Abstract
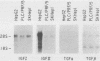
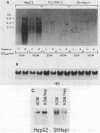
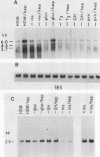
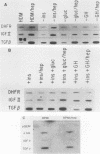
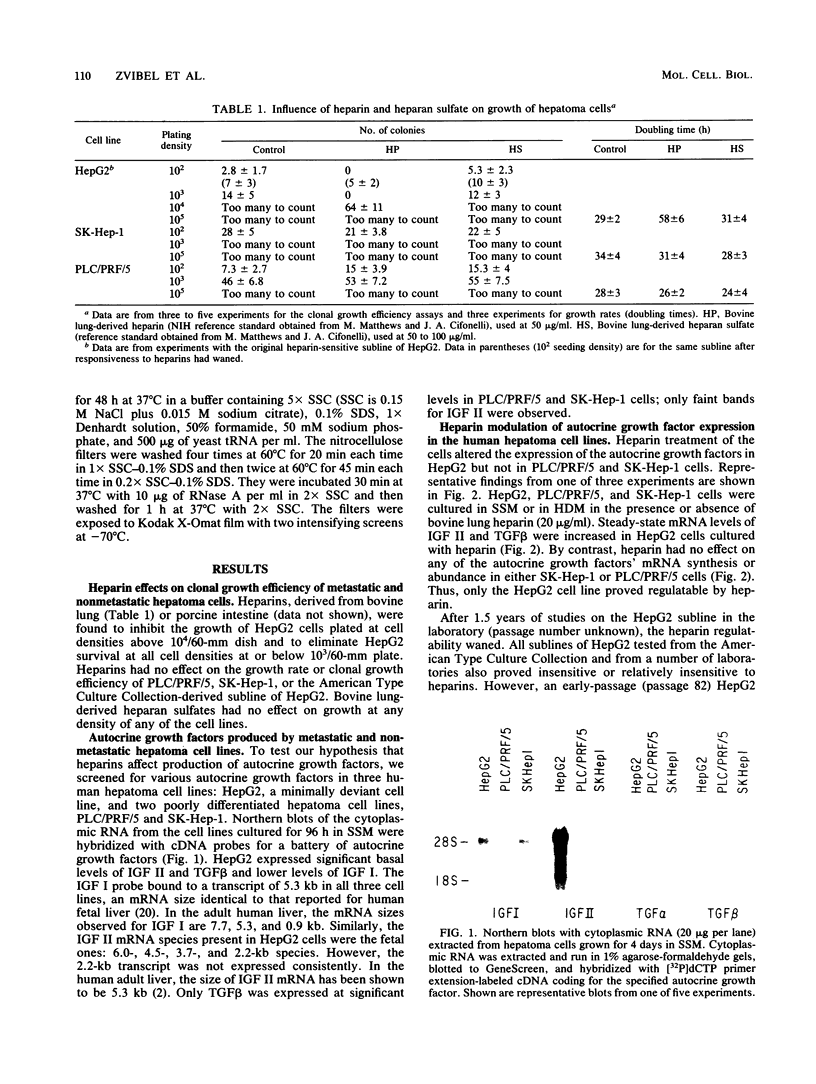
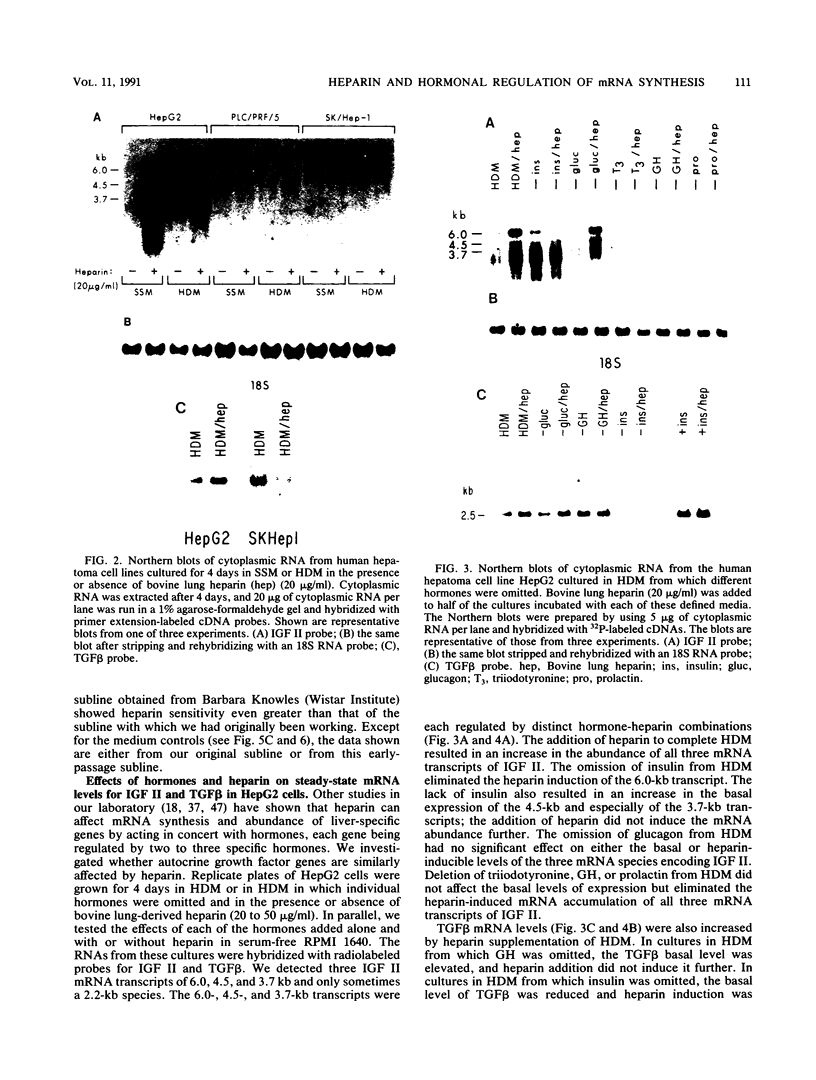
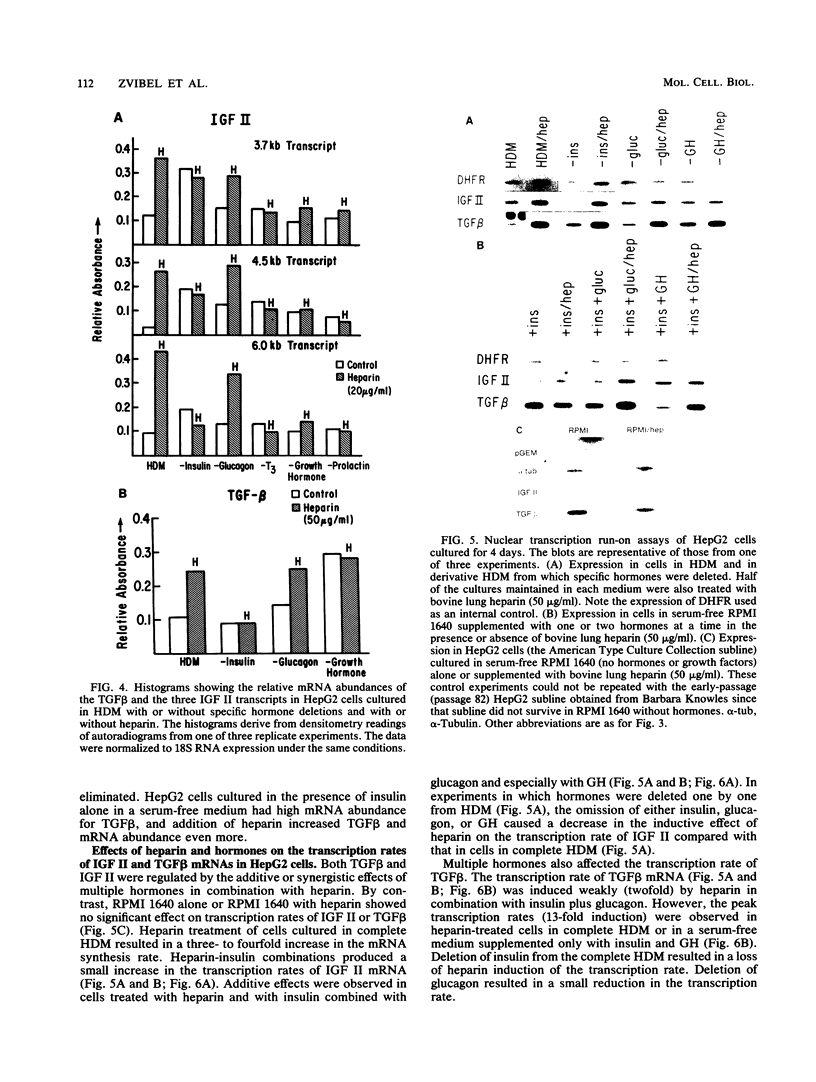
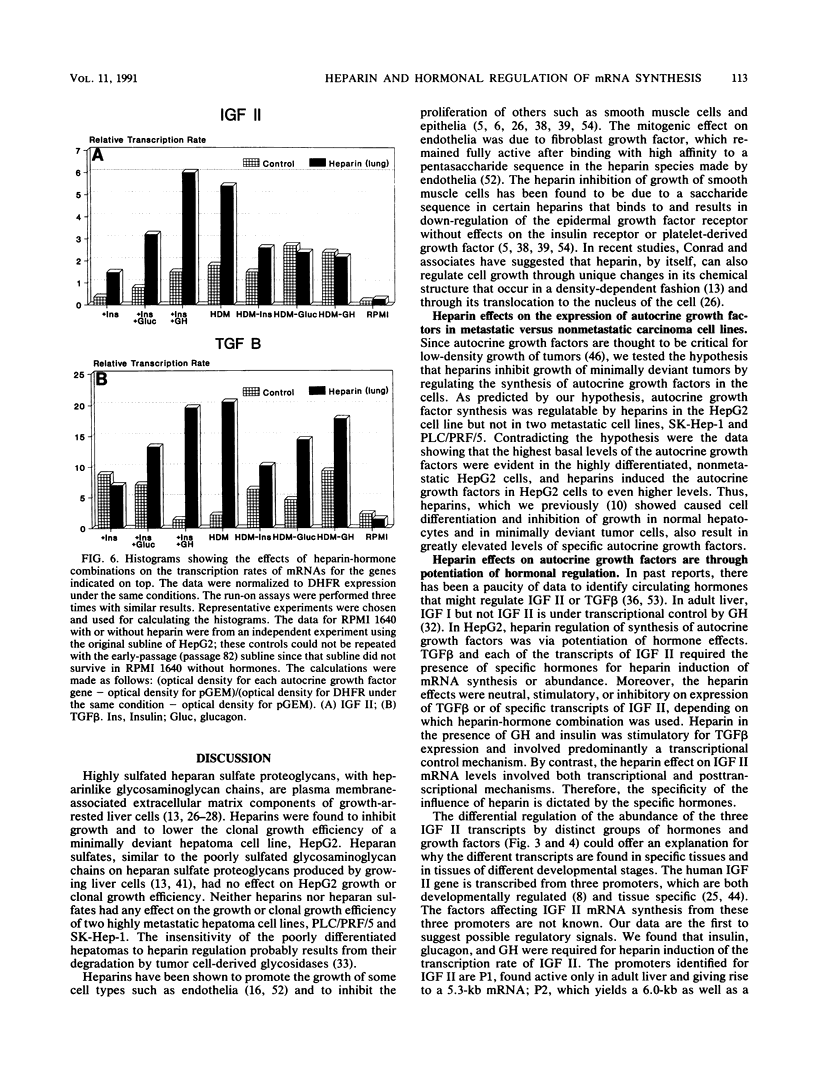
Highly sulfated, heparinlike species of heparan sulfate proteoglycans, with heparinlike glycosaminoglycan chains, are extracellular matrix components that are plasma membrane bound in growth-arrested liver cells. Heparins were found to inhibit the growth and lower the clonal growth efficiency of HepG2, a minimally deviant, human hepatoma cell line. Heparan sulfates, closely related glycosaminoglycans present in the extracellular matrix around growing liver cells, had no effect on the growth rate or clonal growth efficiency of HepG2 cells. Neither heparins nor heparan sulfates had any effect on the growth rate or clonal growth efficiency of two poorly differentiated, highly metastatic hepatoma cell lines, SK-Hep-1 and PLC/PRF/5. Heparin's inhibition of growth of HepG2 cells correlated with changes in the mRNA synthesis and abundance of insulinlike growth factor II (IGF II) and transforming growth factor beta (TGF beta). HepG2 cells expressed high basal levels of mRNAs encoding IGF II and TGF beta that were inducible, through transcriptional and posttranscriptional mechanisms, to higher levels by specific heparin-hormone combinations. For both IGF II and TGF beta, the regulation was multifactorial. Transcriptionally, IGF II was regulated by the additive effects of insulin, glucagon, and growth hormone in combination with heparin; TGF beta was regulated primarily by the synergistic effects of insulin and growth hormone in combination with heparin. Posttranscriptionally, the mRNA abundance of the IGF II 4.5- and 3.7-kb transcripts was affected by insulin. Heparin induction of all IGF II transcripts was also dependent on triiodotyronine and prolactin, but it is unknown whether their induction by heparin was via transcriptional or posttranscriptional mechanisms. Heparin-insulin combinations regulated TGF beta posttranscriptionally. The poorly differentiated hepatoma cell lines PLC/PRF/5 and SK-Hep-1 either did not express or constitutively expressed low basal levels of IGF I, IGF II, and TGF beta, whose mRNA synthesis and abundance showed no response to any heparin-hormone combination. We discuss the data as evidence that matrix chemistry is a variable determining the expression of autocrine growth factor genes and the biological responses to them.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Gerhard D. S., Fong N. M., Sanchez-Pescador R., Rall L. B. Isolation of the human insulin-like growth factor genes: insulin-like growth factor II and insulin genes are contiguous. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6450–6454. doi: 10.1073/pnas.82.19.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Merryweather J. P., Sanchez-Pescador R., Stempien M. M., Priestley L., Scott J., Rall L. B. Sequence of a cDNA clone encoding human preproinsulin-like growth factor II. 1984 Aug 30-Sep 5Nature. 310(5980):775–777. doi: 10.1038/310775a0. [DOI] [PubMed] [Google Scholar]
- Brown A. L., Graham D. E., Nissley S. P., Hill D. J., Strain A. J., Rechler M. M. Developmental regulation of insulin-like growth factor II mRNA in different rat tissues. J Biol Chem. 1986 Oct 5;261(28):13144–13150. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Choay J., Lormeau J. C., Petitou M., Sache E., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. II. Evidence for a pentasaccharide sequence that contains a 3-O-sulfate group. J Cell Biol. 1986 May;102(5):1979–1984. doi: 10.1083/jcb.102.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Cochran D. L., Karnovsky M. J. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol. 1985 Jul;124(1):21–28. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr R., Zvibel I., Chiuten D., D'Olimpio J., Reid L. M. Clonal growth of tumors on tissue-specific biomatrices and correlation with organ site specificity of metastases. Cancer Res. 1989 Jan 15;49(2):384–392. [PubMed] [Google Scholar]
- Enat R., Jefferson D. M., Ruiz-Opazo N., Gatmaitan Z., Leinwand L. A., Reid L. M. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N., Mead J. E., Braun L., Thompson N. L., Panzica M., Goyette M., Bell G. I., Shank P. R. Proto-oncogene expression and growth factors during liver regeneration. Symp Fundam Cancer Res. 1986;39:69–86. [PubMed] [Google Scholar]
- Fedarko N. S., Conrad H. E. A unique heparan sulfate in the nuclei of hepatocytes: structural changes with the growth state of the cells. J Cell Biol. 1986 Feb;102(2):587–599. doi: 10.1083/jcb.102.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fu X. X., Su C. Y., Lee Y., Hintz R., Biempica L., Snyder R., Rogler C. E. Insulinlike growth factor II expression and oval cell proliferation associated with hepatocarcinogenesis in woodchuck hepatitis virus carriers. J Virol. 1988 Sep;62(9):3422–3430. doi: 10.1128/jvi.62.9.3422-3430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatmaitan Z., Jefferson D. M., Ruiz-Opazo N., Biempica L., Arias I. M., Dudas G., Leinwand L. A., Reid L. M. Regulation of growth and differentiation of a rat hepatoma cell line by the synergistic interactions of hormones and collagenous substrata. J Cell Biol. 1983 Oct;97(4):1179–1190. doi: 10.1083/jcb.97.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han V. K., Lund P. K., Lee D. C., D'Ercole A. J. Expression of somatomedin/insulin-like growth factor messenger ribonucleic acids in the human fetus: identification, characterization, and tissue distribution. J Clin Endocrinol Metab. 1988 Feb;66(2):422–429. doi: 10.1210/jcem-66-2-422. [DOI] [PubMed] [Google Scholar]
- Hotta M., Baird A. The inhibition of low density lipoprotein metabolism by transforming growth factor-beta mediates its effects on steroidogenesis in bovine adrenocortical cells in vitro. Endocrinology. 1987 Jul;121(1):150–159. doi: 10.1210/endo-121-1-150. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Cruise J. L., Michalopoulos G. Norepinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary rat hepatocyte cultures. J Cell Physiol. 1988 Jun;135(3):551–555. doi: 10.1002/jcp.1041350327. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Irminger J. C., Rosen K. M., Humbel R. E., Villa-Komaroff L. Tissue-specific expression of insulin-like growth factor II mRNAs with distinct 5' untranslated regions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6330–6334. doi: 10.1073/pnas.84.18.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M., Fedarko N. S., Conrad H. E. Transport of heparan sulfate into the nuclei of hepatocytes. J Biol Chem. 1986 Oct 15;261(29):13575–13580. [PubMed] [Google Scholar]
- Kjellén L., Oldberg A., Hök M. Cell-surface heparan sulfate. Mechanisms of proteoglycan-cell association. J Biol Chem. 1980 Nov 10;255(21):10407–10413. [PubMed] [Google Scholar]
- Kjellén L., Pettersson I., Hök M. Cell-surface heparan sulfate: an intercalated membrane proteoglycan. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5371–5375. doi: 10.1073/pnas.78.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Liaw Y. F., Pao C. C., Chu C. M., Sheen I. S., Huang M. J. Changes of serum hepatitis B virus DNA in two types of clinical events preceding spontaneous hepatitis B e antigen seroconversion in chronic type B hepatitis. Hepatology. 1987 Jan-Feb;7(1):1–3. doi: 10.1002/hep.1840070102. [DOI] [PubMed] [Google Scholar]
- Little M. H., Ablett G., Smith P. J. Enhanced expression of insulin-like growth factor II is not a necessary event in Wilms' tumour progression. Carcinogenesis. 1987 Jun;8(6):865–868. doi: 10.1093/carcin/8.6.865. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Norstedt G., Palmiter R. D. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Di Ferrante D., Di Ferrante N., Nicolson G. L. Heparan sulfate degradation: relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science. 1983 May 6;220(4597):611–613. doi: 10.1126/science.6220468. [DOI] [PubMed] [Google Scholar]
- Norstedt G., Levinovitz A., Möller C., Eriksson L. C., Andersson G. Expression of insulin-like growth factor I (IGF-I) and IGF-II mRNA during hepatic development, proliferation and carcinogenesis in the rat. Carcinogenesis. 1988 Feb;9(2):209–213. doi: 10.1093/carcin/9.2.209. [DOI] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasharma K., Li C. H. Human pituitary and placental hormones control human insulin-like growth factor II secretion in human granulosa cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2643–2647. doi: 10.1073/pnas.84.9.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L. M. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990 Feb;2(1):121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Antiproliferative effects of heparin on vascular smooth muscle cells are reversed by epidermal growth factor. J Cell Physiol. 1987 May;131(2):149–157. doi: 10.1002/jcp.1041310203. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Heparin inhibition of smooth muscle cell proliferation: a cellular site of action. J Cell Physiol. 1986 Oct;129(1):11–19. doi: 10.1002/jcp.1041290103. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Viti M., Hök M. Structure and properties of an under-sulfated heparan sulfate proteoglycan synthesized by a rat hepatoma cell line. J Cell Biol. 1984 Mar;98(3):946–953. doi: 10.1083/jcb.98.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett. 1983 Sep 5;161(1):117–121. doi: 10.1016/0014-5793(83)80742-x. [DOI] [PubMed] [Google Scholar]
- Schwarz L. C., Gingras M. C., Goldberg G., Greenberg A. H., Wright J. A. Loss of growth factor dependence and conversion of transforming growth factor-beta 1 inhibition to stimulation in metastatic H-ras-transformed murine fibroblasts. Cancer Res. 1988 Dec 15;48(24 Pt 1):6999–7003. [PubMed] [Google Scholar]
- Shen S. J., Daimon M., Wang C. Y., Jansen M., Ilan J. Isolation of an insulin-like growth factor II cDNA with a unique 5' untranslated region from human placenta. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1947–1951. doi: 10.1073/pnas.85.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Torti F., Roth R. A. Characterization of the insulin and insulin-like growth factor receptors and responsitivity of a fibroblast/adipocyte cell line before and after differentiation. Biochem Biophys Res Commun. 1986 May 29;137(1):552–558. doi: 10.1016/0006-291x(86)91246-5. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Fujita M., Saez J. C., Choi H., Watanabe T., Hertzberg E., Rosenberg L. C., Reid L. M. Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol. 1987 Jul;105(1):541–551. doi: 10.1083/jcb.105.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen S. E., Sadow J. L., Rotwein P. Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1543–1547. doi: 10.1073/pnas.86.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoli J. V., Rall L. B., Karakousis C. P., Herrera L., Petrelli N. J., Bell G. I., Shows T. B. Enhanced levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res. 1986 Dec;46(12 Pt 1):6169–6173. [PubMed] [Google Scholar]
- Ueno T., Takahashi K., Matsuguchi T., Endo H., Yamamoto M. Transcriptional deviation of the rat insulin-like growth factor II gene initiated at three alternative leader-exons between neonatal tissues and ascites hepatomas. Biochim Biophys Acta. 1988 Sep 7;950(3):411–419. doi: 10.1016/0167-4781(88)90138-8. [DOI] [PubMed] [Google Scholar]
- Ueno T., Takahashi K., Matsuguchi T., Ikejiri K., Endo H., Yamamoto M. Reactivation of rat insulin-like growth factor II gene during hepatocarcinogenesis. Carcinogenesis. 1988 Oct;9(10):1779–1783. doi: 10.1093/carcin/9.10.1779. [DOI] [PubMed] [Google Scholar]
- Uhlrich S., Lagente O., Choay J., Courtois Y., Lenfant M. Structure activity relationship in heparin: stimulation of non-vascular cells by a synthetic heparin pentasaccharide in cooperation with human acidic fibroblast growth factors. Biochem Biophys Res Commun. 1986 Sep 14;139(2):728–732. doi: 10.1016/s0006-291x(86)80051-1. [DOI] [PubMed] [Google Scholar]
- Underwood L. E., D'Ercole A. J. Insulin and insulin-like growth factors/somatomedins in fetal and neonatal development. Clin Endocrinol Metab. 1984 Mar;13(1):69–89. doi: 10.1016/s0300-595x(84)80009-2. [DOI] [PubMed] [Google Scholar]
- Wright T. C., Jr, Johnstone T. V., Castellot J. J., Karnovsky M. J. Inhibition of rat cervical epithelial cell growth by heparin and its reversal by EGF. J Cell Physiol. 1985 Dec;125(3):499–506. doi: 10.1002/jcp.1041250320. [DOI] [PubMed] [Google Scholar]
- Yee D., Cullen K. J., Paik S., Perdue J. F., Hampton B., Schwartz A., Lippman M. E., Rosen N. Insulin-like growth factor II mRNA expression in human breast cancer. Cancer Res. 1988 Dec 1;48(23):6691–6696. [PubMed] [Google Scholar]
- de Pagter-Holthuizen P., Jansen M., van Schaik F. M., van der Kammen R., Oosterwijk C., Van den Brande J. L., Sussenbach J. S. The human insulin-like growth factor II gene contains two development-specific promoters. FEBS Lett. 1987 Apr 20;214(2):259–264. doi: 10.1016/0014-5793(87)80066-2. [DOI] [PubMed] [Google Scholar]