Abstract
The aim of the study was to seek evidence for the production of IL-12 by CD4+ T lymphocytes in in vitro and ex vivo trials. We performed in vitro trials with spleen cells from mice subjected to carcinogenesis, as well as ex vivo trials with cells obtained from the peripheral blood of healthy individuals and cancer patients. We were able to verify a significantly increased expression of IL-12 in CD4+ T lymphocytes from mice and patients with tumors, compared to controls. Follow-up studies are needed to clarify whether this difference is related to being in a chronic disease state or whether it is an attempt by the immune system to produce an anti-tumor response, since T lymphocytes from healthy donors were not able to produce IL-12 when in contact with polyclonal stimuli. We concluded that, in cancer, T helper cells are capable of synthesizing IL-12, raising the question of whether we are faced with another profile, Th12.
Keywords: cancer, immune response, T lymphocyte, interleukin 12
Introduction
Around 1986, Mosmann and colleagues1 conducted studies in which they identified subsets of the T helper (CD4+) cells (lymphocytes) that were classified as type 1 (Th1) and type 2 (Th2) T helper lymphocytes.2–4 In this generic context, Th1 cells mainly secrete interleukin (IL)-2 and interferon (IFN)-γ, which induce cell immunity, while clones of the Th2 cells liberate IL-4, IL-5, IL-10, and IL-13 and are related to humoral immunity.3–6 Recently, the distinction between helper lymphocyte subtypes has become more and more precise, based on the description of transcription factors in each of subtype; a fine equilibrium is responsible for the differentiation of these cells into a clone or something else that exercises their effector functions. For instance, high intracellular expression of T-bet, STAT1, and STAT4 characterizes the differentiation of Th1 clones, whereas expression of STAT6 and GATA-3 are related to Th2 identity.4
Today, other subpopulations are known, namely iTreg, Th17, Th22, and Th9.7–10 The Th17 profile preferentially produces IL-17A, IL-21, IL-22, IL-23, IL-6, and TNF-α, which are known as pro-inflammatory factors and characteristically promote or maintain inflammatory processes and act during infections.6,11,12 Th9 is induced mainly through the stimulus of TGF-β and IL-4, with IL-9 being the main cytokine secreted; its biological action and signaling pathways are still being clarified.13 Meanwhile, iTreg subpopulations are involved in the main processes by which immune responses are inhibited, through TGF-β synthesis.9 Independent of the profile to which they belong, in reality, the synthesis and actions of cytokines form an intricate web, wherein balances between different concentrations can lead to success or failure in confronting pathogen assaults.
The year 1989 saw the discovery of one of the most important cytokines responsible for the process of activating cellular immunity, IL-12.14 This cytokine is a heterodimeric protein composed of two disulphide glycoside-linked chains, known as p40 (40 kDa) and p35 (35 kDa).15 The IL-12 cytokine plays an important role in regulating innate immunological responses and determining the type and duration of acquired immune responses. When stimulated by pathogens, monocytes, macrophages and dendritic cells start to produce IL-12, which together with IL-18 acts on the NK (natural killer) cells and T lymphocytes in a synergetic way, increasing cytolytic activity and priming immunity.16,17
In this context, it is a crucial regulator of adaptive immunity as mediated by Th1 cells, which is one means of achieving protection against neoplasias. Studies in animals have provided clear evidence and support for IL-12’s beneficial role in protecting against neoplasias;15,18,19 clinical studies with humans subjects show better clinical results in situations where IL-12 is present, such as in immunotherapies.20
To date, there is only limited evidence that CD4+ T lymphocytes can synthesize IL-12. A study performed by our group contains a description of cancer patients who expressed IL-12 in CD4+ T lymphocytes.21 We did not find any similar findings in our literature review. In light of these data, this study’s objective was to seek evidence for the production of IL-12 by CD4+ T cells in in vitro and ex vivo trials.
Materials and Methods
In vitro trials with human cells
Cell culture from peripheral blood
For this study, we randomly selected 10 healthy volunteers (five of each sex) at the IPON. We told the volunteers what the objectives of the research were and confirmed that they consented to participate in the study. The Research Ethics Committee (REC) of UFTM approved the study (record number 683-2006).
We collected peripheral blood (10 mL) from the volunteers and submitted it to hemolysis. The blood was added immediately to a lysis solution (BD Biosciences FACS™) at a proportion of 1:20 mL, incubated for 20 min, and centrifuged (290 × g, 10 min, 4 °C). The lysis solution excess was removed by washing the cells 3 times with phosphate buffered saline (PBS) (290 × g, 10 min, 4 °C).
The whole cells were then re-suspended in 15 mL of incomplete RPMI (SIGMA) and incubated on a plate in 5% CO2 at 37 °C, at a concentration of 5 × 106 cells/mL in RPMI (SIGMA) containing 0.24% HEPES, 10% SBF, 1% L-glutamine, 1% gentamicin/streptomycin, 0.1% 2-mercaptoethanol, 0.22% bicarbonate, and 0.1% sodium pyruvate. Next, the cultures were stimulated with LPS (lipopolysaccharide) at 10 μg/mL or PHA (phytohemaglutinin) at 5 μg/mL, and cultivated for 24 hours so that cytometry could subsequently be performed. The use of LPS was necessary for monocyte stimulation, well as stimulation of lymphocytes to PHA, according to reports in literature.22,23
Ex vivo trials with humans
We selected 5 healthy volunteers (2 women and 3 men, mean age 25.6 ± 2.7 years) and 11 cancer patients, 10 women and 1 man (mean age, 61 ± 16.5 years). Of the 11 cancer patients, 4 had invasive breast carcinoma, 1 had vaginal carcinoma in situ and 2 invasive, and there was 1 case each of invasive cervical carcinoma, uterine sarcoma, vaginal melanoma, and lung cancer. All the cancers were advanced or recidivist, without any treatment for at least 60 days as per the protocol for dendritic cell vaccines.21 We communicated the objectives of the research to those involved and obtained their consent to participate in the study. The REC of UFTM approved the study (record number 683-2006). We obtained peripheral blood cells according to the protocol cited above. The cells underwent marking for flow cytometry.
Collection of spleen cells from mice with tumors
We divided 14 adult female mice (Balb/c), 8 weeks old, from the IPON into two groups: a Control group (no induction of tumor) and a Tumor group (chemical carcinogenesis with 7,12-dimethylbenzanthracene [DBMA] at a concentration of 1 mg/mL, delivered orally by gavage, over six consecutive weeks). The animals were maintained in plastic cages, with adequate space to accommodate them, in a 12 hour light/dark environment, at a controlled temperature (21 °C ± 3 °C), with food and water ad libitum. The mice were euthanized 16 weeks after completion of the 6 week tumor induction protocol, the time needed for the development of tumors, using an overdose of the ketamine chloride (90 mg/kg) and xylazine (15 mg/kg). The Ethics Committee on Animal Research (ECAR) of UFTM approved the study (record number 160). Three experiments were conducted separately.
After euthanasia, we removed, mechanical disruption, and homogenized the murine spleens. We washed the cells three times by centrifuging them at 290 × g for 10 min at 4 °C with RPMI 1640 and then submitting them to flow cytometry procedures, in accordance with the approved protocol as described below.
Flow cytometry
The flow cytometry protocol was essentially the same for all of the experiments, differing only in terms of the antibodies used. In brief, after obtaining the animals’ spleen cells, cultured cells from healthy individuals, and peripheral blood cells (PBCs) from Controls and Tumor patients, the samples were washed 3 times with PBS for centrifugation (290 × g, 10 min, 4 °C). We added 1 mL of PBS to the cells’ precipitate and 2 μL of protein transfer inhibitor (BD Golgistop™) for each 3 mL of cell solution. We incubated these suspensions for 20 min at 4 °C and then washed the cells by centrifugation with PBS to remove excess protein.
We then transferred the cell suspensions to test tubes after performing extracellular labeling. The tubes were divided into control isotypes and others for identification of total T (CD3+) lymphocytes, T helper (CD4+) lymphocytes, and macrophages (CD14+), with this last demarcation to confirm the hypothesis that in the selected gate, there were only CD4+ cells; we also performed double labeling of the previously identified cell populations with the antibody IL-12p70. Cytometry protocols and antibodies were deployed in accordance with those suggested by the manufacturer (BD Biosciences, San Diego, CA, USA).
Membrane permeabilization was performed using a BD cyitofix™ kit and intracellular labeling was done with an anti-IL-12 antibody. We then re-incubated the cells at 4 °C for 30 minutes in the dark and washed them in buffer solution (BD Perm/Wash™ Buffer) to remove excess markers. Finally, we re-suspended the cells in 500 uL of PBS to read them in the BD FACSCalibur™ cytometer.
Statistical analysis
We used Mann–Whitney tests and parametric t-tests. For qualitative percentage analysis of the separation between the groups, we used the χ2 test. We considered the differences observed to be of significance when the probability of rejecting the null hypothesis was lower than 0.05.
Results
In evaluating the expression of IL-12 by CD4+ cells in vitro, that is, through cultures of whole cells from peripheral blood samples obtained from healthy individuals, we did not find evidence of IL-12 expression by these cells following stimulation in culture with LPS and PHA (data not shown). To analyze lymphocytes, we performed cytometry of PBMC obtained from healthy individuals and cancer patients (ex vivo) after labeling them with specific antibodies for isotype and cellular type, and defining the area of analysis (FSC versus SSC) in the region corresponding to the lymphocyte gate (Fig. 1). Figure 2A illustrates the percentages of CD4/IL-12 double labeling in the lymphocyte gate. Note that there was a significantly higher percentage of double labeled cells in Tumor patient samples than in Control samples (P = 0.002). The fluorescence intensities for CD4 (Fig. 2B) and IL-12 (Fig. 2C) single labeling were also significantly greater in Tumor patient samples than in Control samples (P = 0.001 and P = 0.002, respectively).
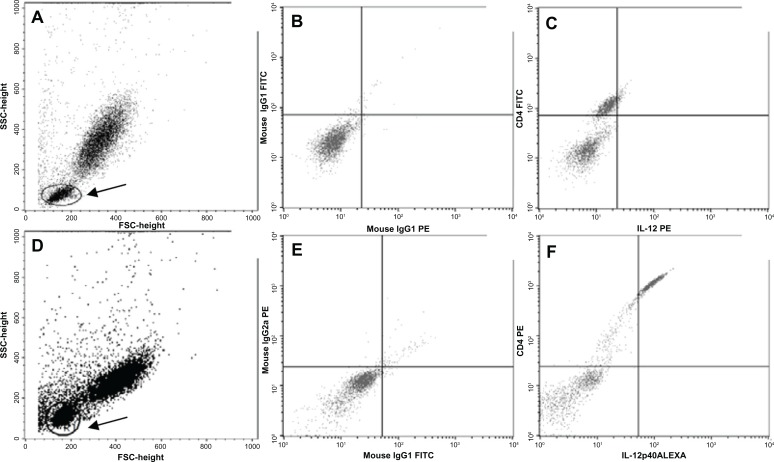
Figure 1.
(A) Granularity (SSC) and size (FSC) distribution of PBMC, with cells to be analyzed with CD4/IL-12, in healthy volunteer; double labeling (gate 1) indicated with a circle and shaft. (B) Isotype control for double labeling (C) Double labeling (CD4+ and IL-12) showed a right upper quadrant. In (A–C) the sample is of a healthy volunteer. (D) Granularity (SSC) and size (FSC) distribution of PBMC, with cells to be analyzed with CD4/IL-12, in a cancer patient, double labeling (gate 1) indicated with a circle and shaft. (B) Isotype control for double labeling (C) Double labeling (CD4+ and IL-12) showed a right upper quadrant. In (D–F) the sample from a cancer patient.
Note: Arrow: lymphocytes subpopulation.
Figure 2.
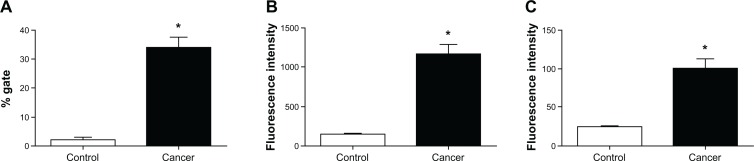
Expression of IL-12 by the CD4+ T lymphocytes in ex vivo control PBMC and tumor patient PBMC. (A) Percentages of double-labeled cells. (B) Intensity of CD4+ fluorescence for each group. (C) IL-12 fluorescence intensity for each group.
Note: *P < 0.002 versus control group.
In order to verify whether CD4+ T lymphocytes from mice with tumors also express IL-12, we labeled spleen cells from the animals that had undergone chemical carcinogenesis. As shown in Table 1, for the group subjected to tumor induction by DMBA (Cancer) compared to the Control group, there was a higher percentage of CD4/IL-12 double-labeled cells in the region corresponding to lymphocytes (P < 0.02).
Table 1.
Distribution in percentage of the expression of IL-12 by splenetic CD4+ T lymphocytes in breast carcinogenesis mice experiment.
| Marking | Control (mean percentage of gate) | Tumor (mean percentage of gate) | P |
|---|---|---|---|
| CD4 + IL-12 | 0.23 | 0.42 | 0.02 |
Notes: Used χ2 to the mean percentage of gate. Three experiments were conducted separately.
Discussion
In our studies, we established that CD4+ T cells have an ability to express intracellular IL-12. In patients with cancer, the levels of IL-12 synthesized by CD4+ T cells were significantly higher than in healthy individuals. This finding leads us to question whether this observation is related to chronic disease, such as the cancer in this study, and/or whether it is a way in which the immune system adapts to reduce tumor activity. We could not find data in the literature about IL-12 expression in CD4+ T lymphocytes.
Evidence in the literature suggests that IL-12 may be secreted by antigen presenting cells (APCs) when a stimulus for the differentiation of naïve CD4+ T cells occurs in Th1 effector cells, thus indirectly participating in the production of IFN-γ. IFN-γ and IL-12 are the main cytokines that stimulate and maintain the Th1 standard and consequently inhibit the Th2 standard.4 Moreover, studies have demonstrated the effects of IL-12, observed via the actions of CD4+ cells, on the production of IFN-γ and NK cells, both in terms of their role as a signal for the differentiation of CD8+ T cells and in their ability to act as an important factor in the reactivation and survival of CD4+ memory T cells.24 This phenomenon is relevant to the repolarization of CD4+ cells toward antitumor activities, as the Th2 profile is converted to Th1.25 A review of IL-12’s action on antitumor immunity and in immunotherapy performed by Colombo et al26 and studies performed by Stoppacciaro and colleagues27 report that IL-12’s antitumor and anti-metastatic activities have been demonstrated in mouse models of melanoma, breast carcinoma, colon carcinoma, renal carcinoma, and sarcoma. Some of these studies have broached the question of the local production of IL-12. In this context, the production of IL-12 in the location of the tumor (by neoplastic cells modified to liberate IL-12 via vectors) induces the rejection of neoplastic cells by CD8+ T cells, in association with macrophage infiltration, damage to the vessels, and necrosis.28
We could not find data in the literature about IL-12 expression in CD4+ T lymphocytes in both in vitro and ex vivo trials, but our results in experiments with mice shown higher percentage of CD4/IL-12 double-labeled cells in the region corresponding to lymphocytes. Kerkar and colleagues24 reported the importance of CD8+ T cells isolated from tumors in producing IL-12 at supra-physiological levels, when transfected with the IL-12-producing vector. However, the reasons for the limited clinical effectiveness of IL-12 as a factor in modifying biological response in cancer patients are still clear.
IL-12 seems to be a fundamental part of antitumor immunity, whereas studies conducted by our group using different immunotherapies have led to the conclusion that tumor regression necessarily involves the production of this cytokine. In immunotherapy using IFN-alphain, patients with 3 grade cervical intraepithelial neoplasia (CIN III) who exhibited good clinical response and subsequent regression of the lesion had significant increases in systemic and locally synthesis of IL-12.29–31 We also demonstrated that the use of immunotherapy with dendritic cells in patients with different tumors leads to increased synthesis of systemic IL-12 in patients with good clinical response.21
One of the main mechanisms by which the defense cells of the acquired immune response are activated is the synthesis of cytokines produced by T lymphocytes,1 whose naïve cells are highly heterogeneous, allowing them to differentiate themselves according to a stimulus into Th1, Th2, Treg, Th17, or Th9 patterns; this leads to distinct immune response functions when confronted by pathogens or chronic disease. The present findings support the notion that we may have an additional profile of T helper lymphocytes, namely Th12. Yet studies of IL-12 synthesis by CD4+ T cells are needed to clarify by what means these cells express increased levels of IL-12 in chronic illness states such as cancer; the result may be a more effective tumor response through polarization of the Th1 profile. More experiments with purified T cells from human and mice samples are needed, including IL-12 mRNA and protein expression assays after proper stimulation. It is also suggested that further studies are necessary in patients with early-stage cancer to demonstrate whether these produce a similar result. Another point to consider is the possibility of the sharing of the p40 subunit between IL-12 and IL-23, which is common to these two cytokines; we are already investigating this possibility. Taken together, our results suggest that CD4 lymphocytes obtained from humans or mice with cancer do indeed express intracellular IL-12.
Footnotes
Author Contributions
Conceived and designed the experiments: MAM, EFCM. Analyzed the data: MAM, EFCM, DRA. Wrote the first draft of the manuscript: MAM, EFCM, DRA, AARA. Contributed to the writing of the manuscript: MAM, DRA, AARA, EFCM. Agree with manuscript results and conclusions: MAM, DRA, AARA, EFCM. Jointly developed the structure and arguments for the paper: MAM, DRA, AARA, EFCM. Made critical revisions and approved final version: MAM, DRA, AARA, EFCM. All authors reviewed and approved of the final manuscript.
Funding
The authors would like to thank the Studies and Projects Funding Body (Financiadora de Estudos e Projetos, FINEP), the Foundation for Research Assistance of the State of Minas Gerais (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG), the National Council for Scientific and Technical Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq), and the Uberaba Foundation for Teaching and Research (Fundação de Ensino e Pesquisa de Uberaba, FUNEPU) for financial assistance.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Dileepan KN, Page JC, Li Y, Stechschulte DJ. Direct activation of murine peritoneal macrophages for nitric oxide production and tumor cell killing by interferon-gamma. J Interferon Cytokine Res. 1995;15(5):387–94. doi: 10.1089/jir.1995.15.387. [DOI] [PubMed] [Google Scholar]
- 3.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8(3):223–46. [PubMed] [Google Scholar]
- 4.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 5.Bais AG, Beckmann I, Ewing PC, et al. Cytokine release in HR-HPV(+) women without and with cervical dysplasia (CIN II and III) or carcinoma, compared with HR-HPV(−) controls. Mediators Inflamm. 2007;2007:24147. doi: 10.1155/2007/24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podgaec S, Abrao MS, Dias JA, et al. Endometriosis: an inflammatory disease with a Th2 immune response component. Human Reproduction. 2007;22(5):1373–9. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453(7198):1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elyaman W, Bradshaw EM, Uyttenhove C. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;31:12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Rudensky A. Controlo f regulatory T cell lineage commitent and maintenance. Immunity. 2009;30:616–25. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH-1 and TH-2 cells. Nat Immunol. 2009;10:864–71. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 11.Guedes PMM, Gutierrez FRS, Maia FL, et al. IL-17 produced during trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS Negl Trop Dis. 2010;4:e604. doi: 10.1371/journal.pntd.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Rostami A. IL-9: Basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neur Pharmacology. 2010;5:198–209. doi: 10.1007/s11481-009-9186-y. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhu N, Wang Q, et al. MEKK3 overexpression contributes to the hyperresponsiveness of IL-12-overproducing cells and CD4+ T conventional cells in nonobese diabetic mice. J Immunol. 2010;185:3554–63. doi: 10.4049/jimmunol.1000431. [DOI] [PubMed] [Google Scholar]
- 16.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin-12 and interleukin-18. Cytokine. 1999;11(11):822–30. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 17.Robinson D, Shibuya K, Mui A, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NF-γB. Immunity. 1997;7(4):571–81. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 18.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatsumi T, Huang J, Gooding WE, et al. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer Res. 2003;63:78–86. [PubMed] [Google Scholar]
- 20.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues CM, Matias BF, Murta EF, Michelin MA. The role of T lymphocytes in cancer patients undergoing immunotherapy with autologous dendritic cells. Clin Med Insights Oncol. 2011;5:107–15. doi: 10.4137/CMO.S6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leavitt RD, Felsted RL, Bachur NR. Biological and biochemical properties of Phaseolus vulgaris isolectinas. J Biol Chem. 1977;252(9):2961–6. [PubMed] [Google Scholar]
- 23.Ovstebo R, Olstad OK, Brusletto B, et al. Identification of genes particularly sensitive to lipopolysaccharide (LPS) in human monocytes induced by wild-type versus LPS-deficient Neisseria meningitidis strains. Infect Immun. 2008;76:2685–95. doi: 10.1128/IAI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70(17):6725–34. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wesa A, Kalinski P, Tatsumi T, et al. Polarized type-1 cells (DC1) producing high levels of IL-12 family members rescue patient Th1-type anti-melanoma CD4+ T cell responses in vitro. J Immunother. 2007;30:75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 26.Colombo MP, Trinchieri G. Interleukin-12 in antitumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–68. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 27.Zilocchi C, Stoppacciaro A, Chiodoni C, et al. Interferon gamma-independent rejection of interleukin 12-transduced carcinoma cells requires CD4+ T cells and Granulocyte/Macrophage colony-stimulating factor. J Exp Med. 1998;188(1):133–43. doi: 10.1084/jem.188.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavallo F, Signorelli P, Giovarelli M, et al. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049–58. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 29.Ramos MC, Mardegan MC, Peghini BC, Adad SJ, Michelin MA, Murta EF. Expression of cytokines in cervical stroma in patients with high-grade cervical intraepithelial neoplasia after treatment with intralesional interferon α-2b. Eur J Gynaecol Oncol. 2010;31(5):522–9. [PubMed] [Google Scholar]
- 30.Mardegan MC, Ramos MC, Adad SJ, Michelin MA, Shimba D, Murta EF. Immunological evaluation of vaginal secretion in patients with high-grade cervical intra-epithelial neoplasia treated with intralesional interferon α-2b. Eur J Gynaecol Oncol. 2011;32:297–302. [PubMed] [Google Scholar]
- 31.Misson DR, Abdalla DR, Borges AM, et al. Cytokine serum levels in patients with cervical intraepithelial neoplasia grade II–III treated with intralesional interferon-α 2b. Tumori. 2011;97:580–6. doi: 10.1177/030089161109700507. [DOI] [PubMed] [Google Scholar]