI.9 These research findings together suggest that bFGF-incorporated GH has the potential for saving damaged neuronal cells and improving angiogenesis after SCI.
BMSC with fibrin scaffolding has been observed to improve survival of transplanted cells after spinal cord hemisection.10 The combination of the neurotrophin-3, platelet-derived growth factor, and fibrin scaffold has been reported to enhance the total number of neural progenitor cells present in the spinal cord lesion 2 weeks after injury.11 The study findings together suggest that the controlled release of growth factor incorporated into a scaffold in conjunction with cell transplantation has the potential to improve the survival of transplanted cells and enhancing locomotor recovery after SCI.
In the present study, we sought to establish the safety of bFGF-incorporated GH in humans. Our study protocol was to inject bFGF-incorporated GH and GH without bFGF into contused spinal cords in rats and to measure locomotion for 9 weeks after SCI, as well to estimate two types of allodynia before and after SCI.
Methods
Experimental groups
The 18 animal subjects were randomly assigned to two groups: (1) the bFGF + GH group (bFGF – GH, n = 8), which received an injection of bFGF + GH mixture into the spinal cord; (2) the GH-only group (GH, n = 10), which received an injection of GH without bFGF into the spinal cord.
bFGF-incorporated GH treatment
bFGF-incorporated GH
A frozen aliquot of bFGF (10 µg/μl) was diluted 1:1 with phosphate-buffered saline (PBS) and incubated overnight at 4°C (5 µg/μl). GH (2 mg) was mixed with a 20-μl aliquot of bFGF and incubated at 37°C for 1 hour. Just before injection, bFGF-incorporated GH was diluted by 20 µl of PBS. We injected 8 µl bFGF-incorporated GH into the injured spinal cord.
Animal surgery
Our experimental SCI protocol utilized a total of 18 8-week-old female Sprague–Dawley rats (SLC, Hamamatsu, Japan). Rats were anesthetized with 1.6% halothane in 0.5 l/minute oxygen. We performed a laminectomy at the T9–T10 levels and induced a contusion injury of the spinal cord with the infinite horizon impactor (IH impactor, 200 kdyn, Precision Systems and Instrumentation, Lexington, NY, USA). Rats were group-housed in the animal facility and maintained under conditions of constant temperature and humidity. Food and water were provided ad libitum. Manual bladder expression was performed twice a day until recovery of the bladder reflex. All animals were given antibiotics (500 µl/day; Bactramin, Chugai Pharmaceutical, Tokyo, Japan) by subcutaneous administration once a day for 3 days. Body weight after SCI was measured weekly, from which we calculated body weight ratios by dividing each post-injury body weight by the body weight before surgery.
Seven days after injury, we re-exposed the injury site and injected the same volume (8 µl) of bFGF-incorporated GH, or GH only, into the center of the injured spinal cord using a micro-glass pipette needle attached to a 10-μl Hamilton syringe (Hamilton Company, Reno, NV, USA) under microscopy. We performed the injection at multiple depths (2, 1.5, 1.0, and 0.5 mm) during drawback, and the needle was left in the spinal cord for 3 minutes following the last injection in order to minimize reflux. None of the animals showed abnormal behavior. All the experimental procedures were performed in compliance with the guidelines established by the Animal Care and Use Committee of Chiba University.
Assessments of sensory motor functions
Basso, Beattie, Bresnahan open field locomotor test
Hind limb function was assessed in an open field (100 cm × 60 cm plastic pool) using the Basso, Beattie, Bresnahan (BBB) open field locomotor test.12 Measurements were performed weekly thereafter for 9 weeks. Tests were videotaped for 5 minutes and scored by a trained observer who was unaware of the treatment group to which each subject was assigned.
Inclined plane test
Each animal was placed in head-up, transverse, and head-down positions on an inclined plane and the angle of slope gradually increased. The angle at which the animal fell down from the slope was recorded for each position, two trials per animal, after SCI. The better of the two trial results for each subject were combined and compared among the three groups. We performed these measurements before surgery and then 4, 6, and 8 weeks after SCI.
Rota-rod test
Four and 6 weeks after SCI, animals were placed on a 5 cm-wide turning cylinder (Rota-rod MK-630B, Muromachi Kikai, Japan) and forced to walk on it. The speed of rotation was gradually accelerated from 3 rpm (rotations per minute) to 30 rpm, and then maintained at 30 rpm for 5 minutes (Mode A1, 3–30 rpm). The time when the animal fell from the Rota-rod was recorded. Preoperatively, animals were able to stay on the Rota-rod for a mean duration of 199.3 seconds.
Sensory tests
Thermal nociceptive thresholds in rat hind limbs were evaluated using a Hargreaves device (Ugo Basile, Varese, Italy). The rats were placed in individual transparent acrylic boxes with the floor maintained at 28°C. A heat stimulus (150 mcal/seconds/cm2) was delivered using a 0.5 cm-diameter radiant heat source positioned under the plantar surface of the hind limb. The heat source was placed alternately under each hind limb to avoid anticipation by the animal. A cutoff time of 22 seconds was used, as we had previously ascertained that no tissue damage would result within this time period. The withdrawal threshold was calculated as the average value of three consecutive tests. Mechanical withdrawal thresholds in rat hind limbs were tested using a dynamic plantar aesthesiometer (Ugo Basile), in which a mechanical stimulus was applied via an actuator filament (0.5 mm diameter), which under computer control applied a linear ramp 5.0 g/seconds to the plantar surface of the hind limb. The withdrawal threshold was calculated as the average of six consecutive tests. Both tests were performed pre-injury and then 5, 7, and 9 weeks after contusion.
Anterograde labeling of the cortico-spinal tract with biotinylated dextran amine; immunohistochemical; and histological assessments
Nine weeks after contusion, the cortico-spinal tract was bilaterally traced under halothane anesthesia with 2.0 µl biotinylated dextran amine (BDA, molecular weight: 10 000, 10% in 0.01 M PBS, Molecular Probes, Carlsbad, CA). A micro-glass pipette needle attached to a 2-μl Hamilton syringe was stereotaxically guided, and BDA was slowly injected into four sites in the sensorimotor cortex for the hind limb at a 1-mm depth: Bregma 2 mm, sagittal suture 2 mm; Bregma 2 mm, sagittal suture 3 mm; Bregma 2.5 mm, sagittal suture 3 mm; Bregma 2.5 mm, sagittal suture 2.5 mm. The needle was left in place for 1 minute following each injection to minimize reflux.
Histology
Animals were subjected to trans-cardiac perfusion with 4% paraformaldehyde in PBS (pH 7.4) 14 days after BDA injection. The spinal cords were dissected and immersed overnight in 4% paraformaldehyde and then stored in 20% sucrose in PBS. The spinal cords were cut into 20-mm lengths (10 mm rostral to and 10 mm caudal to the lesion site) and embedded in optimal cutting temperature (OCT) compound (Tissue Tek, Sakura Finetechnical, Tokyo, Japan). We sectioned each block in the sagittal plane (25 µm) using a cryostat and mounted eight consecutive sections on poly-l-lysine-coated slides (Matsunami, Tokyo, Japan) to make serial sections. The sections on each slide were sliced at 150 µm intervals; each eight section slide therefore covered approximately 1200 µm of the lesion site. We performed histological or immunohistochemical staining on the slides.
To evaluate lesion size, we stained three slices from each animal with cresyl violet. We determined the cavity size of each section using Photoshop 5.5 software (Adobe, San Jose, CA, USA). We calculated a mean cavity size from these three values for each animal and compared cavity sizes between groups.
For anterograde labeling of the cortico-spinal tract with BDA, sections were incubated with Alexa Fluor 594-conjugated streptavidin (1:800; Molecular Probes). We selected seven consecutive sections from the rostral edge of the lesion center, which we photographed with a 20 × objective lens using a fluorescence microscope (DP71, Olympus, Tokyo, Japan). We added up the number of fibers and compared the fiber counts between the groups.
Three slices per animal, centered on the lesion epicenter, were incubated with rabbit anti-calcitonin gene-related peptide (CGRP) antibody (1:1000, ImmunoStar, Inc. Hudson, WI, USA) or rabbit anti-von Willebrand factor (1:400, Dako Cytomation, Glostrup, Denmark), then reacted with Alexa-Fluor 594 goat anti-rabbit IgG secondary antibody. Slices were photographed on the rostral and caudal edges of the lesion epicenter with a 10× objective lens using a fluorescence microscope (DP71, Olympus). The numbers of CGRP-positive immunoreactive fibers or von Willebrand factor-positive immunoreactive vessels were counted and averaged.
Statistical analysis
For histological studies and for assessments of sensory motor functions at each time point, we performed a Mann–Whitney U test. For the 9-week locomotor scale, we performed repeated-measures analysis of variance (ANOVA). Data were reported as mean values ± SEM. Differences with P values <0.05 were considered statistically significant.
Results
We measured body weight ratios every week after SCI. Rats were treated with bFGF – GH, or GH, 7 days after SCI. Weight loss was severe at 7 days after SCI: weight loss ratios for the bFGF – GH and the GH groups were 0.958 ± 0.020 and 0.938 ± 0.013, respectively. At the end of 9 weeks, the weights of the animals had increased to 1.322 ± 0.039 and 1.319 ± 0.034, respectively, for two groups. No statistically significant increase in the body weight ratio was observed during the entire experiment.
BBB locomotor scores 7 days after SCI were 1.0 ± 0.49 and 0.6 ± 0.34, respectively, for the bFGF – GH and the GH groups, and the intergroup difference was not statistically significant. BBB scores at 9 weeks were 10.5 ± 0.54 and 10.2 ± 0.58, respectively, for the two groups, and again no statistically significant difference between the groups was observed (Fig. 1). Repeated-measures ANOVA also failed to detect any statistically significant intergroup differences in BBB scores over the entire experiment period (P = 0.27).
Figure 1.
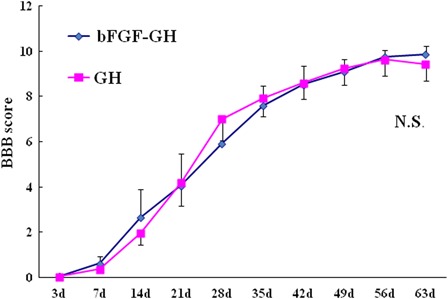
BBB locomotor scores during the first 9 weeks after SCI. The differences between the groups were not statistically significant (P = 0.27).
Inclined plane testing showed that before SCI, rats could keep their body on an inclined plane at 61.04 ± 0.43° in a head-up position, 57.29 ± 0.44° in a transverse position, and 45.83 ± 0.25° in a head-down position. The differences were not statistically significant between groups at 4, 6, and 8 weeks after SCI (data not shown). The Rota-rod test also showed no statistically significant differences between groups at 4, 6, and 8 weeks after SCI (data not shown).
Analysis of the pre-injury data for the Hargreaves device revealed a mean thermal latency of 16.9 ± 0.4 seconds (n = 18). Thermal latency decreased to mean values of 13.5 ± 0.9 seconds at 5 weeks, 14.7 ± 1.0 seconds at 7 weeks, and 14.0 ± 1.1 seconds at 9 weeks in the bFGF – GH group, and 13.2 ± 1.0 seconds at 5 weeks, 13.9 ± 0.9 seconds at 7 weeks, and 13.4 ± 0.7 seconds at 9 weeks in the GH group. Although thermal latency decreased in both groups after SCI compared with normal pre-injury rats, the differences did not reach statistical significance. In addition, none of the differences in mean thermal latency between the groups at any time period were statistically significant.
Mechanical thresholds using a dynamic plantar aesthesiometer had a mean pre-injury value of 31.5 ± 1.4 g (Fig. 2: n = 18). The mean values decreased to 26.2 ± 1.5 g at 5 weeks, 27.2 ± 1.2 g at 7 weeks, and 28.5 ± 1.9 g at 9 weeks in the bFGF – GH group, and 22.9 ± 2.1 g at 5 weeks, 25.2 ± 2.0 g at 7 weeks, and 28.5 ± 2.2 g at 9 weeks in the GH group. The GH group exhibited significantly more mechanical allodynia compared with pre-injury rats at 5 and 7 weeks (P = 0.006 and P = 0.021, respectively). The decreases in mechanical thresholds in the bFGF – GH group were not statistically significant over the course of the entire experiment.
Figure 2.
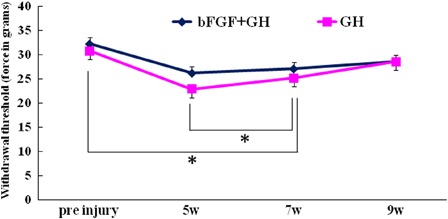
Mechanical thresholds using a dynamic plantar aesthesiometer were performed pre-injury and also 5, 7, and 9 weeks after contusion. The GH group showed significantly more mechanical allodynia compared with pre-injury rats at 5 and 7 weeks (P = 0.006 and P = 0.021, respectively). However, the bFGF – GH group showed no statistically significant decrease over the entire experiment.
To elucidate the efficacy of bFGF – GH or GH for tissue protection or tissue sparing after SCI, we measured the area of the cystic cavity with cresyl violet staining 9 weeks after injury (Fig. 3). The differences between the groups did not reach statistical significance (Fig. 3C, P = 0.94).
Figure 3.
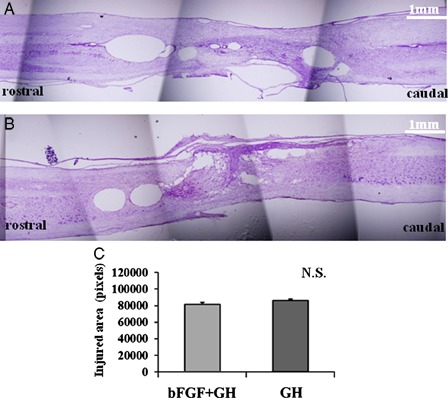
Cresyl violet staining 9 weeks after SCI did not show statistically significant cavity size differences between the two groups. The cavity size of each section was analyzed. Representative figures of each group from the bFGF + GH group (A) and the GH group (B) are presented. The differences among groups did not reach statistical significance (C, P = 0.94). Bar = 1 mm for figures A, B.
The BDA signals rostral to the lesion epicenter were 49.1 ± 13.7 and 38.9 ± 11.9 for the bFGF – GH and GH groups, respectively (Fig. 4). The differences between the two groups did not reach statistical significance (Fig. 4C, P = 0.22). In the same way, we analyzed von Willebrand factor-positive signals at the lesion epicenter. Values were 23 ± 12.0, and 17.6 ±5.2, for the bFGF – GH and GH groups, respectively. No statistically significant difference between groups was observed (P = 0.83).
Figure 4.
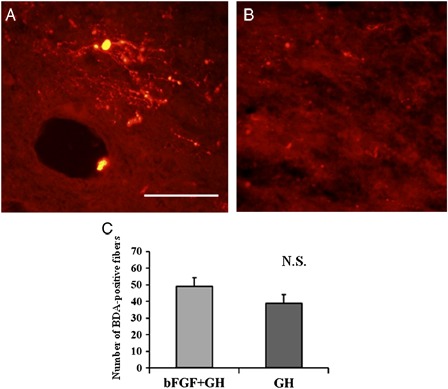
Biotinylated dextran amine tracing 8 weeks after SCI. The BDA signals at the rostral edge of the lesion epicenter were analyzed. The differences between the groups did not reach statistical significance (C, P = 0.22). Bar = 100 µm for figures A, B.
The CGRP signals from the posterior funiculus on the rostral and caudal sides of the lesion epicenter were analyzed and compared. CGRP-positive fiber counts were 7.1 ± 2.6 and 35.2 ± 6.5 for the bFGF–GH and GH groups, respectively, and none of the differences were statistically significant (P = 0.17).
Discussion
Although we injected bFGF-incorporated GH 1 week after SCI in our experimental model, the optimal timing of bFGF injection remains an unresolved issue. Several studies, though, have provided suggestive data. For instance, one study detected significant increases in various molecular forms of FGF2 protein 4 days after SCI.13 Another study showed significant up-regulation of bFGF 3 days after SCI, when cell proliferation is maximal.14 A third study tracked bFGF mRNA, which initially was detected 1 hour post-injury, increased between 6 hours and 3 days, declined thereafter, and returned to baseline levels by 21 days.15 These reports together indicate that up-regulation of bFGF is maximal 3 days after SCI and gradually decreases after that, from which we deduce that in terms of timing, it is best to wait until after bFGF up-regulation has peaked before injecting bFGF. Furthermore, another study showed that epidermal growth factor and FGF2 injection immediately after SCI had no impact on BBB scores for 8 weeks.16 On the basis of all these studies, we decided to inject bFGF-incorporated GH 7 days after SCI.
We measured BBB scores for 9 weeks after SCI and also performed Rota-rod and inclined plane testing at several time points after SCI. The locomotor measurement data showed no statistically significant recovery in this study. bFGF – GH and GH-only injections appear to have had almost identical effects on injured spinal cords. In other words, both the bFGF – GH and the GH injections may have improved the ability of injured rats to perform weight-bearing activity. While the saline-injected SCI model rats that suffered the same contusion injury of the spinal cord did not reach weight-bearing levels in their BBB scores in our previous study,1 rats of both groups in this study were able to perform weight-bearing activity. This result implies the possibility that GH itself has neuroprotective effects. Further investigation is needed to clarify this point. To examine associated histological changes, we assessed cortico-spinal tract tracing 2 weeks before sacrifice. Comparisons of BDA signals did not show statistically significant differences between groups. This histological finding supports the locomotor assessments in which bFGF – GH and GH-only injections showed no statistically significant recovery in this study.
We measured two types of allodynia using a Hargreaves device and a dynamic plantar aesthesiometer. We observed no statistically significant differences between groups and in comparison with pre-injury rats, in mean thermal latency using the Hargreaves device. With respect to mechanical allodynia using the dynamic plantar aesthesiometer, while the GH injection group showed significantly more mechanical allodynia than the pre-injury data, the bFGF – GH group showed no statistically significant threshold changes compared with pre-injury. Although no statistically significant differences in the posterior funiculus CGRP-positive fiber counts between rostral and caudal sides of the lesion epicenter were observed, CGRP-positive fiber counts were lower in the bFGF – GH group. The histology data thus show some correspondence with the mechanical allodynia testing data, i.e. the bFGF – GH injection group had significantly less sensitivity to mechanical allodynia.
Conclusion
To summarize, the findings of this study revealed that the bFGF – GH group showed no statistically significant threshold changes compared with pre-injury, whereas the GH-alone group showed significantly more mechanical allodynia than the pre-injury data for that group. We had hoped to provide evidence that bFGF – GH could create a better environment for spinal cord regeneration. In the present study, although the bFGF – GH group showed almost identical amounts of recovery in comparison with GH group, we conclude that bFGF – GH created better conditions for decreasing sensory abnormalities.
Conclusion
Although we found few significant effects of bFGF – GH therapy, our results did provide evidence that bFGF-incorporated hydrogel treatment may possibly relieve mechanical allodynia following SCI and should be comparatively safe in future clinical use.
Acknowledgement
This research was supported by a grant-in-aid for Japanese scientific research grant 20591736.
References
- 1.Furuya T, Hashimoto M, Koda M, Okawa A, Murata A, Takahashi K, et al. Treatment of rat spinal cord injury with a Rho-kinase inhibitor and bone marrow stromal cell transplantation. Brain Res 2009;1295:192–202 [DOI] [PubMed] [Google Scholar]
- 2.Marui A, Tabata Y, Kojima S, Yamamoto M, Tambara K, Nishina T, et al. A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I-IIa study. Circ J 2007;71(8):1181–6 [DOI] [PubMed] [Google Scholar]
- 3.Iwakura A, Fujita M, Kataoka K, Tambara K, Sakakibara Y, Komeda M, et al. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels 2003;18(2):93–9 [DOI] [PubMed] [Google Scholar]
- 4.Iwakura A, Tabata Y, Miyao M, Ozeki M, Tamura N, Ikai A, et al. Novel method to enhance sternal healing after harvesting bilateral internal thoracic arteries with use of basic fibroblast growth factor. Circulation 2000;10219 Suppl. 3:III307–11 [DOI] [PubMed] [Google Scholar]
- 5.Aimoto T, Uchida E, Matsushita A, Tabata Y, Takano T, Miyamoto M, et al. Controlled release of basic fibroblast growth factor promotes healing of the pancreaticojejunal anastomosis: a novel approach toward zero pancreatic fistula. Surgery 2007;142(5):734–40 [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem 1995;65(4):1740–51 [DOI] [PubMed] [Google Scholar]
- 7.Kirschner PB, Henshaw R, Weise J, Trubetskoy V, Finklestein S, Schulz JB, et al. Basic fibroblast growth factor protects against excitotoxicity and chemical hypoxia in both neonatal and adult rats. J Cereb Blood Flow Metab 1995;15(4):619–23 [DOI] [PubMed] [Google Scholar]
- 8.Anderson KJ, Dam LS, Cotman CW. Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature 1998;332(6162):360–1 [DOI] [PubMed] [Google Scholar]
- 9.Teng YD, Mocchetti I, Wrathall JR. Basic and acidic fibloblast growth factors protect spinal motor neurons in vivo after experimental spinal cord injury. Eur J Neurosci 1998;10(2):798–802 [DOI] [PubMed] [Google Scholar]
- 10.Itosaka H, Kuroda S, Shichinohe H, Yasuda H, Yano S, Kamei S, et al. Fibrin matrix provides a suitable scaffold for bone marrow stromal cells transplanted into injured spinal cord: a novel material for CNS tissue engineering. Neuropathology 2009;29(3):248–57 [DOI] [PubMed] [Google Scholar]
- 11.Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 and platelet derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant 2010;19(1):89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12(1):1–21 [DOI] [PubMed] [Google Scholar]
- 13.Mocchetti I, Rabin SJ, Colangelo AM, Whittemore SR, Wrathall JR. Increased basic fibroblast growth factor expression following contusive spinal cord injury. Exp Neurol 1996;141(1):154–64 [DOI] [PubMed] [Google Scholar]
- 14.Zai LJ, Yoo S, Wrathall JR. Increased growth factor expression and cell proliferation after contusive spinal cord injury. Brain Res 2005;1052(2):147–55 [DOI] [PubMed] [Google Scholar]
- 15.Lee YL, Shih K, Bao P, Ghirnikar RS, Eng LF. Cytokine chemokine expression in contused rat spinal cord. Neurochem Int 2000;36(4–5):417–25 [DOI] [PubMed] [Google Scholar]
- 16.Jimenez Hamann MC, Tator CH, Shoichet MS. Injectable intrathecal delivery system for localized administration of EGF and FGF-2 to the injured rat spinal cord. Exp Neurol 2005;194(1):106–19 [DOI] [PubMed] [Google Scholar]


