Abstract
Objective
To demonstrate the effect of a passive abdominal functional electrical stimulation (AFES) training program on unassisted respiratory measures in tetraplegia.
Design
Longitudinal feasibility study.
Setting
National spinal injuries unit in a university teaching hospital.
Participants
Twelve patients with tetraplegic spinal cord injury, who could breathe independently, with reduced vital capacity and no visible abdominal movement.
Intervention
Three weeks of abdominal muscle conditioning using transcutaneous AFES.
Main outcome measures
Forced vital capacity (FVC), forced exhaled volume in 1 second (FEV1), peak expiratory flow rate (PEF), and maximum exhaled pressure (MEP).
Results
Mean (SD) FVC increased by 0.36 l (0.23) during training (P = 0.0027). Mean (SD) FEV1 and PEF tended to increase by 0.18 l (0.16) and 0.39 l/seconds (0.35), respectively, but this was not significant. No significant change was found in the outcome measures during a 1-week pre-training control phase and during a 3-week post-training phase.
Conclusions
The increase in FVC over the training period and the absence of change before or after training suggest that passive abdominal FES training can be used for respiratory rehabilitation in tetraplegia.
Keywords: Spinal cord injuries, Tetraplegia, Functional electrical stimulation, Rehabilitation, Respiratory, Paralysis, Ventilatory capacity
Introduction
A spinal cord injury (SCI) to the cervical region results in tetraplegia. Depending on the level and severity of injury, a degree of paralysis and a loss of sensation will affect all four limbs and the trunk. Included in this paralysis are the main breathing muscles: the diaphragm, intercostal muscles and abdominal muscles. Generally, in motor complete injuries below C3, partial diaphragm function will be maintained, allowing respiration without the use of artificial ventilation. However, paralysis of the intercostal and abdominal muscles will result in decreased ventilatory capacity, which is a major factor in rehospitalization for respiratory complications.1
Common measures of ventilatory capacity include forced vital capacity (FVC), forced exhaled volume in 1 second (FEV1), peak expiratory flow rate (PEF), and maximum expiratory pressure (MEP). These have been shown to be substantially reduced in individuals with tetraplegia caused by SCI compared to normal values for an able-bodied population.2 Furthermore, an improvement in these indicators has been shown to positively correlate with a reduction in respiratory complications.3
Previous studies have shown the above measures can be improved in tetraplegia using respiratory muscle training incorporating transcutaneous functional electrical stimulation (FES).3,4 Zupan et al.4 combined breathing exercises with abdominal FES (AFES), using an active training program that required patient interaction. Cheng et al.3 applied a repeating pattern of FES to the pectoral and abdominal muscles, using a passive training program that did not require patient interaction. It is unknown whether a passive training program incorporating only stimulation of the abdominal muscles could also be of benefit to these patients. However, such a program could be very practical as passive training allows patients to complete other activities at the same time and FES applied only to the abdominal muscles is simple and quick.
In this study, the feasibility of using a passive AFES training program for respiratory rehabilitation is investigated. It was hypothesized that the training program would promote an increase in unassisted FVC, FEV1, PEF, and MEP.
Methods
The study involved 16 patients with tetraplegia, 12 of whom completed the study, who could breathe independently, but had no useful abdominal movement and reduced vital capacity. The subjects recruited were current inpatients and outpatients of a university teaching hospital. The local research ethics committee approved all procedures and all subjects gave written informed consent. Throughout the study inpatients received their regular rehabilitation that did not include any formal respiratory training sessions.
Study protocol
A longitudinal design was employed for this study. An outline of the protocol is shown in Fig. 1. The study consisted of three phases: a control phase (week 1), a training phase (weeks 2–4), and a follow-up phase (weeks 5–7). Each subject was asked to participate in six assessment sessions (A1–A6), which took place at the hospital and during which respiratory function was measured. Starting at the beginning of week 2, each subject underwent 3 weeks of abdominal FES muscle training. Four training sessions were prescribed per week that took place in between assessment sessions. Training sessions incremented from 20 minutes per day in the first week to 60 minutes per day in the third week. FES was administered during the training sessions by a researcher for inpatients of the hospital and was left to the responsibility of the patient if they were living at home. In both cases, a training diary was maintained. For both the training and the assessment sessions, abdominal binders were removed if present.
Figure 1.
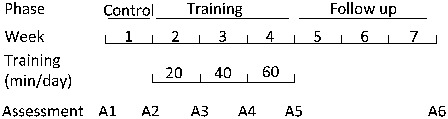
Outline of the study design.
FVC and MEP assessments
MEP was assessed using a mouth pressure meter (MPM, Micro Medical Ltd, Chatham, UK). To perform the test, the subject was instructed to inhale to total lung capacity (TLC) before exhaling as forcefully as possible for at least 2 seconds. FVC, FEV1, FEV1/FVC, and PEF were determined from a FVC test using a portable spirometer (Micro-Loop) connected to a low dead space full face mask (Hans Rudolph, Hans Rudolph Inc., Kansas, Shawnee, USA). When performing the FVC test, the subject was asked to inhale to TLC before exhaling as quickly and completely as possible. The MEP test preceded the FVC test and both tests were performed with the subjects sitting. Subjects were asked to perform each test five times or until three valid attempts had been collected. Attempts were counted as valid when measurements were within 20% of each other. The same researcher conducted all assessments and the subjects were encouraged to use maximum effort throughout each test.
FVC and MEP assessments
Transcutaneous abdominal FES was applied using a stimulator (RehaStim, HASOMED GmbH, Magdeburg, Germany) that delivered bi-phasic electrical stimulation at 30 Hz over the rectus abdominis and the external oblique muscle groups. Electrodes (PALS Platinum, Nidd Valley Medical Ltd, North Yorkshire, UK) were placed as shown in Fig. 2. The initial stimulation intensity for each week of training was set at the end of the preceding assessment session (e.g. A2 for week 1 of training) using the following procedure. At a constant pulse-width of 50 microseconds the stimulation current was adjusted for each channel individually so that a strong, even contraction of the abdomen was observed. Contraction was assessed visually using the change in girth of the abdomen and the tone of the abdominal wall as cues. Following this, the stimulation pulse-width was increased during MEP attempts assisted with AFES until no further gains in MEP were achieved. Throughout a training session, stimulation pulse-width was increased to maintain the same level of visual muscle contraction. During training sessions, an onboard program on the stimulator provided periodic stimulation, which was set so that stimulation was approximately synchronized with the subject's exhalation.
Figure 2.
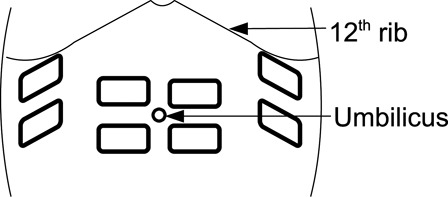
Electrode placement.
Analysis and outcome measures
The mean MEP, FVC, FEV1, FEV1/FVC, and PEF from the best three attempts collected were used for further analysis. The data from each assessment session were tested for normality using the Shapiro–Wilks test. Based on the outcome of this test, absolute data were transformed using the natural logarithm for statistical analysis. Missing data points (see section Abdominal FES) were replaced by the value from the previous assessment (last value carried forward). Repeated measures analysis of variance (ANOVA) with the Greenhouse Geisser correction was used to test for longitudinal changes in the outcome measures through the study. In the case of significance, post hoc multiple comparisons were performed using the Tukey–Kramer honestly significant difference procedure. For all tests a P value of <0.05 was regarded as statistically significant.
Results
Subjects
The details of the subjects who completed the study are given in Table 1.
Table 1.
Details of the subjects who completed the study. Outpatients, who completed their training at home, are marked with an asterisk (*)
| ID | Sex | Age (years) | Height (cm) | Weight (kg) | Level of injury | AIS | Post-injury (months) | Smoker |
|---|---|---|---|---|---|---|---|---|
| S1 | M | 18 | 183 | 90 | C4/5 | A | 5 | N |
| S2 | M | 31 | 180 | 89 | C5/6 | C | 2 | N |
| S3 | M | 73 | 180 | 91 | C4 | A | 5 | N |
| S4* | M | 24 | 168 | – | C4 | A | 94 | N |
| S5 | M | 54 | 187 | 70 | C6 | C | 9 | N |
| S6 | M | 53 | 178 | 76 | C3 | C | 4 | N |
| S7* | M | 18 | 173 | 53 | C6 | A | 27 | N |
| S8 | M | 21 | 183 | 74 | C6 | A | 5 | Y |
| S9 | M | 18 | 183 | 70 | C6 | C | 3 | N |
| S10 | M | 68 | 168 | 89 | C4 | A | 3 | N |
| S11 | F | 53 | 183 | 75 | C6 | C | 3 | N |
| S12* | M | 32 | 178 | – | C5 | A | 36 | Y |
| Median | – | 31 | 180 | 76 | – | – | 5 | – |
| Range | – | 18–73 | 168–187 | 53–91 | – | – | 2–94 | – |
In most cases, AFES was tolerated well and a strong even contraction of the abdomen was achieved. The exceptions to this were S5 and S6 who both had non-symmetrical bi-lateral contractions. In the case of S5, one side of the abdomen did not respond well to stimulation even at high intensity; for S6, intact sensation on one side of the abdomen limited the stimulation current that could be used. The range of currents that were used for each subject is given in Table 2.
Table 2.
Training duration and current settings
| Training duration (minutes) |
Current (mA) |
||||||
|---|---|---|---|---|---|---|---|
| Subject | Week 1 | Week 2 | Week 3 | R-EO | R-RA | L-RA | L-EO |
| S1 | 60 | 120 | 180 | 50 | 40 | 40 | 50 |
| S2 | 80 | 160 | 240 | 70 | 60 | 60 | 70 |
| S3 | 80 | 90 | 240 | 60 | 50 | 50 | 60 |
| S4 | 90 | 230 | 475 | 80–100 | 50 | 50 | 90–100 |
| S5 | 80 | 140 | 190 | 80–100 | 80–100 | 120 | 120 |
| S6 | 80 | 200 | 190 | 30–40 | 0–30 | 15–35 | 20–40 |
| S7 | 80 | 160 | 240 | 30 | 30 | 30 | 35 |
| S8 | 60 | 160 | 230 | 45 | 40 | 45 | 30 |
| S9 | 100 | 150 | 240 | 40 | 40 | 35 | 30 |
| S10 | 80 | 160 | 200 | 100 | 50 | 50 | 80 |
| S11 | 80 | 160 | 240 | 120 | 120 | 100 | 100 |
| S12 | 80 | 165 | 240 | 120 | 120 | 100 | 100 |
| Mean | 180 | 158 | 241 | ||||
| SD | 11 | 34 | 77 | ||||
SD, standard deviation; R-EO and L-EO, right and left external oblique muscles; R-RA and L-RA, right and left rectus abdominis muscles.
The mean (standard deviation) number of days between each assessment session was: 8.7 (4.3) between A1 and A2; 7.3 (1.4) between A2 and A3; 7.5 (1) between A3 and A4; 8.7 (2.8) between A4 and A5; and 21.8 (2.4) between A5 and A6. S9 missed assessment A3 due to personal time constraints. However, he did move from the 20-minute training sessions to the 40-minute training sessions at the correct point in the intervention. The number of minutes of AFES received during each week of training is given in Table 2.
FVC and MEP assessments
The MEP test results for S1, S2, and S3 were not collected due to technical problems and the MEP results from S6 were discarded as unreliable because this subject had difficulties performing this test. The FVC test results were discarded for S8 as he started swinging his upper body forward while performing the test in later assessment sessions. FVC results were discarded for S1 on A3, and for S2 and S3 on A2 because only one usable attempt was collected.
The individual subject results are given in Fig. 3. As can be seen in the figure there was considerable inter- and intra-subject variability for all of the outcome measures. The mean results for the overall group as well as for the subjects with incomplete tetraplegia and the subjects with complete tetraplegia are shown in Fig. 4. There was very little change over the control phase (between A1 and A2) for all of the outcome measures. Over the training phase (between A2 and A5) FVC, FEV1, and PEF all increased while there was little change in FEV1/FVC and MEP. In the follow-up phase (between A5 and A6), there was very little change in FVC, FEV1, FEV1/FVC, and PEF while MEP increased slightly. Over the whole study the response for the subjects with incomplete and complete tetraplegia were comparable.
Figure 3.
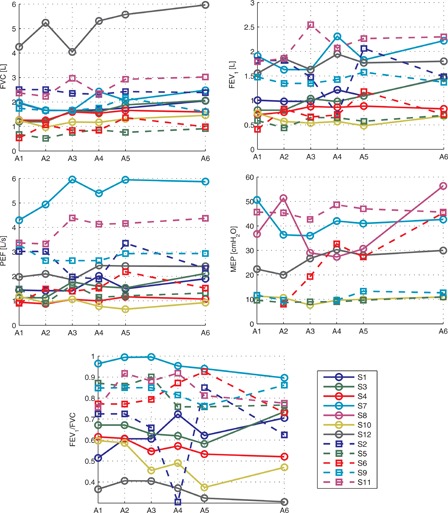
Individual subject results for each outcome measure. Each line represents absolute results for one subject. Subjects with motor complete tetraplegia have been drawn using solid lines and subjects with motor incomplete tetraplegia are have been drawn with a dashed line.
Figure 4.
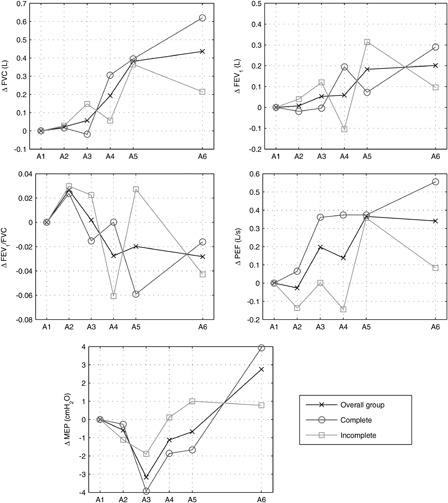
Mean results for the overall group, subjects with incomplete tetraplegia and subjects with complete tetraplegia. Results are presented as absolute change relative to the first assessment session A1.
The ANOVA found the longitudinal change in FVC to be significant (P = 0.0027) and multiple comparison testing showed a difference from A1 to A5 and A6 and from A2 to A5 and A6. No significant difference was shown over the control period (between A1 and A2) or over the follow-up period (between A5 and A6). Statistical significance was not found for changes in FEV1 (P = 0.196), FEV1/FVC (P = 0.435), PEF (P = 0.205), and MEP (P = 0.164).
Discussion
In this feasibility study the effect of a passive AFES training program on respiratory function in tetraplegia was investigated. The results show a significant improvement in FVC following training. While there was also a tendency for FEV1and PEF to increase this was not significant. In a 1-week pre-training control phase and a 3-week follow-up phase, no significant changes in any of the outcome measures were found. This suggests that the change in FVC over the training phase was a response to the intervention.
This study addresses an important topic for patients with tetraplegia. Respiratory complications, including in particular pneumonia and atelectasis, are a major cause of morbidity and a leading cause of death in this population.1,5,6 Although the effect of the intervention on respiratory complications was not measured, FVC has previously been shown to be a strong predictor of respiratory complications.5 Furthermore, in a similar study, incorporating FES training of the pectoral and abdominal muscles, there was a positive correlation between the outcome measures used in this study and a fall in respiratory complications.3
In this study, the feasibility of AFES training was investigated over a short period of 3 weeks. However, the increase in FVC, FEV1, and PEF did not plateau, which suggests that further benefits from the intervention might be achieved if training was continued over a longer period. In the case of FEV1 and PEF this may lead to a statistically significant change.
This study included a heterogeneous sample of patients with a mix of injury levels, time post injury, and AIS grade leading to large inter-subject variability. Despite this complex case mix the results show that FVC improved for all but one subject over the training phase. Furthermore, the absolute changes relative to baseline over the study were comparable for those subjects with motor complete and incomplete tetraplegia. This suggests that this technique is applicable for a wide demographic of people with tetraplegia.
The majority of subjects in this study were patients in hospital with busy rehabilitation programs. However, subjects managed to follow the training program, and three of the subjects completed their training sessions at the same time as other activities. Furthermore, several subjects voluntarily reported that they felt the AFES training had had a positive impact and asked if it was possible to continue stimulation for personal use. This suggests that the passive training program adopted in this study is practical to be used as a rehabilitation tool in tetraplegia.
Caution should be taken when interpreting the results of this study, as a matched control group was not employed. This is because previous work has shown that FVC, FEV1, and PEF increase considerably within the first 3 months of injury due to natural recovery.7 While it is not possible to rule out natural recovery for the changes seen in this study the lack of change in respiratory function during the week before training and 3 weeks post training do not support this notion. In addition, previous work, including subjects with a similar time post injury to this study, found no changes in respiratory function over a period of 4 weeks.3
There was considerable intra-subject variability seen in some of the subjects. This could be attributable to fluctuating general health of the patients, but might also be attributed to test–retest reliability. In this study, the mean of three attempts that were within 20% of each other was used for analysis. This protocol was inline with American Thoracic Society/European Respiratory Society (ATS/ERS) recommendations for the MEP test8 but we were not able to follow these recommendations for the FVC test.9 Although previous work has shown ATS/ERS standards for spirometry can be applied in SCI,10 this only included subjects with chronic injuries (>2 years). In the present study it was necessary to accept this large variation between attempts to make data collection possible as most of our subjects were in the early stages of injury and found it difficult to produce consistent results.
Increases in FVC, FEV1, and PEF of similar magnitude, in response to training which utilizes AFES, have been shown before.3,4 However, decreases in FVC have also been observed.11 A possible explanation for the increases over the training phase may be increased abdominal muscle mass and tone which has been shown to be reduced in tetraplegia.12 This would lead to greater support of the abdominal contents, which act as a fulcrum as the diaphragm contracts,13 placing the diaphragm in a better mechanical position to expand the lower lung. Thus, inhalation capacity would be increased. This explanation contradicts the results found by Hascakova-Bartova et al.11 where the authors agree that training increased abdominal bulk but they interpret that this would have a negative impact on the diaphragm. One major difference between the present study and the study by Hascakova-Bartova et al.11 is that the initial FVC of the subjects was considerably greater in the later study.
Although previous similar studies have shown similar improvements in FVC3,4 the technique used in this paper offers several advantages. This is because it is passive, meaning that subjects can use AFES at the same time as other activities, and because it requires stimulation of only one group of muscles, which makes it easier to apply.
Conclusion
The results of this feasibility study show the potential of passive AFES as a rehabilitation tool for respiratory function in tetraplegia. Since a control group was not employed the conclusions drawn should be taken as preliminary; however, the results provide the basis for future research of passive AFES in a follow-up study.
References
- 1.Cardenas D, Hoffman J, Kirshblum S, Mckinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 2004;85(11):1757–63 [DOI] [PubMed] [Google Scholar]
- 2.Linn WS, Adkins RH, Gong H, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 Southern California adult outpatients. Arch Phys Med Rehabil 2000;81(6):757–63 [DOI] [PubMed] [Google Scholar]
- 3.Cheng P, Chen C, Wang C, Chung C. Effect of neuromuscular electrical stimulation on cough capacity and pulmonary function in patients with acute cervical cord injury. J Rehabil Med 2006;38(1):32–6 [DOI] [PubMed] [Google Scholar]
- 4.Zupan A, Šavrin R, Erjavec T, Kralj A, Karčnik T, Škorjanc T, et al. Effects of respiratory muscle training and electrical stimulation of abdominal muscles on respiratory capabilities in tetraplegic patients. Spinal Cord 1997;35(8):540–5 [DOI] [PubMed] [Google Scholar]
- 5.Reines HD, Harris RC. Pulmonary complications of acute spinal cord injuries. Neurosurgery 1987;21(2):193–6 [DOI] [PubMed] [Google Scholar]
- 6.Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med 2007;30(4):319–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledsome JR, Sharp JM. Pulmonary function in acute cervical cord injury. Am Rev Respir Dis 1981;124:41–4 [DOI] [PubMed] [Google Scholar]
- 8.Gibson GJ, Whitelaw W, Siafakas N, Supinski GS, Fitting JW, Bellemare F, et al. ATS/ERS Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med 2002;166(4):518–624 [DOI] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–38 [DOI] [PubMed] [Google Scholar]
- 10.Kelley A, Garshick E, Gross ER, Lieberman SL, Tun CG, Brown R. Spirometry testing standards in spinal cord injury. Chest 2003;123(3):725–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hascakova-Bartova R, Dinant J-F, Parent A, Ventura M. Neuromuscular electrical stimulation of completely paralyzed abdominal muscles in spinal cord-injured patients: a pilot study. Spinal Cord 2008;46(6):445–50 [DOI] [PubMed] [Google Scholar]
- 12.Estenne M, Pinet C, Troyer AD. Abdominal muscle strength in patients with tetraplegia. Am J Respir Crit Care Med 2000;161(3 Pt 1):707–12 [DOI] [PubMed] [Google Scholar]
- 13.Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 2003;82(10):803–14 [DOI] [PubMed] [Google Scholar]


