Abstract
Background
Ischemic/reperfusion (I/R) injury of the spinal cord is a serious complication that can result from thoracoabdominal aortic surgery.
Objective
To investigate the neuroprotective effect of curcumin against I/R injury in a rabbit model.
Methods
A total of 36 rabbits were randomly divided into three groups: sham, I/R, and curcumin-treated group. Rabbits were subject to 30-min aortic occlusion to induce transient spinal cord ischemia. Neurological function was observed after reperfusion and spinal cord segment (L3–L5) was collected for histopathological evaluation. Malondialdehyde (MDA) and total superoxide dismutase (SOD) activity were also assayed.
Results
Rabbits in I/R group were induced to paraplegia. While after 48-hour treatment, compared with I/R group, curcumin significantly improved neurological function, reduced cell apoptosis and MDA levels as well as increased SOD activity (P < 0.05).
Conclusions
The results suggest that curcumin, at least in an animal model, can attenuate transient spinal cord ischemic injury potentially via reducing oxidative damage, which may provide a novel approach in the treatment of spinal cord ischemic injury.
Keywords: Curcumin, Spinal cord, Ischemia/reperfusion injury, Rabbits, Paraplegia, Aneurysm, Abdominal, Aortic, Neuroprotection
Introduction
Paraplegia is a major unpredictable complication after surgical repair of thoracoabdominal aortic aneurysms,1 which is thought to be related to multiple factors. Among these factors, ischemia and reperfusion injury of the spinal cord is regarded as the principal root. Unfortunately, although many interventions have been used to protect the spinal cord, the complication still cannot be prevented completely.2–4 Given its poor prognosis, it is necessary to develop new approaches to prevent or treat ischemic/reperfusion (I/R) injury of the spinal cord.
Curcumin, a yellow-orange dye extracted from the spice turmeric, possessing both anti-inflammatory antioxidant and anti-carcinogenic effect,5 is a potent stimulator of the stress-induced expression of heat shock protein 70. Recently, it was reported that administration of curcumin could ameliorate I/R injury in rat kidney, myocardium, and nervous tissue.6–8 However, to the best of our knowledge, little is known about its effect on I/R injured spinal cord. In this study, we examined its neuro-protective effect and potential mechanism in a rabbit model with I/R injury.
Materials and methods
Animals and treatment
All procedures were approved by the animal care committee of Jining Medical University. A total of 36 male New Zealand rabbits, weighting from 2.0 to 2.5 kg (mean 2.25 ± 0.25 kg) were obtained from the center of experimental animals of Jining Medical University. Water was available ad libitum for all rabbits. Standard chow (22.6% protein, 53.8% carbohydrate, 5.6% fat, 6.6% mineral and vitamin mixture, and 3.3% fiber) was used for this study. The animal room was kept on a 12-hour reverse light/dark cycle (dark, 7:00 am to 7:00 pm; light, 7:00 pm to 7:00 am), at a constant temperature (22 ± 1°C) and a relative humidity of 55 ± 5% throughout the experimental period.
After two weeks’ acclimatization of laboratory conditions, rabbits were randomly divided into three groups: (1) sham group (n = 12): animals were under surgical procedure but not aorta occluded; (2) I/R group (n = 12): abdominal aorta was occluded for 30 minutes, followed by reperfusion; and (3) curcumin group (n = 12): curcumin was infused 10 minutes before abdominal aorta occlusion and was occluded for 30 minutes, followed by reperfusion.
Experimental I/R spinal cord injury
Rabbits were anesthetized with 30 mg/kg sodium pentobarbital and mechanically ventilated with room air. Surgical procedures were performed aseptically. A polyethylene catheter (0.90-mm lumen diameter) was inserted into the left femoral artery for continuous blood pressure monitoring. Parameters including the arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), pH, and plasma glucose were all recorded throughout the experiment until 20 minutes after reperfusion by means of the OMNI Modular System.9 Drugs and saline were administered through the ear vein. The animals were placed in supine position and an 8-cm midline incision was made to expose the abdomen. After giving 400 units of heparin, the abdominal aorta was occluded for 30 minutes using atraumatic vascular clamps at the level of the left renal artery. At the end of the occlusion period, the clamp was removed, and the abdominal wall was subsequently closed in layers using 4–0 nylon suture. Curcumin group animals were injected with 50 mg/kg curcumin dissolved in 1-mL dimethyl sulfoxide solution into a branch of the glossopharyngeal vein 10 minutes before abdominal aorta occlusion; the sham and I/R group was injected with the same volume of saline solution.
Neurological evaluation
Neurological function was observed at 48 hours after reperfusion. Modified Tarlov criteria were used to grade the motor function of hind limbs as previously described.10 Evaluation was scored independently by two investigators, who did not know any details of our study, according to the following scale: 0 – no movement of the lower limbs; 1 – minimal movement; 2 – good movement but unable to stand; 3 – able to stand and walk but unable to hop normally; and 4 – normal recovery.
Histological analysis
After neurological evaluation, eight rabbits from each group were euthanized. Spinal cord segments (L3–L5) were collected and immersed in 4% of paraformaldehyde in 0.1 M phosphate-buffered saline and stored at 4°C for two weeks. After dehydration in graded ethanol, the samples were embedded in paraffin. Coronal sections of spinal cord segments were cut about 5 µm and hematoxylin and eosin (HE) were used for evaluation of structural changes. Injured neurons were identified as intensely eosinophilic cytoplasm, loss of Nissl substance, and pyknotic nuclei. The residual normal neurons in the ischemic ventral spinal cord in each animal, judged by their morphological appearance, were counted in three sections selected randomly from the rostral, middle, and caudal levels of the L5 segment and then averaged.
TUNEL assay
Sections were prepared as described for HE staining, and the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed according to the manufacturer's instructions as we previously reported.11 The results were observed under a Leica photograph microscope. The number of apoptotic cells in five unfolded continuous fields in each section was counted by the same observer, who was blinded to the protocol. The total number of apoptotic cells was expressed as the average percentage of TUNEL positive cells.
Biochemical analysis
Spinal cord tissues were washed two times with cold saline solution and stored in –30°C until use. Tissue malondialdehyde (MDA) levels were determined by the method described by Nazari et al.12 Briefly, MDA was reacted with thiobarbituric acid by incubating for 1 hour at 95–100°C and fluorescence intensity was measured in the n-butanol phase with a fluorescence spectrophotometry (Hitachi, Model F-4010, Japan), by comparing with a standard solution of 1, 1, 3, 3-tetramethoxypropane. The results were expressed in terms of nmol/g wet tissue.
Total (Cu-Zn and Mn) superoxide dis mutase (SOD) activity was measured according to reduction of nitroblue tetrazolium by xanthine–xanthine oxidase system as described previously.13 Enzyme activity leading to 50% inhibition was regarded as one unit. The results were expressed as U/mg of protein. Protein concentrations were determined according to Lowry's method.14
Statistical analysis
Statistical analysis was performed using SPSS 10.0 software. Physiological parameters, MDA levels, SOD activity, and apoptotic levels were performed with a one-way analysis of variance followed by least significant difference test. Neurological scores were analyzed with non-parametric method (Kruskal–Wallis test) followed by the Mann–Whitney U test with Bonferroni correction. Data were expressed as mean ± SD and P < 0.05 was considered as statistically significant.
Results
Physical parameters
The distal blood pressure was about 80–90 mmHg before blocking abdominal aorta and decreased by 10–15 mmHg during the period of ischemia. Among the three groups, there were no significant differences in terms of hemodynamics, rectal temperature, arterial pH, PaCO2, and PaO2 values during the experiment.
Neurological function evaluation
As shown in Table 1, compared with animals in the sham group, animals in the I/R group displayed significantly reduced neurological scores at 48 hours after reperfusion (P < 0.05). However, after treatment with curcumin, although the neurological score was still lower than that of the sham group, it was significantly higher than that of the I/R group (P < 0.05).
Table 1.
Neurological scores of rabbits 48 hour after reperfusion
| Groups | N | Motor score |
Average motor score | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Sham group | 12 | 0 | 0 | 0 | 0 | 12 | 5 |
| I/R group | 12 | 5 | 4 | 3 | 0 | 0 | 1.76 ± 0.76* |
| Curcumin group | 12 | 2 | 5 | 4 | 1 | 0 | 3.26 ± 0.76** |
*P < 0.05, compared with sham group. **P < 0.05, compared with I/R group.
Histopathological examination
The representative micrographs of HE staining in spinal cord segment were shown in Fig. 1. No abnormalities were seen in the sham group (Fig. 1A); however, the I/R group exhibited necrotic changes with pronounced vacuolization, intensely eosinophilic cytoplasm, loss of Nissl granule, and pyknosis (Fig. 1B). Furthermore, many normal neurons were seen in the curcumin-treated group when compared with the I/R group (Fig. 1C).
Figure 1.

Histological changes of spinal cord segment stained with HE after curcumin treatment. (A) Sham group; (B) I/R group; (C) Curcumin group (magnification, × 400).
TUNEL staining identified a few dead cells in the cord sections of sham-operated animals. In the spinal cords of I/R group, numerous cells were strongly positive for TUNEL staining. However, in the curcumin-treated group, some cells were positive for TUNEL staining but fewer than in the I/R group. Using quantitative analysis, both positive and negative cells for TUNEL staining were counted and the results were shown in Fig. 2. It showed that the curcumin-treated group had significantly fewer positive cells than in I/R group (P < 0.05), which suggests curcumin may alleviate apoptosis following ischemia/reperfusion.
Figure 2.

Percentage of TUNEL positive cells in rabbit spinal cord after curcumin treatment. *P < 0.01 vs. sham group; **P < 0.05 vs. I/R group.
Biochemical function
To determine the local oxidative/antioxidative levels, we further measured the MDA and SOD activities in spinal cord collected from the three groups. As shown in Figs. 3 and 4, significantly higher MDA levels and decreased SOD activities were found in the I/R group compared with those in the sham group (both P < 0.01). While after treatment with curcumin, although still not recovered thoroughly, curcumin indeed reduced the local oxidative stress as shown by decreased MDA levels and increased SOD activities.
Figure 3.
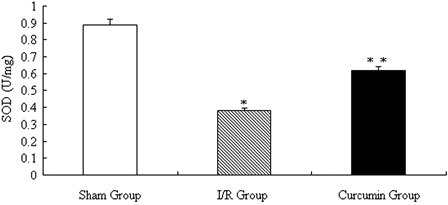
SOD activities in collected spinal cord after curcumin treatment. *P < 0.01 vs. sham group; **P < 0.05 vs. I/R group.
Figure 4.

MDA levels in rabbit spinal cord after curcumin treatment. *P < 0.01 vs. sham group; **P < 0.05 vs. I/R group.
Discussion
The present study demonstrated that treatment with curcumin significantly attenuated spinal cord injury induced by I/R in an animal model. The protective effect of curcumin is potentially related to its antioxidative capacity, thus it reduces neuronal apoptosis.
Reactive oxygen species (ROS) are a class of chemical properties of active oxygen atoms or group of atoms, which include all the active forms of oxygen. Under physiological conditions, the scavenging of ROS is performed by a large number of antioxidant systems including antioxidant enzymes (such as SOD) and non-enzymatic antioxidants. The imbalance between oxidant and antioxidant status, resulting from either the increased production of ROS or inactivation and excessive consumption of antioxidant systems, causes oxidative stress. Under conditions of oxidative stress, damage to cellular biomolecules such as lipids, proteins, and DNA occurs and participates in many pathological processes.15 As we all know, oxidative stress plays an important role in the spinal cord injury induced by I/R,16 which is the major cause of paraplegia suffered in patients after repair of thoracoabdominal aortic aneurysms. Curcumin, a herbal medicine with powerful antioxidant,17 has been widely used as a therapeutic agent for a variety of diseases from cancer to Alzheimer's to infectious diseases,18 especially for I/R related injury in different tissues.6,19–21 For example, just recently, Avci et al.20 reported that curcumin treatment significantly revealed I/R injury in skeletal muscle, and it has more potent antioxidant activity than vitamin E in the skeletal muscle I/R. In the nerve system, curcumin has also been proven to have neuroprotective effects in a variety of preclinical stroke models, and is regarded as an acute monotherapy or can be used in conjunction with thrombolytics for acute ischemic stroke.22 In this study, we found that curcumin treatment significantly reduced MDA levels and improved SOD activities in local spinal cord after I/R. Since both MDA (the product of peroxidation of lipids) and SOD (an oxygen radical scavenger) are often used to evaluate the extent of oxidative stress, our results provided new evidence that curcumin can also significantly decrease I/R induced oxidative stress in spinal cords. Furthermore, as noted, numerous dead cells were observed in the spinal cords of the I/R model animals, while the total number of TUNEL-positive cells was significantly reduced in the curcumin group, which suggested that curcumin can further provide neuronal protection through antiapoptotic mechanisms to prevent cell apoptosis and improve physical and neurological function. Our results suggest that curcumin may protect against neuronal injury and has a potential clinical benefit in the future.
Curcumin increased the mitochondrial levels of Bcl-2 protein and decreased the subsequent cytosolic translocation of cytochrome c, thus affect downstream pathways such as caspases.6,7,23 In this study, although reduced cell apoptosis was achieved after treatment with curcumin, it still cannot recover to the normal level. This result suggests that there may be other pathways in I/R process besides Bcl-2.24–26 Multiple pathways are involved in spinal cord ischemia. For example, blocking of cytochrome c release and subsequent caspase activation is related to the neuro-protective effects of curcumin in amyotrophic lateral sclerosis mice.25 Furthermore, another study showed the regulatory effect of curcumin on nuclear factor-kappa B and p53,27 both of which are important in regulation of apoptosis. Indeed, our study provided evidence that curcumin can reduce I/R-induced cell apoptosis in the spinal cord, but the exact neuro-protective mechanism of curcumin, such as inhibiting cytochrome c release and following activation of caspase, need further study.
Taken together, our studies provide evidence that curcumin, at least in an animal model, can attenuate spinal cord injury induced by I/R. The protective effect of curcumin is potentially related to its antioxidative and antiapoptotic capacities, which may provide a novel approach in the treatment of spinal cord ischemic injury. Further studies are required to examine the safety and therapeutic potency of curcumin in patients, especially those with high risk of I/R injury.
Acknowledgements
This work was funded by the Young Scientists Star Program of Jinan City (grant no. 09114), Natural Science Foundation (grant no. ZR2009CQ023) and Medical Science Development Plan (grant no. 2009QZ025) of Shandong Province.
Author contributions
Zhi-Qiang Liu and Shan-Shan Xing were responsible for the data collection and were also responsible for the draft of the manuscript. Wei Zhang conceived and designed the study and was also responsible for the statistical analysis.
References
- 1.Ucak A, Onan B, Güler A, Sahin MA, Kılıçkaya O, Oztaş E, Uysal B, Arslan S, Yılmaz AT. Rosuvastatin, a new generation 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, reduces ischemia/reperfusion-induced spinal cord tissue injury in rats. Ann Vasc Surg 2011;25(2):686–95 [DOI] [PubMed] [Google Scholar]
- 2.Herlambang B, Orihashi K, Mizukami T, Takahashi S, Uchida N, Hiyama E, Sueda T. New method for absolute spinal cord ischemia protection in rabbits. J Vasc Surg 2011;54(4):1109–16 [DOI] [PubMed] [Google Scholar]
- 3.Anik I, Kokturk S, Genc H, Cabuk B, Koc K, Yavuz S, Ceylan S, Ceylan S, Kamaci L, Anik Y. Immunohistochemical analysis of TIMP-2 and collagen types I and IV in experimental spinal cord ischemia-reperfusion injury in rats. J Spinal Cord Med 2011;34(3):257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, Bruce IC, Luo BY, Xia Q. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke 2009;39(3):983–90 [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Yang D, Li S, Xu Z, Wang X, Bai C. Effects of curcumin or dexamethasone on lung ischaemia-reperfusion injury in rats. Eur Respir J 2009;3(2):398–404 [DOI] [PubMed] [Google Scholar]
- 6.Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol 2011;42(6):579–87 [DOI] [PubMed] [Google Scholar]
- 7.Yeh CH, Chen TP, Wu YC, Lin YM, Jing Lin P. Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res 2005;125(1):109–16 [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res 2005;82(1):138–48 [DOI] [PubMed] [Google Scholar]
- 9.Prakash T, Kotresha D, Nedendla RR. Neuroprotective activity of Wedelia calendulacea on cerebral ischemia/reperfusion induced oxidative stress in rats. Indian J Pharmacol 2011;43(6):676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi E, Jiang X, Wang L, Akuzawa S, Nakajima Y, Kazui T. Intrathecal injection of hepatocyte growth factor gene-modified marrow stromal cells attenuates neurologic injury induced by transient spinal cord ischemia in rabbits. Anesthesiology 2010;113(5):1109–17 [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Gao L, Sun N, Zhang J, Zhang W, Zhou X, Zhang H, Zhao J. Effects of Kang-Jia-Wan, a Chinese medicinal herb officinal, on apoptosis induction in goiter of rats. J Ethnopharmacol 2009;122(3):533–40 [DOI] [PubMed] [Google Scholar]
- 12.Nazari A, Sadr SS, Faghihi M, Imani A, Moghimian M. The cardioprotective effect of different doses of vasopressin (AVP) against ischemia-reperfusion injuries in the anesthetized rat heart. Peptides 2011;32(12):2459–66 [DOI] [PubMed] [Google Scholar]
- 13.Trocha M, Merwid-Ląd A, Chlebda E, Pieśniewska M, Sozański T, Szeląg A. Effect of simvastatin treatment on rat livers subjected to ischemia/reperfusion. Pharmacol Rep 2010;62(4):757–62 [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Tian LM, Han Y, Ma HY, Wang LC, Guo J, Gao L, Zhao JJ. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med 2009;13(11–12):4636–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floyd RA, Towner RA, He T, Hensley K, Maples KR. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med 2011;51(5):931–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong HL, Zhang Y, Su BX, Zhu ZH, Gu QH, Sang HF, Xiong L. Limb remote ischemic preconditioning protects the spinal cord from ischemia-reperfusion injury: a newly identified nonneuronal but reactive oxygen species-dependent pathway. Anesthesiology 2010;112(4):881–91 [DOI] [PubMed] [Google Scholar]
- 17.Chan WH, Wu HJ. Protective effects of curcumin on methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Acta Pharmacol Sin 2006;27(9):1192–8 [DOI] [PubMed] [Google Scholar]
- 18.Marathe SA, Dasgupta I, Gnanadhas DP, Chakravortty D. Multifaceted roles of curcumin: two sides of a coin! Expert Opin Biol Ther. 2011;11(11):1485–99 [DOI] [PubMed] [Google Scholar]
- 19.Shen SQ, Zhang Y, Xiang JJ, Xiong CL. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J Gastroenterol 2007;13(13):1953–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avci G, Kadioglu H, Sehirli AO, Bozkurt S, Guclu O, Arslan E, Muratli SK. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res 2012;172(1):e39–46 [DOI] [PubMed] [Google Scholar]
- 21.Awad AS, El-Sharif AA. Curcumin immune-mediated and anti-apoptotic mechanisms protect against renal ischemia/reperfusion and distant organ induced injuries. Int Immunopharmacol 2011;11(8):992–6 [DOI] [PubMed] [Google Scholar]
- 22.Lapchak PA. Neuroprotective and neurotrophic curcuminoids to treat stroke: a translational perspective. Expert Opin Investig Drugs 2011;20(1):13–22 [DOI] [PubMed] [Google Scholar]
- 23.Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca AF, Sahin S, Yildirim ME, Kaya A, Cimentepe E, Akcay A. Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J Urol 2008;26(3):285–91 [DOI] [PubMed] [Google Scholar]
- 24.Li WJ, Shin MK, Oh SJ. Time dependent bladder apoptosis induced by acute bladder outlet obstruction and subsequent emptying is associated with decreased MnSOD expression and Bcl-2/Bax ratio. J Korean Med Sci 2010;25(11):1652–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Paola R, Esposito E, Mazzon E, Paterniti I, Galuppo M, Cuzzocrea S. GW0742, a selective PPAR-beta/delta agonist, contributes to the resolution of inflammation after gut ischemia/reperfusion injury. J Leukoc Biol 2010;88(2):291–301 [DOI] [PubMed] [Google Scholar]
- 26.Ryu H, Smith K, Camelo SI, Carreras I, Lee J, Iglesias AH, Dangond F, Cormier KA, Cudkowicz ME, Brown RH, Jr, Ferrante RJ. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem 2005;93(5):1087–98 [DOI] [PubMed] [Google Scholar]
- 27.Watanabe FT, Chade DC, Reis ST, Piantino C, Dall’ Oglio MF, Srougi M, Leite KR. Curcumin, but not Prima-1, decreased tumor cell proliferation in the syngeneic murine orthotopic bladder tumor model. Clinics (Sao Paulo) 2011;66(12):2121–4 [DOI] [PMC free article] [PubMed] [Google Scholar]


