Abstract
Spinal cord injury (SCI) results in motor and sensory impairments that can be identified with the American Spinal Injury Association (ASIA) Impairment Scale (AIS). Although, SCI may disrupt autonomic neural transmission, less is understood regarding the clinical impact of decentralized autonomic control. Cardiovascular regulation may be altered following SCI and the degree of impairment may or may not relate to the level of AIS injury classification. In general, persons with lesions above T1 present with bradycardia, hypotension, and orthostatic hypotension; functional changes which may interfere with rehabilitation efforts. Although many individuals with SCI above T1 remain overtly asymptomatic to hypotension, we have documented deficits in memory and attention processing speed in hypotensive individuals with SCI compared to a normotensive SCI cohort. Reduced resting cerebral blood flow (CBF) and diminished CBF responses to cognitive testing relate to test performance in hypotensive non-SCI, and preliminary evidence suggests a similar association in individuals with SCI. Persons with paraplegia below T7 generally present with a normal cardiovascular profile; however, our group and others have documented persistently elevated heart rate and increased arterial stiffness. In the non-SCI literature there is evidence supporting a link between increased arterial stiffness and cognitive deficits. Preliminary evidence suggests increased incidence of cognitive impairment in individuals with paraplegia, which we believe may relate to adverse cardiovascular changes. This report reviews relevant literature and discusses findings related to the possible association between decentralized cardiovascular autonomic control and cognitive dysfunction in persons with SCI.
Keywords: Spinal cord injuries, Cerebral blood flow, Brain injury, Traumatic, Cognitive dysfunction, Arterial pressure, Heart rate, Arterial stiffness, Autonomic dysreflexia, Tetraplegia, Paraplegia, Neuropsychological testing, Rehabilitation
Introduction
Several investigators have reported that from 10 to 60% of individuals with spinal cord injury (SCI) demonstrate cognitive deficits in the areas of attention,1–4 concentration,2–5 memory,2,3,5 abstract reasoning,5 verbal learning,2,3,5 and information processing.6 Reports suggest that these cognitive deficits impair comprehensive rehabilitation efforts and social integration in persons with SCI.6,7 Despite these findings, few studies have attempted to clearly define the etiology of the cognitive impairments in individuals with chronic SCI, and most reports attribute the deficits to concomitant traumatic brain injury (TBI) or pre-morbid conditions.1,5,8,9 A single report suggests that, although TBI was likely in a sample of individuals with SCI (injuries due to motor vehicle accidents), the pattern of the cognitive deficits suggested multiple etiologies.10 It is conceivable that, analogous to observations in the non-SCI population, cardiovascular and cerebral vascular dysfunction may contribute to the cognitive deficits observed in persons with SCI. Specifically, in the non-SCI population, cognitive deficits are reported in association with altered autonomic cardiovascular regulation,11,12 systemic hypotension,13–15 and cerebral hypoperfusion,16,17 increased basal blood pressure (BP)18–21 and arterial stiffness.22–27 These adverse systemic and cerebral hemodynamic and regulatory changes will be discussed as they relate to the SCI population which, we propose, may contribute to the cognitive deficits observed.
Autonomic cardiovascular function and cognitive performance
Evidence in non-SCI individuals suggests that adrenergic stimulation with exogenous epinephrine infusion enhances long-term memory consolidation28 whereas; non-selective beta-blockade with propranolol significantly impairs long-term memory for emotionally arousing stimuli.29 However, the relationship between cognitive function and adrenergic stimulation appears to be inversely U-shaped because memory and attention may be diminished at relatively low levels of sympathetic activation but are also impaired with higher levels of stimulation.28–31 Individuals with SCI above T6 are reported to have diminished sympathetic responses to stress, particularly during upright positioning,32–34 exercise,35 and mental activation.36 The impact of diminished sympathetic responses to stress on cognitive function is currently unknown in persons with lesions above T6, although an association seems likely.
The relationship between cardiac vagal tone and cognitive processes has been studied in infants and children, and, in general, higher basal levels of vagal tone are associated with improved sustained attention.37,38 In adults, findings of resting heart rate variability (HRV), an indirect estimate of cardio-vagal tone, and cognitive function suggest improved working memory and faster reaction times in individuals with relatively higher resting HRV.39 Moreover, a negative correlation was established between resting HRV and the number of false-positive responses, suggesting that the effects of vagal tone were specific to tasks of executive function.39 Although anatomically intact, we have demonstrated diminished amplitudes within the high-frequency bandwidth of HRV (i.e. an indirect cardio-vagal estimate) with increasing level and severity of SCI.40,41 More recently, during a laboratory test of the diving reflex (i.e. cold face test), which should stimulate cardio-vagal efferent activity and elicit bradycardia, we demonstrated a paradoxical increase in HR in subjects with tetraplegia and paraplegia suggesting vagal pathophysiology,42 regardless of the level of lesion. Evidence is not available on the possible relationship between alterations in vagal function and cognitive performance in persons with SCI, and investigation into this possible association should be considered, regardless of the level of lesion.
In healthy volunteers, an inverse relationship between baroreceptor reflex sensitivity (BRS) and cognition has been suggested, such that lower resting BRS tone (i.e. smaller change in HR for a given change in BP) related to improved attentional performance.11 However, a subsequent report suggested that the association between BRS and cognitive performance was dependent on tonic BP.43 Optimal task performance during cognitive testing is related to precise regulation of autonomic cardiovascular control, which is comprised of adrenergic, vagal, and baroreceptor reflex influences. Given the complexity of the association between cognitive performance and autonomic function, the cognitive deficits reported in persons with SCI may reflect, in part, impairment in global cardiovascular autonomic regulation.
Autonomic cardiovascular regulation is compromised following SCI, the degree of which may or may not relate to the level and completeness of the spinal lesion as assessed by the American Spinal Injury Association (ASIA) Impairment Scale (AIS).40,41,44–46 Although it is likely that the cardiovascular alterations after SCI relate to the degree of de-centralized sympathetic cardiovascular control, accurate assessment of the extent of damage to the spinal sympathetic pathways following SCI is not currently possible,47,48 and the impact on cognitive function is not known. However, the consequence of de-centralized sympathetic innervation seems to influence cardiovascular function differentially by the level of lesion. Specifically, individuals with a spinal lesion above T1 generally present with bradycardia, hypotension, and orthostatic hypotension (OH),47,49–52 but may also display substantial, unpredictable, and potentially life-threatening increases in BP during bouts with autonomic dysreflexia (AD).36,53–55 On the other hand, persons with paraplegia below T6 often present with a clinically normal cardiovascular profile, although reports have suggested chronically elevated resting HR and arterial stiffness.35,56–59 Thus, the cardiovascular consequences of decentralized autonomic cardiovascular control may play a role in cognitive dysfunction in persons with SCI, regardless of the level of lesion.
The relationship between hypotension, cerebral hypoperfusion, and cognitive performance
There is a growing body of evidence to support an association between chronic hypotension and cognitive deficits. In otherwise healthy non-SCI individuals, hypotension was associated with slowed cognitive speed,60 fewer word recall,61 decreased accuracy of response,13 limited attention,61 prolonged reaction times,13,15,60 and reduced memory and concentration capacity13,15 compared to normotensive controls. Chronic hypotension has also been linked to poorer reporting of general health,62,63 disturbances in mental state,63 increased reporting of fatigue,62–64 impaired social wellbeing,65 loss of appetite,65 and a high incidence of depression62 in otherwise healthy individuals compared to age-matched normotensive individuals. In addition to static BP, findings on circadian BP variations in the general population suggest an association between the absence of a nocturnal dip in BP and cognitive impairments.66
Individuals with SCI above T1 tend to be persistently hypotensive with periods of episodic orthostatic hypotension.44,45,47,49–52,67–69 Our preliminary data suggest that systolic blood pressure (SBP) is persistently lower in individuals with tetraplegia over a typical 24-hour period compared to persons with high paraplegia (T2–T5) and non-SCI controls (Fig. 1), and as a consequence, the nocturnal dip in SBP (SBPdip) was significantly attenuated in individuals with tetraplegia compared to the groups with paraplegia (T2–T5 and T7–T12) or non-SCI controls (Fig. 2).70 As previously reported in non-SCI subjects,13,14,17,66,71 persistent and episodic hypotension and diminished nocturnal SBPdip may predispose these individuals to cognitive deficits, and we have documented significantly reduced memory and marginally reduced attention and processing speed in hypotensive individuals with SCI compared to a normotensive SCI cohort.72
Figure 1.
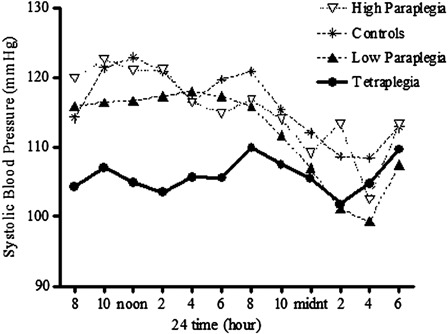
SBP over a 24-hour observation period in subjects with tetraplegia (C3–C8: closed circles), high paraplegia (T2–T5: open triangles), low paraplegia (T7–T12: closed triangles), and non-SCI controls (asterisks). The interaction effect was significant; P < 0.01, post hoc analysis revealed that SBP was significantly lower in the tetraplegic group compared to the non-SCI and high-paraplegic groups (P < 0.05). Recreated from Ref. 70.
Figure 2.
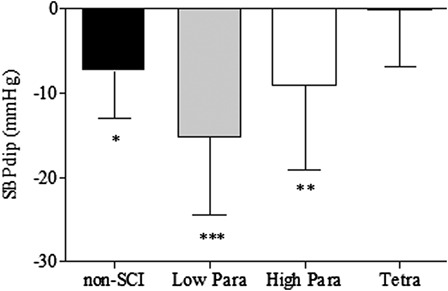
The nocturnal dip in BP (SBPdip) among the non-SCI, low-paraplegic, high-paraplegic, and tetraplegic groups; *P < 0.05; **P < 0.01; ***P < 0.001 versus tetraplegic group. Reported in Ref. 70.
There is evidence to support the notion that chronic and episodic cerebral hypoperfusion, stemming from systemic hypotension, results in cognitive deficits.17 Supporting data suggest that elevation of BP with midodrine (0.4 mg/kg) increased resting cerebral blood flow (CBF) and improved reaction times in hypotensive non-SCI individuals, and the degree of increase in CBF was associated with improvement in cognitive performance.14 We have preliminary evidence of reduced seated BP at rest and during cognitive testing in individuals with tetraplegia compared to non-SCI controls and subjects with paraplegia (Fig. 3).73 Although the hypotension did not relate to CBF responses to cognitive testing, on average, both the paraplegic and tetraplegic groups demonstrated a reduction in CBF during cognitive testing compared to a mean increase in the non-SCI controls (Fig. 4).73 Owing to the relatively small sample size, these group differences did not attain statistical significance; however, 8 of the 13 individuals with SCI tested displayed an inappropriate fall in CBF during cognitive testing compared to an increase in CBF during testing in 12 of the 16 non-SCI control subjects (Fig. 5).73
Figure 3.
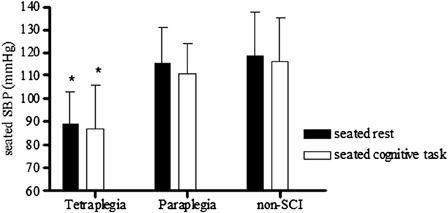
Seated SBP among the non-SCI, paraplegia, and tetraplegia groups. *P < 0.01 versus similar condition in paraplegic and non-SCI groups. Reported in Ref. 73.
Figure 4.
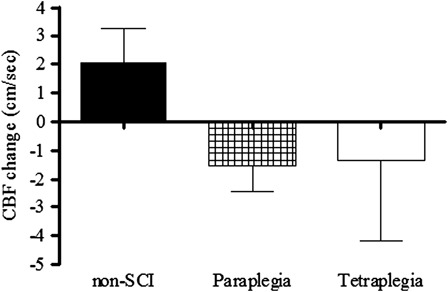
Mean change in CBF from seated rest to seated cognitive testing. Recreated from Ref. 73.
Figure 5.
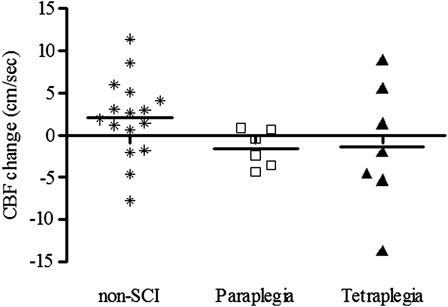
Individual change scores in CBF (cm/second) from seated rest to seated cognitive testing among the non-SCI, paraplegia, and tetraplegia groups. Reported in Ref. 73.
To determine the possible cerebral vascular etiology responsible for the paradoxical CBF response to cognitive testing in persons with SCI, we examined cerebral vascular resistance index (CVRi: an estimate of the cerebral micro-vascular response to testing). CVRi is the ratio between Mean Arterial Pressure (MAP) and CBF and, as expected, most of the non-SCI subjects demonstrated a reduction in CVRi, suggesting vasodilatation of the downstream cerebral vasculature during cognitive testing. CVRi was not reduced during testing in the paraplegic or tetraplegic groups, and the majority of subjects with SCI had an inappropriate increase in CVRi during cognitive testing (Fig. 6).73 Furthermore, the relationship between change in CVRi and cognitive score on the Stroop color task (a test of processing speed) was significant in the non-SCI group, but no association was apparent in the SCI groups (Fig. 7).73 Although preliminary, these observations suggest adversely altered systemic and cerebral hemodynamic function, which are not appropriately modified during cognitive testing.
Figure 6.
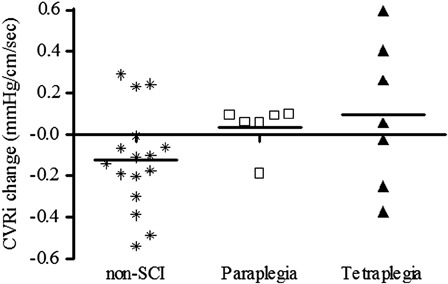
Individual change scores in CVRi (cm/second) from seated rest to seated cognitive testing among the non-SCI, paraplegic, and tetraplegic groups. Recreated from Ref. 73.
Figure 7.
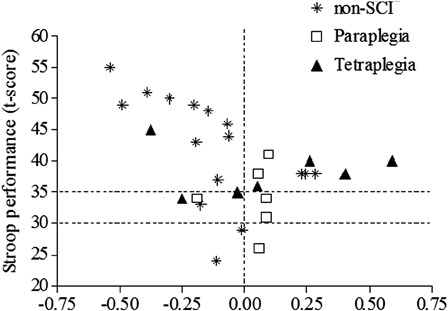
The relationship between change in CVRi and t-score on the Stroop color task was significant in the non-SCI controls subjects (r = 0.579; P = 0.0187), but there was no significant association in either the paraplegic or tetraplegic groups. Recreated from Ref. 73.
The relationship between systemic hemodynamics and cerebral circulatory responses to cognitive testing may be altered in individuals with SCI, regardless of the level of injury, and further exploration into the possible mechanisms involved and the impact on cognitive performance should be a priority. We believe that chronic and/or episodic hypotension may adversely alter cerebral vascular hemodynamics and cognitive performance in persons with tetraplegia. However, the pathophysiological CBF responses to cognitive testing in persons with paraplegia cannot be explained by hypotension and may relate to increased resting HR,35,56,57 arterial stiffening,58,59 and vasculature dysfunction.74,75
The relationship between increased resting HR and arterial stiffening to cognitive performance
Reports in the general literature suggest that persistent elevation in HR augments systolic blood flow pulsatility and may lead to endothelial dysfunction76 and increased arterial stiffness,77 reflecting autonomic imbalance.78,79 Cross-sectional evidence supports an association between large vessel stiffening and HR, and a longitudinal observation found that increased resting HR was a powerful predictor of the accelerated progression of pulse wave velocity (a surrogate measure of arterial stiffening).77,80 We have preliminary evidence of significantly elevated resting HR over a typical 24-hour period in individuals with high and low paraplegia compared to non-SCI controls (Fig. 8).70 Daytime HR was significantly elevated in both paraplegic groups compared to the controls, but of significant importance, nighttime HR remained elevated in the low paraplegic group compared to the tetraplegic and control groups (P < 0.01). Persistently elevated HR in persons with paraplegia may contribute to the increased arterial stiffness reported in these individuals,58,59 thus leading to the cognitive deficits observed.
Figure 8.
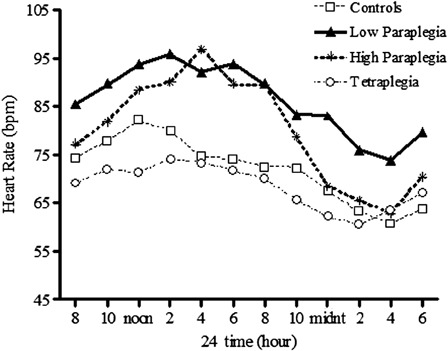
HR over a 24-hour observation period in subjects with tetraplegia (C3–C8: open circles), high paraplegia (T2–T5: asterisks), low paraplegia (T7–T12: closed triangles), and non-SCI controls (open squares). The interaction effect was significant; P < 0.0001, post hoc analysis revealed that HR was significantly higher in the high- and low-paraplegic groups compared to the non-SCI (P < 0.05) and tetraplegic group (P < 0.01). Recreated from Ref. 70.
The notion that increased arterial stiffness and pulse pressure contribute to cognitive decline with aging in the absence of dementia has been reported in the non-SCI literature.22,23,25,27,81 Several large population-based studies document an association between increased arterial stiffness (AS) and impaired cognitive function in elderly patients with known vascular dementia,82 mild cognitive impairment,83 and memory loss,24 as well as in otherwise healthy subjects.25 Specifically, cross-sectional observations documenting the association between increased arterial stiffness and cognitive performance report significant deficits in memory,24,25 psychomotor speed,22,25 global cognitive function (mini mental state examination score),23,26 and increased prevalence and severity of white matter hyperintensities (WMH: suggestive of ischemic changes).24 In a longitudinal study, over a 2-year span in individuals without dementia who had no history of cerebral vascular or renal disease, higher baseline pulse pressure, and pulse wave velocity was associated with an accelerated rate of decline in verbal and non-verbal memory, attention, and concentration capacities.27 A significant relationship between cognitive status and arterial stiffness was reported after co-varying for age, gender, systolic BP, education, cardiovascular disease, and antihypertensive therapy in a large cohort of elderly subjects (mean age 78 ± 8 years) who were categorically divided by their cognitive status (normal, mild cognitive impairment, Alzheimer's disease, and vascular dementia).23 Although the majority of work has been reported in populations with specific co-morbid conditions, such as hypertension or diabetes mellitus, there is evidence that cognitive dysfunction may be present in overtly asymptomatic populations due to sub-clinical vascular remodeling. In addition to changes in vascular morphology with chronic diseases and conditions, arterial stiffness increases with aging22,26 and sub-clinical changes in vascular morphology has been implicated in the age-associated change in cognitive function.22 Thus, persistently elevated HR documented in individuals with paraplegia may predispose these individuals to premature arterial stiffness78,79,84 and accelerated age-associated declines in cognitive function.22,23,25,27 We propose that although individuals with paraplegia do not present with clinically overt cardiovascular pathophysiology, insidious sub-clinical changes in vascular morphology resulting from persistently elevated resting HR may predispose these individuals to cognitive deficits.
The relationship of AD to cognition
Although most individuals with lesions above T6 have chronically low to normal BP, many of these individuals also struggle with episodes of severe increase in BP due to AD.36,53–55 Recent evidence in an animal model of SCI, in which AD was induced via repetitive colorectal distension, suggests structural changes in the peripheral vasculature that may have significant implication to cardiovascular function.85 In addition, wide fluctuations in BP due to AD may be detrimental to the cerebral microvasculature, because an association between rapid changes in BP and cerebral hypoperfusion during dialysis was reported in individuals with stable end-stage renal failure,86 and it is believed that silent cerebral infarctions ensue.87 More recently, Wu et al.88 reported significantly increased risk of stroke in individuals with SCI (5.96 per 1000) compared to a age-, gender-, and propensity-score-matched controls (2.04 per 1000). Although comparison of several risk factors for stroke (smoking, obesity, alcohol consumption, carotid artery stenosis, and metabolic syndrome) and the anatomic level of injury were not reported, the incidence of ischemic stroke was higher than hemorrhagic stroke (3.42 [95% confidence interval (CI): 1.89–6.54], P < 0.001) in the subjects with SCI, which implicates long-term cardiovascular and cerebral vascular dysfunction as potential contributors.88 Despite the lack of acute neurological symptoms, silent ischemic infarctions predict long-term adverse outcomes, such as dementia and steeper decline in cognitive function with aging.87,89 Thus, it is conceivable that persons with SCI who experience frequent bouts of AD in the presence of chronic hypotension will have significant evidence of white matter ischemic changes on neuro-imaging and accelerated decrements in cognitive function with aging. A single preliminary report on the potential association between AD and cognitive function in persons with SCI suggests that the intensity of AD, as reported using a customized questionnaire, was inversely associated with performance on tests of executive function.90 Although substantiating evidence is lacking, the potential association between AD and cognitive impairment seems likely and should be an area of high priority in future research endeavors.
Final considerations
Several factors, other than cardiovascular and cerebral vascular dysregulation, may also contribute to the cognitive deficits reported in persons with SCI. Some of these factors include an increased incidence of disorders of carbohydrate and lipid metabolism, diabetes, and sleep apnea. Disorders of carbohydrate and lipid metabolism are common findings in the SCI population,91 and our group has reported that individuals with SCI develop these disorders at relatively younger ages than controls.92 It is well appreciated that dyslipidemia, glucose intolerance, and diabetes mellitus predispose to micro- and macro-vascular disease, which adversely affect tissue perfusion. These adverse affects may result in persistent or episodic cerebral hypoperfusion, thus contributing to the cognitive deficits reported in persons with SCI. The prevalence of obstructive sleep apnea (OSA) is increased in persons with SCI,93 particularly those with tetraplegia94 compared to age-matched non-SCI controls. As cognitive deficits are associated with OSA in the general population,95–97 individuals with SCI with OSA may be at increased risk of cognitive impairments. Given the increased prevalence of metabolic disorders and OSA in the SCI population, studies addressing the possible association between these disorders and cognitive impairments should be considered a high priority.
Summary and conclusions
Cognitive deficits in the areas of memory, attention and processing speed, and executive function are prominent in individuals with SCI, regardless of the level of injury. It has generally been assumed that these cognitive deficits are, in large measure, accounted for by concomitant TBI or pre-morbid conditions. However, the growing body of evidence to support associations between cognitive deficits and adverse cardiovascular and cerebral vascular changes in the non-SCI literature may be extrapolated to the SCI population. Investigation into the pathophysiological conditions that impair cognitive function in persons with SCI, and the identification of sub-groups at risk within the population, will permit appropriate treatment interventions, as well as the development of novel therapies to improve cardiovascular, cerebral vascular, and cognitive function. These strategies may prevent and/or ameliorate further deterioration in cognitive function as these individuals age. Such a proactive approach in persons with SCI should result in improved employability, self-reliance, independence, and quality of life.
Acknowledgements
This research was supported by the Veterans Affairs Rehabilitation. Research and Development Service (Grants: A6161W, B3203R, and B4162C).
References
- 1.Davidoff G, Morris J, Roth E, Bleiberg J. Cognitive dysfunction and mild closed head injury in traumatic spinal cord injury. Arch Phys Med Rehabil 1985;66(8):489–91 [PubMed] [Google Scholar]
- 2.Davidoff G, Roth E, Thomas P, Doljanac R, Dijkers M, Berent S, et al. Depression and neuropsychological test performance in acute spinal cord injury patients: lack of correlation. Arch Clin Neuropsychol 1990;5(1):77–88 [PubMed] [Google Scholar]
- 3.Davidoff GN, Roth EJ, Haughton JS, Ardner MS. Cognitive dysfunction in spinal cord injury patients: sensitivity of the Functional Independence Measure subscales vs neuropsychologic assessment. Arch Phys Med Rehabil 1990;71(5):326–9 [PubMed] [Google Scholar]
- 4.Roth E, Davidoff G, Thomas P, Doljanac R, Dijkers M, Berent S, et al. A controlled study of neuropsychological deficits in acute spinal cord injury patients. Paraplegia 1989;27(6):480–9 [DOI] [PubMed] [Google Scholar]
- 5.Wilmot CB, Cope DN, Hall KM, Acker M. Occult head injury: its incidence in spinal cord injury. Arch Phys Med Rehabil 1985;66(4):227–31 [DOI] [PubMed] [Google Scholar]
- 6.Dowler RN, O'Brien SA, Haaland KY, Harrington DL, Feel F, Fiedler K. Neuropsychological functioning following a spinal cord injury. Appl Neuropsychol 1995;2(3–4):124–9 [DOI] [PubMed] [Google Scholar]
- 7.Davidoff GN, Roth EJ, Richards JS. Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch Phys Med Rehabil 1992;73(3):275–84 [PubMed] [Google Scholar]
- 8.Richards JS, Brown L, Hagglund K, Bua G, Reeder K. Spinal cord injury and concomitant traumatic brain injury. Results of a longitudinal investigation. Am J Phys Med Rehabil 1988;67(5):211–6 [DOI] [PubMed] [Google Scholar]
- 9.Davidoff G, Thomas P, Johnson M, Berent S, Dijkers M, Doljanac R. Closed head injury in acute traumatic spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil 1988;69(10):869–72 [PubMed] [Google Scholar]
- 10.Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc 1997;3(5):464–72 [PubMed] [Google Scholar]
- 11.Duschek S, Muckenthaler M, Werner N, del Paso GA. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol 2009;81(2):110–7 [DOI] [PubMed] [Google Scholar]
- 12.Duschek S, Dietel A, Schandry R, Reyes Del Paso GA. Increased baroreflex sensitivity and reduced cardiovascular reactivity in individuals with chronic low blood pressure. Hypertens Res 2008;31(10):1873–8 [DOI] [PubMed] [Google Scholar]
- 13.Duschek S, Matthias E, Schandry R. Essential hypotension is accompanied by deficits in attention and working memory. Behav Med 2005;30(4):149–58 [DOI] [PubMed] [Google Scholar]
- 14.Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res 2007;17(2):69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duschek S, Weisz N, Schandry R. Reduced cognitive performance and prolonged reaction time accompany moderate hypotension. Clin Auton Res 2003;13(6):427–32 [DOI] [PubMed] [Google Scholar]
- 16.Duschek S, Hadjamu M, Schandry R. Enhancement of cerebral blood flow and cognitive performance following pharmacological blood pressure elevation in chronic hypotension. Psychophysiology 2007;44(1):145–53 [DOI] [PubMed] [Google Scholar]
- 17.Duschek S, Schandry R. Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology 2004;41(6):905–13 [DOI] [PubMed] [Google Scholar]
- 18.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347(9009):1141–5 [DOI] [PubMed] [Google Scholar]
- 19.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001;322(7300):1447–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flicker L. Cardiovascular risk factors, cerebrovascular disease burden, and healthy brain aging. Clin Geriatr Med 2010;26(1):17–27 [DOI] [PubMed] [Google Scholar]
- 21.Obisesan TO, Obisesan OA, Martins S, Alamgir L, Bond V, Maxwell C, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc 2008;56(3):501–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol Aging 2009;24(1):154–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke 2005;36(10):2193–7 [DOI] [PubMed] [Google Scholar]
- 24.Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke 2009;40(4):1229–36 [DOI] [PubMed] [Google Scholar]
- 25.Pase MP, Pipingas A, Kras M, Nolidin K, Gibbs AL, Wesnes KA, et al. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens 2010;28(8):1724–9 [DOI] [PubMed] [Google Scholar]
- 26.Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, et al. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens 2009;22(5):525–30 [DOI] [PubMed] [Google Scholar]
- 27.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008;51(1):99–104 [DOI] [PubMed] [Google Scholar]
- 28.Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem 2003;79(2):194–8 [DOI] [PubMed] [Google Scholar]
- 29.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature 1994;371(6499):702–4 [DOI] [PubMed] [Google Scholar]
- 30.Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behav Neurosci 2004;118(2):420–8 [DOI] [PubMed] [Google Scholar]
- 31.McGaugh JL. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annu Rev Neurosci 1989;12:255–87 [DOI] [PubMed] [Google Scholar]
- 32.Mathias CJ, Christensen NJ, Corbett JL, Frankel HL, Goodwin TJ, Peart WS. Plasma catecholamines, plasma renin activity and plasma aldosterone in tetraplegic man, horizontal and tilted. Clin Sci Mol Med 1975;49(4):291–9 [DOI] [PubMed] [Google Scholar]
- 33.Mathias CJ, Christensen NJ, Frankel HL, Peart WS. Renin release during head-up tilt occurs independently of sympathetic nervous activity in tetraplegic man. Clin Sci (Lond) 1980;59(4):251–6 [DOI] [PubMed] [Google Scholar]
- 34.Wecht JM, Weir JP, Bauman WA. Blunted heart rate response to vagal withdrawal in persons with tetraplegia. Clin Auton Res 2006;16(6):378–83 [DOI] [PubMed] [Google Scholar]
- 35.Schmid A, Huonker M, Barturen JM, Stahl F, Schmidt-Trucksass A, Konig D, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol 1998;85(2):635–41 [DOI] [PubMed] [Google Scholar]
- 36.Karlsson AK, Friberg P, Lonnroth P, Sullivan L, Elam M. Regional sympathetic function in high spinal cord injury during mental stress and autonomic dysreflexia. Brain 1998;121(Pt 9):1711–9 [DOI] [PubMed] [Google Scholar]
- 37.Richards JE. Infant visual sustained attention and respiratory sinus arrhythmia. Child Dev 1987;58(2):488–96 [PubMed] [Google Scholar]
- 38.Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology 1994;31(1):17–22 [DOI] [PubMed] [Google Scholar]
- 39.Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol 2003;48(3):263–74 [DOI] [PubMed] [Google Scholar]
- 40.Grimm DR, De Meersman RE, Almenoff PL, Spungen AM, Bauman WA. Sympathovagal balance of the heart in subjects with spinal cord injury. Am J Physiol 1997;272(2 Pt 2):H835–42 [DOI] [PubMed] [Google Scholar]
- 41.Grimm DR, DeMeersman RE, Garofano RP, Spungen AM, Bauman WA. Effect of provocative maneuvers on heart rate variability in subjects with quadriplegia. Am J Physiol 1995;268(6 Pt 2):H2239–45 [DOI] [PubMed] [Google Scholar]
- 42.Wecht JM, Weir JP, DeMeersman RE, Schilero GJ, Handrakis JP, LaFountaine MF, et al. Cold face test in persons with spinal cord injury: age versus inactivity. Clin Auton Res 2009;19(4):221–9 [DOI] [PubMed] [Google Scholar]
- 43.Del Paso GA, Gonzalez MI, Hernandez JA, Duschek S, Gutierrez N. Tonic blood pressure modulates the relationship between baroreceptor cardiac reflex sensitivity and cognitive performance. Psychophysiology 2009;46(5):932–8 [DOI] [PubMed] [Google Scholar]
- 44.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 2006;23(12):1713–25 [DOI] [PubMed] [Google Scholar]
- 45.Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 2006;44(6):341–51 [DOI] [PubMed] [Google Scholar]
- 46.Inoue K, Ogata H, Hayano J, Miyake S, Kamada T, Kuno M, et al. Assessment of autonomic function in traumatic quadriplegic and paraplegic patients by spectral analysis of heart rate variability. J Auton Nerv Syst 1995;54(3):225–34 [DOI] [PubMed] [Google Scholar]
- 47.Krassioukov AV, Karlsson AK, Wecht JM, Wuermser LA, Mathias CJ, Marino RJ. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J Rehabil Res Dev 2007;44(1):103–12 [DOI] [PubMed] [Google Scholar]
- 48.Alexander MS, Biering-Sorensen F, Bodner D, Brackett NL, Cardenas D, Charlifue S, et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord 2009;47(1):36–43 [DOI] [PubMed] [Google Scholar]
- 49.Christ JE. An analysis of circadian rhythmicity of heart rate in tetraplegic human subjects. Paraplegia 1979;17(2):251–8 [DOI] [PubMed] [Google Scholar]
- 50.Goswami R, Krishan K, Suryaprakash B, Vaidyanathan S, Rao K, Rao MS, et al. Circadian desynchronization in pulse rate, systolic and diastolic blood pressure, rectal temperature and urine output in traumatic tetraplegics. Indian J Physiol Pharmacol 1985;29(4):199–206 [PubMed] [Google Scholar]
- 51.Krum H, Louis WJ, Brown DJ, Jackman GP, Howes LG. Diurnal blood pressure variation in quadriplegic chronic spinal cord injury patients. Clin Sci (Lond) 1991;80(3):271–6 [DOI] [PubMed] [Google Scholar]
- 52.Munakata M, Kameyama J, Kanazawa M, Nunokawa T, Moriai N, Yoshinaga K. Circadian blood pressure rhythm in patients with higher and lower spinal cord injury: simultaneous evaluation of autonomic nervous activity and physical activity. J Hypertens 1997;15(12 Pt 2):1745–9 [DOI] [PubMed] [Google Scholar]
- 53.Dolinak D, Balraj E. Autonomic dysreflexia and sudden death in people with traumatic spinal cord injury. Am J Forensic Med Pathol 2007;28(2):95–8 [DOI] [PubMed] [Google Scholar]
- 54.Krassioukov A, Warburton DE, Teasell R, Eng JJ. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 2009;90(4):682–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Somani BK. Autonomic dysreflexia: a medical emergency with spinal cord injury. Int J Clin Pract 2009;63(3):350–2 [DOI] [PubMed] [Google Scholar]
- 56.Houtman S, Oeseburg B, Hughson RL, Hopman MT. Sympathetic nervous system activity and cardiovascular homeostatis during head-up tilt in patients with spinal cord injuries. Clin Auton Res 2000;10(4):207–12 [DOI] [PubMed] [Google Scholar]
- 57.Jacobs PL, Mahoney ET, Robbins A, Nash M. Hypokinetic circulation in persons with paraplegia. Med Sci Sports Exerc 2002;34(9):1401–7 [DOI] [PubMed] [Google Scholar]
- 58.Wecht JM, Weir JP, DeMeersman RE, Spungen AM, Bauman WA. Arterial stiffness in persons with paraplegia. J Spinal Cord Med 2004;27(3):255–9 [DOI] [PubMed] [Google Scholar]
- 59.Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med 2009;32(1):72–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weisz N, Schandry R, Jacobs AM, Mialet JP, Duschek S. Early contingent negative variation of the EEG and attentional flexibility are reduced in hypotension. Int J Psychophysiol 2002;45(3):253–60 [DOI] [PubMed] [Google Scholar]
- 61.Costa M, Stegagno L, Schandry R, Bitti PE. Contingent negative variation and cognitive performance in hypotension. Psychophysiology 1998;35(6):737–44 [PubMed] [Google Scholar]
- 62.Barrett-Connor E, Palinkas LA. Low blood pressure and depression in older men: a population based study. BMJ 1994;308(6926):446–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pilgrim JA, Stansfeld S, Marmot M. Low blood pressure, low mood?. BMJ 1992;304(6819):75–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wessely S, Nickson J, Cox B. Symptoms of low blood pressure: a population study. BMJ 1990;301(6748):362–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosengren A, Tibblin G, Wilhelmsen L. Low systolic blood pressure and self perceived wellbeing in middle aged men. BMJ 1993;306(6872):243–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamoto Y, Akiguchi I, Oiwa K, Hayashi M, Ohara T, Ozasa K. The relationship between 24-hour blood pressure readings, subcortical ischemic lesions and vascular dementia. Cerebrovasc Dis 2005;19(5):302–8 [DOI] [PubMed] [Google Scholar]
- 67.Blackmer J. Orthostatic hypotension in spinal cord injured patients. J Spinal Cord Med 1997;20(2):212–7 [DOI] [PubMed] [Google Scholar]
- 68.Illman A, Stiller K, Williams M. The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord 2000;38(12):741–7 [DOI] [PubMed] [Google Scholar]
- 69.Lopes P, Figoni S. Current literature on orthostatic hypotension and training in SCI patients. Am Correct Ther J 1982;36(2):56–9 [PubMed] [Google Scholar]
- 70.Rosado-Rivera D, Radulovic M, Handrakis JP, Cirnigliaro CM, Jensen AM, Kirshblum S, et al. Comparison of 24-hour cardiovascular and autonomic function in paraplegia, tetraplegia, and control groups: implications for cardiovascular risk. J Spinal Cord Med 2011;34(4):395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwartz GL, Bailey KR, Mosley T, Knopman DS, Jack CR, Jr, Canzanello VJ, et al. Association of ambulatory blood pressure with ischemic brain injury. Hypertension 2007;49(6):1228–34 [DOI] [PubMed] [Google Scholar]
- 72.Jegede AB, Rosado-Rivera D, Bauman WA, Cardozo CP, Sano M, Moyer JM, et al. Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res 2010;20(1):3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wecht JM, Rosado-Rivera D, Jegede A, Cirnigliaro CM, Jensen MA, Kirshblum S, et al. Systemic and cerebral hemodynamics during cognitive testing. Clin Auton Res 2012;22(1):25–33 [DOI] [PubMed] [Google Scholar]
- 74.Popa C, Popa F, Grigorean VT, Onose G, Sandu AM, Popescu M, et al. Vascular dysfunctions following spinal cord injury. J Med Life 2010;3(3):275–85 [PMC free article] [PubMed] [Google Scholar]
- 75.Olive JL, Dudley GA, McCully KK. Vascular remodeling after spinal cord injury. Med Sci Sports Exerc 2003;35(6):901–7 [DOI] [PubMed] [Google Scholar]
- 76.Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science 1984;226(4671):180–2 [DOI] [PubMed] [Google Scholar]
- 77.Sa Cunha R, Pannier B, Benetos A, Siche JP, London GM, Mallion JM, et al. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J Hypertens 1997;15(12 Pt 1):1423–30 [DOI] [PubMed] [Google Scholar]
- 78.Palatini P, Parati G. Persistently elevated heart rate accelerates the progression of arterial stiffness. J Hypertens 2010;28(4):653–6 [DOI] [PubMed] [Google Scholar]
- 79.Chen W, Srinivasan SR, Berenson GS. Differential impact of heart rate on arterial wall stiffness and thickness in young adults: The Bogalusa Heart Study. J Am Soc Hypertens 2008;2(3):152–7 [DOI] [PubMed] [Google Scholar]
- 80.Albaladejo P, Asmar R, Safar M, Benetos A. Association between 24-hour ambulatory heart rate and arterial stiffness. J Hum Hypertens 2000;14(2):137–41 [DOI] [PubMed] [Google Scholar]
- 81.Benetos A, Buatois S, Salvi P, Marino F, Toulza O, Dubail D, et al. Blood pressure and pulse wave velocity values in the institutionalized elderly aged 80 and over: baseline of the PARTAGE study. J Hypertens 2010;28(1):41–50 [DOI] [PubMed] [Google Scholar]
- 82.Mizushima Y, Oobasawa H, Yoshida S, Irie H, Urata T, Shimoda H. Pulse wave velocity in persons with vascular dementia. J Am Geriatr Soc 2003;51(9):1329–30 [DOI] [PubMed] [Google Scholar]
- 83.Nagai K, Akishita M, Machida A, Sonohara K, Ohni M, Toba K. Correlation between pulse wave velocity and cognitive function in nonvascular dementia. J Am Geriatr Soc 2004;52(6):1037–8 [DOI] [PubMed] [Google Scholar]
- 84.Park BJ, Lee HR, Shim JY, Lee JH, Jung DH, Lee YJ. Association between resting heart rate and arterial stiffness in Korean adults. Arch Cardiovasc Dis 2010;103(4):246–52 [DOI] [PubMed] [Google Scholar]
- 85.Moniri N, Ramer MS, Laher I, Krassioukov A. Vascular changes in spinal cord injured animals with repetative episodes of autonomic dysreflexia. Top Spinal Cord Inj Rehabil 2012;18Suppl. 1:22023459106 [Google Scholar]
- 86.Stefanidis I, Bach R, Mertens PR, Liakopoulos V, Liapi G, Mann H, et al. Influence of hemodialysis on the mean blood flow velocity in the middle cerebral artery. Clin Nephrol 2005;64(2):129–37 [DOI] [PubMed] [Google Scholar]
- 87.Seliger SL, Sarnak MJ. Subclinical vascular disease of the brain in dialysis patients. Am J Kidney Dis 2007;50(1):8–10 [DOI] [PubMed] [Google Scholar]
- 88.Wu JC, Chen YC, Liu L, Chen TJ, Huang WC, Cheng H, et al. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology 2012;78(14):1051–7 [DOI] [PubMed] [Google Scholar]
- 89.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348(13):1215–22 [DOI] [PubMed] [Google Scholar]
- 90.Krassioukov A, Gao F, Li J, Pak M, Chanm C. Cognitive function among spinal cord injured individuals with autonomic dysreflexia: a pilot study. Top Spinal Cord Inj Rehabil 2012;18Suppl. 1:206 [Google Scholar]
- 91.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77 [DOI] [PubMed] [Google Scholar]
- 92.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–56 [DOI] [PubMed] [Google Scholar]
- 93.Short DJ, Stradling JR, Williams SJ. Prevalence of sleep apnoea in patients over 40 years of age with spinal cord lesions. J Neurol Neurosurg Psychiatry 1992;55(11):1032–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burns SP, Little JW, Hussey JD, Lyman P, Lakshminarayanan S. Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil 2000;81(10):1334–9 [DOI] [PubMed] [Google Scholar]
- 95.Kiratli PO, Demir AU, Volkan-Salanci B, Demir B, Sahin A. Cerebral blood flow and cognitive function in obstructive sleep apnea syndrome. Hell J Nucl Med 2010;13(2):138–43 [PubMed] [Google Scholar]
- 96.Alchanatis M, Zias N, Deligiorgis N, Amfilochiou A, Dionellis G, Orphanidou D. Sleep apnea-related cognitive deficits and intelligence: an implication of cognitive reserve theory. J Sleep Res 2005;14(1):69–75 [DOI] [PubMed] [Google Scholar]
- 97.Decary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep 2000;23(3):369–81 [PubMed] [Google Scholar]


