Abstract
Background
Alcohol use disorders are chronic disabling conditions for which existing pharmacotherapies have only modest efficacy. In the present review, derived from the 2012 Behavior, Biology and Chemistry “Translational Research in Addiction” symposium, we summarize the anti-relapse potential of corticotropin-releasing factor type 1 (CRF1) receptor antagonists to reduce negative emotional symptoms of acute and protracted alcohol withdrawal and stress-induced relapse to alcohol seeking.
Methods
We review the biology of CRF1 systems, the activity of CRF1 receptor antagonists in animal models of anxiolytic and antidepressant activity, and experimental findings in alcohol addiction models. We also update the clinical trial status of CRF1 receptor antagonists, including pexacerfont (BMS-562086), emicerfont (GW876008), verucerfont (GSK561679), CP316311, SSR125543A, R121919/NBI30775, R317573/19567470/CRA5626, and ONO-2333Ms. Finally, we discuss the potential heterogeneity and pharmacogenomics of CRF1 receptor pharmacotherapy for alcohol dependence.
Results
The evidence suggests that brain penetrant-CRF1 receptor antagonists have therapeutic potential for alcohol dependence. Lead compounds with clinically desirable pharmacokinetic properties now exist, and longer receptor residence rates (i.e., slow dissociation) may predict greater CRF1 receptor antagonist efficacy. Functional variants in genes that encode CRF system molecules, including polymorphisms in Crhr1 (rs110402, rs1876831, rs242938) and Crhbp genes (rs10055255, rs3811939) may promote alcohol seeking and consumption by altering basal or stress-induced CRF system activation.
Conclusions
Ongoing clinical trials with pexacerfont and verucerfont in moderately to highly severe dependent anxious alcoholics may yield insight as to the role of CRF1 receptor antagonists in a personalized medicine approach to treat drug or alcohol dependence.
Keywords: corticotropin-releasing factor or hormone receptor antagonist, CRF or CRH, anxiety disorder, major depression, alcohol or ethanol, drug addiction or alcoholism or alcohol dependence or alcohol use disorder or binge drinking, acute or protracted withdrawal or abstinence, treatment or clinical trial, stress-induced relapse or reinstatement or craving
1. INTRODUCTION
Each year, almost half of all American adults (47%) suffer from an addictive disorder (Sussman et al., 2011). Alcohol misuse alone has an annual prevalence of 10% (Sussman et al., 2011) and accounts for 10% of total disability in developed countries (Rehm et al., 2009). Most of the disability and cost to society results from alcohol dependence, or alcoholism, which has lifetime prevalence in the United States of more than 12% (Hasin et al., 2007). Available pharmacotherapies for alcoholism have only modest long-term efficacy and are underutilized (Heilig et al., 2011). Novel treatment options are needed. Here, we review the current state of therapeutically targeting corticotropin-releasing factor (CRF) systems to prevent alcohol dependence and relapse.
Brain and pituitary CRF1 receptors mediate many endocrine, behavioral, and autonomic responses to stress (Heinrichs and Koob, 2004). Accordingly, the pharmaceutical industry has sought to develop blood-brain barrier-penetrating CRF1 receptor antagonists for stress-related psychiatric conditions, including anxiety disorders and major depression (Holsboer and Ising, 2008; Koob and Zorrilla, 2012; Zorrilla and Koob, 2004, 2010). Indeed, the search for CRF receptor antagonists began from the time that Vale and colleagues isolated the stress-secreted CRF peptide in 1981 (Vale et al., 1981). In addition to CRF, the CRF/urocortin (Ucn) system includes genes encoding three CRF paralogs (Ucn 1, Ucn 2, and Ucn 3) and two G-protein coupled receptors (CRF1, CRF2), with which the CRF/Ucn peptides interact (Fekete and Zorrilla, 2007). Extensive, previously reviewed preclinical data support the therapeutic potential of blood-brain barrier penetrating CRF1 receptor antagonists for different facets of alcohol dependence (Breese et al., 2011; Ciccocioppo et al., 2009; Heilig et al., 2010a, 2011; Heilig and Koob, 2007; Heilig et al., 2010b; Koob and Zorrilla, 2010, 2012; Le and Shaham, 2002; Logrip et al., 2011; Martin-Fardon et al., 2010; Shalev et al., 2010; Zorrilla and Koob, 2010). Here, we will focus on (i) recent developments in the medicinal chemistry of CRF1 receptor antagonists, (ii) the progress of specific small molecule CRF1 receptor antagonists in clinical trials, and (iii) issues related to the potential heterogeneity and pharmacogenomics of CRF1 receptor pharmacotherapy for alcoholism. The review is derived from our 2012 Behavior, Biology and Chemistry “Translational Research in Addiction” symposium in San Antonio, Texas.
2. BIOLOGY OF CRF/UROCORTIN RECEPTOR SYSTEMS
2.1 CRF/Urocortin system molecules
CRF1 and CRF2 receptors are class B1 (“secretin-like”) G-protein coupled receptors that share ~70% sequence identity with one another. The CRF1 receptor exists in multiple isoforms (e.g., CRF1a-CRF1h), with the CRF1(a) subtype being the major functional isoform. In humans, the CRF2 receptor has three known membrane-associated functional subtypes -- CRF2(a), CRF2(b), and CRF2(c). CRF peptide has preferential agonist activity for CRF1 vs. CRF2 receptors. Ucn 1 is a high-affinity agonist at both receptors, but the type 2 urocortins (Ucn 2 and Ucn 3) are much more selective agonists for CRF2 receptors. A CRF-binding protein (CRF-BP) putatively sequesters CRF and Ucn 1 with equal or greater affinity than do CRF receptors. Most CRF receptor antagonists do not bind the CRF-BP, because of the different structural requirements for binding to CRF receptors vs. the CRF-BP (Fekete and Zorrilla, 2007; Zorrilla and Koob, 2004).
2.2 Hypothalamic vs. extrahypothalamic CRF systems
CRF initiates the hypothalamic-pituitary-adrenal (HPA) axis neuroendocrine stress response by binding CRF1 receptors in the anterior pituitary after release into portal blood. In addition, CRF1 receptors are widely distributed in stress-responsive brain regions, including the neocortex, central extended amygdala, medial septum, hippocampus, thalamus, cerebellum, and autonomic midbrain and hindbrain nuclei (Grigoriadis et al., 1996; Primus et al., 1997; Sanchez et al., 1999; Van Pett et al., 2000). The CRF1 receptor distribution resembles the distribution of its natural ligands CRF and Ucn 1 and accounts for the dissociable role of extrahypothalamic CRF1 systems (i.e., outside the HPA-axis) to mediate behavioral and autonomic stress responses (Fekete and Zorrilla, 2007; Kozicz et al., 1998; Swanson et al., 1983; Zorrilla and Koob, 2004).
3. CRF1 RECEPTOR ANTAGONISTS IN ANIMAL MODELS OF ANXIETY AND DEPRESSION
3.1 CRF1 receptor antagonists in anxiety models
CRF1 receptor antagonists produce anxiolytic-like effects in animal models of anxiety. In rodents, CRF1 receptor antagonists reduced acoustic startle responding (Chen et al., 1997; Schulz et al., 1996), conditioned fear (Hikichi et al., 2000; Kikusui et al., 2000), shock-induced freezing (Weninger et al., 1999), and defensive burying behavior (Heinrichs et al., 2002; Richardson et al., 2008; Zhao et al., 2007a; Zorrilla et al., 2003). CRF1 receptor antagonists also produced anxiolytic-like effects in exploration models of anxiety, but only under high anxiety baseline conditions (Gilligan et al., 2000; Griebel et al., 2002; Lelas et al., 2004; Okuyama et al., 1999; Zorrilla et al., 2002b). CRF1 receptor antagonists blunted the anxiogenic-like effects of social stressors; they reduced ultrasonic vocalization responses of rat pups to neonatal isolation (Griebel et al., 2002; Kehne et al., 2000) and reduced anxiogenic-like responses of rodents (Millan et al., 2001; Overstreet and Griebel, 2004) and non-human primates (Habib et al., 2000) to unfamiliar conspecifics. A CRF1 receptor antagonist (R317573/JNJ19567470/CRA5626) also produced anxiolytic-like effects in a rodent panic model (Shekhar et al., 2011).
3.2 Tolerance and side effect profile of CRF1 receptor antagonists
Little tolerance is seen to the anxiolytic-like actions of CRF1 receptor antagonists (Zorrilla and Koob, 2004). Unlike benzodiazepines, CRF1 receptor antagonists did not have sedative or ataxic effects or impair attention or spatial learning (Hogan et al., 2005; Zorrilla and Koob, 2004; Zorrilla et al., 2002a). CRF1 receptor antagonists may have less addiction liability than benzodiazepines; they did not promote formation of a conditioned place preference (Sahuque et al., 2006; Stinus et al., 2005) or support intravenous self-administration (Broadbear et al., 2002).
3.3 CRF1 receptor antagonists in models of antidepressant activity
The efficacy of CRF1 receptor antagonists in animal models of antidepressant activity is less consistent. Antalarmin reduced forced swim immobility in CRF2 receptor null mutant mice (Bale and Vale, 2003), an antidepressant-like effect, as did antalarmin, SSR125543A, LWH234, and CRA1000 in outbred rats (Griebel et al., 2002; Harro et al., 2001; Jutkiewicz et al., 2005). Subchronic treatment with R121919 and DMP696 also reduced forced swim immobility in mice (Nielsen et al., 2004) as did chronic SSR125543 treatment in Flinder Sensitive Line rats (Overstreet and Griebel, 2004; Overstreet et al., 2004a). R278995 reduced hyperemotionality (Chaki et al., 2004) in the olfactory bulbectomy model of depression (Song and Leonard, 2005). Finally, chronic treatment with antalarmin or SSR125543A reversed negative effects of chronic mild stress on coat appearance and hippocampal neurogenesis (Alonso et al., 2004; Ducottet et al., 2003; Griebel et al., 2002).
However, negative findings also are common. R121919, CP-154,526, and R278995 did not reduce forced-swim immobility in rats (Chaki et al., 2004; Jutkiewicz et al., 2005); antalarmin, CP-154,526, DMP904, R121919, and DMP696 did not reduce forced-swim immobility in mice (Nielsen et al., 2004; Oshima et al., 2003; Yamano et al., 2000). Antalarmin, CP-154,526, DMP904, R121919, DMP696, and R278995 did not produce antidepressant-like effects in the mouse tail-suspension test (Chaki et al., 2004; Liu et al., 2003; Nielsen et al., 2004). Although CP-154,526 Although CRF1 antagonist treatment was initially reported to reverse “learned helplessness” (Mansbach et al., 1997), subsequent studies with CP-154,526, DMP904, DMP696, R2789995, and CRA1000 failed to replicate this finding (Chaki et al., 2004; Li et al., 2005; Takamori et al., 2001). Finally, R278995 did not produce antidepressant-like effects in the rat differential-reinforcement-of-low-rate 72-s model (Chaki et al., 2004).
3.3 Conditions under which CRF1 receptor antagonists are effective
A possible reconciliation of these contradictory findings is that CRF1 receptor antagonists may differentially show antidepressant-like activity in dysfunction models that involve genetic (e.g., CRF2 null mutant mice) or environmental factors (e.g., chronic mild stress) that amplify or disinhibit CRF1 receptor signaling. Alternatively, perhaps CRF1 receptor antagonists are more effective against dynamic, active responses to acute stressors as compared to chronic, sustained stressors (Koob and Zorrilla, 2012). CRF1 receptor antagonists consistently reduce anxiety-like behavior in genetic, environmental and pharmacologically-induced models of high anxiety, but not low anxiety, conditions (Zorrilla and Koob, 2004).
4. RATIONALE FOR TARGETING CRF1 RECEPTORS TO TREAT ALCOHOL DEPENDENCE AND PREVENT RELAPSE
4.1 CRF1 receptor antagonists in withdrawal/negative affect models of alcohol dependence
The reviewed preclinical data suggest that extrahypothalamic CRF1 systems subserve some negative emotional states. Activation of CRF systems may therefore contribute to the withdrawal/negative affect stage of the addiction cycle. Individuals with high levels of innate anxiety or depression may be more likely to consume alcohol for its anxiolytic or dysphoria-relieving effects (Pohorecky, 1991). By reducing dysphoria, CRF1 receptor antagonists may help treat individuals who “self-medicate” antecedent anxiety or depression with alcohol. Consistent with this idea, small molecule CRF1 receptor antagonists reduce alcohol drinking in rodent models with high innate anxiety (Ciccocioppo et al., 2006; Hansson et al., 2007, 2006; Heilig and Koob, 2007; Lodge and Lawrence, 2003; Sommer et al., 2008), at doses that do not alter intake of normal, outbred rodents.
Chronic alcohol use itself, even if initiated for its rewarding effects, can lead to negative emotional symptoms and negatively reinforced alcohol use. An extension of the opponent process theory of affective regulation (Solomon and Corbit, 1974), this hypothesis of addiction proposes that alcohol initially engages brain structures that subserve positive emotional states (e.g., pleasure, contentment). To restore emotional homeostasis, however, a counterregulatory, opponent-process then decrements mood and increases vigilance/tension via downregulation of brain reward circuitry and recruitment of brain stress circuitry (Breese et al., 2011; Heilig et al., 2010a, 2011; Heilig and Koob, 2007; Heilig et al., 2010b; Koob and Zorrilla, 2010, 2012; Logrip et al., 2011). With repeated cycles of intoxication/withdrawal, the opponent-process allostatically predominates over the rewarding primary process. Consequently, progressively more alcohol is required to maintain euthymia. In the absence of alcohol, negative affective signs emerge (i.e., acute withdrawal: anxiety, dysphoria, irritability). With a sufficient alcohol use history, dysphoria can episodically and spontaneously resurge and heightened responses to otherwise innocuous stressors can be seen despite sustained abstinence (i.e., protracted withdrawal). Accordingly, fMRI activation responses to negative affective pictures are sensitized in detoxified alcoholics (Gilman and Hommer, 2008). Under this conceptualization, alcohol use escalates and relapse occurs because alcohol prevents and relieves the intrinsically-determined negative emotional symptoms of acute and protracted withdrawal (Heilig and Koob, 2007; Koob and Zorrilla, 2010).
The opponent process putatively involves activation of otherwise quiescent brain CRF1 receptor stress systems. Accordingly, cerebrospinal CRF levels are elevated in recently withdrawn alcoholics (Adinoff et al., 1996). In animal models, acute alcohol withdrawal activates CRF systems in the central nucleus of the amygdala (Funk et al., 2006; Merlo Pich et al., 1995; Roberto et al., 2010; Zorrilla et al., 2001) and bed nucleus of the stria terminalis (Olive et al., 2002), components of the central extended amygdala. Alcohol exposure also activates the HPA-axis via neuroendocrine CRF-dependent release of ACTH and glucocorticoids (Allen et al., 2011; Ogilvie et al., 1998; Rivier, 1996; Rivier and Plotsky, 1986),
Supporting a functional role for central extended amygdala CRF1 receptor activation in the negative affect/withdrawal stage, site-specific injections of CRF receptor antagonists into the central amygdala reduce anxiety-like behavior, motivational deficits for other reinforcers, and excessive self-administration of addictive substances during acute withdrawal (Heilig and Koob, 2007; Heilig et al., 2010b; Koob and Zorrilla, 2010; Logrip et al., 2011; Parylak et al., 2011). CRF1-dependent activation of the HPA-axis is also implicated because glucocorticoid receptor antagonists reduce the development and expression of excessive alcohol self-administration that results from repeated, intermittent intoxication (Vendruscolo et al., 2012). Consistent with both sets of findings, systemic injections of small molecule CRF1 receptor antagonists reduce the heightened anxiety-like behavior (Breese et al., 2005a, 2005b; Gehlert et al., 2007; Knapp et al., 2004; Overstreet et al., 2004b; Sommer et al., 2008) and escalated alcohol self-administration of dependent rodents acutely withdrawn from alcohol (Chu et al., 2007; Funk et al., 2007; Gehlert et al., 2007; Gilpin et al., 2008; Richardson et al., 2008; Sabino et al., 2006) at doses that do not alter intake of non-dependent animals.
Neuroadaptations in amygdala CRF1 systems still can be observed several weeks following detoxification from repeated cycles of intoxication/withdrawal in animal models (Sommer et al., 2008; Zorrilla et al., 2001). Accordingly, CRF1 receptor antagonists reduce the potentiated anxiogenic-like and ethanol intake behavior responses to otherwise ineffectual stressors seen during protracted withdrawal (Rimondini et al., 2002; Sommer et al., 2008; Valdez et al., 2002, 2003). CRF1 antagonists also attenuate the increased spontaneous anxiety-like behavior (Breese et al., 2005a, 2005b; Overstreet et al., 2002; Sommer et al., 2008; Valdez et al., 2002; Zhao et al., 2007b) and alcohol intake that can be seen in post-dependent rats even under low exteroceptive stress conditions (Rimondini et al., 2002; Sommer et al., 2008; Valdez et al., 2002). Both sets of findings are critical, because the resurgence of negative emotional states during protracted withdrawal, whether in the presence or absence of external stressors, is a major predictor of relapse in alcoholics (Mossberg et al., 1985; Pickens et al., 1985).
4.2 CRF1 receptor antagonists in relapse models of alcohol-seeking
CRF1 receptors also have been implicated in stress-induced reinstatement of alcohol seeking (Le and Shaham, 2002; Shalev et al., 2010). In the reinstatement model of relapse, animals are trained to self-administer drugs and are then subjected to extinction training during which lever presses are not reinforced. Reinstatement of extinguished lever responding (the operational measure of drug seeking) is determined after exposure to drug priming injections, drug-associated cues, or different stressors (Shaham et al., 2003; Stewart and de Wit, 1987), such as intermittent footshock (Le et al., 2000; Liu and Weiss, 2002) or the pharmacological stressor yohimbine (Le et al., 2012; Marinelli et al., 2007). Systemic injection of yohimbine, an alpha-2 adrenoceptor antagonist, induces stress- and anxiety-like responses in humans and laboratory animals (Bremner et al., 1996a, b). Furthermore, yohimbine reinstates drug seeking in rats (Feltenstein et al., 2012; Shepard et al., 2004) and monkeys (Lee et al., 2004) and palatable food seeking in rats via a CRF1 receptor-mediated mechanism (Ghitza et al., 2006). Yohimbine injections also induce alcohol and heroin craving in human drug addicts (Stine et al., 2002; Umhau et al., 2011).
In an initial study, Le et al. (2000) demonstrated that systemic injections of CP154,526 attenuate footshock-induced reinstatement of alcohol seeking in non-dependent rats. Subsequently, Hansson et al (2006) and Gehlert et al. (2007) reported that antalarmin and MTIP also attenuate footshock-induced reinstatement of alcohol seeking and additionally found that the CRF1 receptor antagonists were more effective in alcohol dependent rats or in genetically selected, Marchigian Sardinian alcohol-preferring rats. In the latter rat line, the Crhr1 transcript is innately upregulated in several brain areas, including the amygdala (Hansson et al., 2006). Finally, Marinelli et al. (2007) showed that antalarmin attenuates yohimbine-induced reinstatement of alcohol seeking.
The ability of CRF1 receptor antagonists to reduce stress-induced reinstatement is mediated by extrahypothalamic sites. Intermittent fooshock-induced reinstatement was not affected by adrenalectomy (Le et al., 2000), and antalarmin had no effect on yohimbine-induced corticosterone secretion (Marinelli et al., 2007). Furthermore, blockade of CRF receptors in the median raphe nucleus (Le et al., 2012, 2002) was sufficient to attenuate footshock- and yohimbine-induced reinstatement of alcohol seeking. The results agree with reports that stress-induced reinstatement of cocaine and heroin seeking is mediated by stress-induced activation of extrahypothalamic CRF sites (Blacktop et al., 2011; Erb et al., 1998; Shaham et al., 1997; Wang et al., 2005, 2006).
Taken together, results from the studies described above have established a critical role of CRF1 receptors in three main alcohol addiction-related behaviors in animal models: escalation of alcohol intake by the induction of alcohol dependence, acute and protracted aversive psychological withdrawal symptoms that can occur even in the absence of exteroceptive stress, and stress-induced relapse to alcohol seeking.
5. NONPEPTIDE CRF1-SELECTIVE RECEPTOR ANTAGONISTS
5.1 Pharmacophore and selectivity of nonpeptide CRF1 receptor antagonists
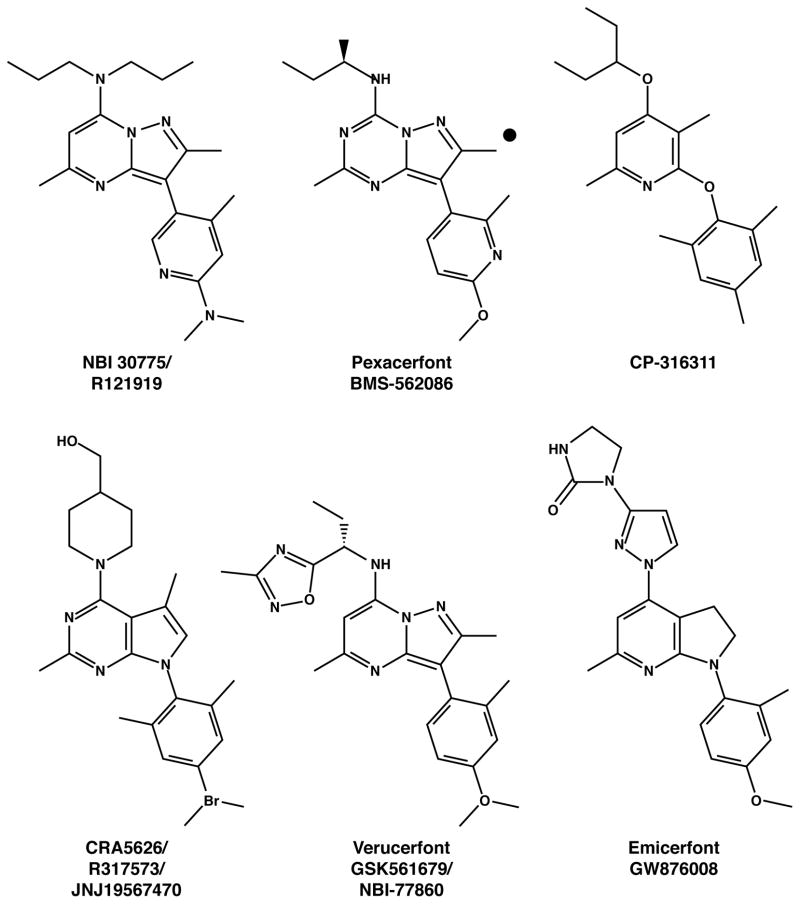
Almost all disclosed nonpeptide CRF1 receptor antagonists conform to a single pharmacophore. Prototypical compounds (Figure 1) share one or two aliphatic top units that occupy a hydrophobic pocket of the receptor, a central mono-, bi-, or tricyclic ring core, and an orthogonal, conformation-stabilizing, di- or -tri-substituted aromatic bottom group. Each ring core contains a putative proton-accepting ring nitrogen separated from the pendant aromatic by a one- or, more commonly, two-atom spacer. The core ring is typically methylated on the opposite position adjacent to the bonding nitrogen. Small molecules of this pharmacophore are potent and selective CRF1 receptor antagonists in relation to their interactions with regions of the third (His-199) and fifth (Met-276) transmembrane CRF1 receptor domains, residues not shared by the CRF2 receptor (Zorrilla and Koob, 2004, 2010).
Figure 1.
Prototypical CRF1 receptor antagonists that have been tested in humans.
Rare exceptions to this pharmacophore include oxo-7H-benzo[e]pyrimidine-4-carboxylic acid derivatives (subtype nonselective CRF receptor antagonists discovered by Alanex), CC 2064460 (a moderately potent arylamidrazone CRF1 receptor antagonist that lacks a central ring core with the customary hydrogen-bond accepting nitrogen), and stereospecific N-phenylphenylglycines (which also lack a ring core but were identified through computational screening based on a classic pharmacophore training set; Zorrilla and Koob, 2004, 2010).
5.2 Identification of “drug-like” CRF1 receptor antagonists
Early CRF1 receptor antagonist leads did not have drug-like physiochemical and pharmacokinetic properties. They violated Lipinski s “Rule of 5” heuristic for clinical drug candidates (e.g., excessively lipophilic and water insoluble with LogP>5) and correspondingly showed poor oral bioavailability (F% < 20%) and undesirably high volumes of distribution at steady state (VD > 10 L/kg) and/or plasma clearance (Clplasma > 45 ml/min/kg; Chen, 2006; Zorrilla and Koob, 2004, 2010). As shown in Table 1, however, concerted medicinal chemistry efforts have procured compounds with more favorable properties. Each of these compounds is active in vivo at minimum effective systemic doses of 2–10 mg/kg in preclinical behavioral or endocrine animal models that are sensitive to CRF1 receptor signaling. Notably, for MTIP and MPZP, these in vivo activities include published positive findings in animal models of alcohol dependence and relapse (Table 1).
Table 1.
Selected nonpeptide CRF1receptor antagonists with “drug-like” properties
| Familiar name | CAS registry | Nominal CRF1 affinity (nM) | cLog P | Oral bioavailability (F%) | Cl plasma (ml/min·kg) | Vd (L/kg) | t1/2 (h) | Notes | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Emicerfont (GW876008) | 786701-83-5 | IC50 = 66 | 1.5 | 66% | 19 | 2.4 | 1.6 | B/P = 3.7; reduced i.c.v. CRF-induced gerbil forepaw treading (10 mg/kg), marmoset defensive postures (10 mg/kg), and rat pup ultrasonic vocalization (30 mg/kg) | (Di Fabio et al., 2008) |
| Verucerfont (NBI-77860/GSK561679A) | 885220-61-1 | pKi=8.2 (~16 nM) | 4.3 | 66% | 14 | 7.5 | B/P=1.6 | (Tellew et al., 2010) | |
| MTIP | 910551-43-8 | Kd = 0.22 | 3.6 | 91% | 4.9 | 1.7 | 3.9 | Reduced conflict behavior; reduced hangover’ anxiety-like behavior in elevated plus-maze (10 mg/kg); reduced alcohol self-administration (10 mg/kg); reduced ethanol-intake in alcohol post-dependent or alcohol-preferring rats; decreased stress-induced reinstatement | (Gehlert et al., 2007; Sommer et al., 2008) |
| MPZP | 202579-76-8 | Kd = 4.9 | 2.9 | — | — | — | — | Reduced defensive burying behavior; reduced ethanol, nicotine and cocaine self-administration in dependence models | (George et al., 2007; Richardson et al., 2008; Specio et al., 2008) |
| Pexacerfont (BMS-562,086) | 459856-18-9 | Kd = 6.1 | 2.9 | 40% | 17.9 | 14.9 | 13.5 | Anxiolytic-like activity in elevated plus-maze and defensive withdrawal models (10 mg/kg, p.o.); solubility of 16 μg/ml in water (pH 7.4) and 16.3 mg/ml in 0.01 N HCl (pH 2.5); good pharmacokinetics in nonhuman primates | (Gilligan et al., 2009a; Zhou et al., 2012) |
| BMS-561,388 | 202578-88-9 (free base) | Kd = 4.7 | 2.2 | 51% | 20 | 14.6 | 9.7 | Anxiolytic-like activity in elevated plus-maze and defensive withdrawal models (10 mg/kg, p.o.); observed logP = 4.76; solubility < 1 μg/ml in water (pH 7.4) and 2.5 mg/ml in 0.01 N HCl (pH 2.5); inferior oral pharmacokinetics in chimpanzee compared with pexacerfont | (Gilligan et al., 2009b) |
Note: Intravenous pharmacokinetic parameters are Cl (clearance from plasma), Vd (volume of distribution at steady state) and t1/2 (plasma half-life).
Abbreviations: ACTH, adrenocorticotropic hormone; B/P, brain/plasma ratio; cLogP, calculated LogP; CRF, corticotropin-releasing factor. cLogP was calculated with Advanced Chemistry Development (ACD/Labs) Software v. 8.19 for Solaris.
Selected CRF1 receptor antagonists that are currently in clinical trials are discussed below (see also Table 1 and Figure 1). GlaxoSmithKline developed moderately potent (IC50 = 32–100 nM) substituted tetrahydrotetraazaacenaphthylenes and diydropyrrolo[2,3-d]pyrimidines with excellent oral bioavailability (52–86%), distribution/clearance balance, and central accumulation (B/P = 2.3–3.7). These include verucerfont (NBI-77860/GSK561679; (Tellew et al., 2010) and emicerfont (GW876008; (Di Fabio et al., 2008). In addition, pexacerfont (Gilligan et al., 2009a), the potent (IC50 = 6.1 nM) lead candidate from Bristol Myers Squibb, is a substituted pyrazolo[1,5-a]-1,3,5-triazine that showed good pharmacokinetics in rat, dog, and nonhuman primate models, no evidence of gastrointestinal or respiratory toxicity, and mild renal effects at doses ~1 order greater than those needed to substantially occupy brain CRF receptors (Gilligan et al., 2009a; Zhou et al., 2012). Supporting its therapeutic potential, pexacerfont was more potent than MTIP in reversing acute alcohol withdrawal-induced anxiety-like behavior of rats in the elevated plus-maze. (Thorsell, Heilig, unpublished results).
5.3 Clinical trials for CRF1 receptor antagonists
5.3.1 Completed trials for psychiatric indications
Clinical anticipation for CRF1 receptor antagonists for stress-related psychiatric disorders has been high since an early, open-label Phase IIa trial found that increasing doses of R121919 normalized sleep EEG and reduced depressive and anxious symptoms in depressed patients with few side effects (Held et al., 2004; Kunzel et al., 2005, 2003; Zobel et al., 2000). Unfortunately, to date, no CRF1 receptor antagonist has successfully completed a Phase III trial for any stress-related psychiatric illness. Development programs for R121919 (Neurocrine Press Release April 5, 2000; Zobel et al., 2000) and PF-00572778 (NCT00580190) were discontinued due to liver enzyme elevations. ONO-2333Ms (NCT00514865; (Ono Pharmaceutical Co Ltd, 2008), verucerfont (GSK561679; GlaxoSmithKline Results Summary for CRS106139, 2010) and CP-316,311 (Binneman et al., 2008) all lacked efficacy in controlled trials for major depression. Pexacerfont (BMS-562086) did not relieve generalized anxiety disorder symptoms (Coric et al., 2010), suicidal ideation in anxious patients (Coric et al., 2009), or diarrhea-predominant irritable bowel syndrome (Sweetser et al., 2009). Emicerfont (GW876008) also lacked efficacy in a Phase II trial for irritable bowel syndrome (GlaxoSmithKline Results Summary for CRI105626, 2008). Trials of verucerfont and emicerfont for social anxiety disorder were completed with undisclosed results (NCT00555139), as were trials of pexacerfont (NCT00135421) and SSR125543 (NCT01034995) for major depression. Finally, verucerfont is still being evaluated for efficacy to reduce post-traumatic stress disorder in women (NCT01059227).
5.3.2 Receptor residence rate and clinical efficacy
Recent pharmacological findings may provide an explanation for the discrepancy between the early positive findings obtained with R121919 and the negative findings more recently obtained with ONO-2333Ms, CP-316,311, and pexacerfont. Specifically, the nominal binding affinities shown in Table 1 that were attributed to each small molecule during compound screening and development were based on room temperature, standard competition assays. Such non-equilibrium single-point measurements do not capture the actual kinetics by which the small molecule binds to and, most importantly, subsequently resides on the human CRF1 receptor in vivo (Fleck et al., 2012; Ramsey et al., 2011). Greater receptor antagonist efficacy is putatively associated with greater residence time on the receptor (Brinkerhoff et al., 2008; Fleck et al., 2012; Tummino and Copeland, 2008; Vauquelin et al., 2006; Vauquelin and Van Liefde, 2006), as exemplified in the greater efficacy and duration of action of candesartan, a slowly dissociating angiotensin II AT1 receptor antagonist (Lacourciere and Asmar, 1999; Verheijen et al., 2004), versus the more rapidly dissociating antagonist losartan (Hansson, 2001). Kinetic analysis of receptor association and dissociation rates at physiological temperatures demonstrated that R121919, with which positive preliminary clinical findings were obtained, exhibits a “kinetic” Ki of ~0.36 nM, a potency underestimated by binding constants for it of 3–4 nM in standard competition assays (Fleck et al., 2012). In contrast, ONO-2333Ms, CP-316,311 and pexacerfont, for which negative clinical findings have been obtained, exhibit “kinetic” Kis of 1–2 orders poorer potency than R121919 (15, 12 and 19 nM, respectively), indicating that their nominal affinities (~2, 2 and 8 nM) may have overestimated their actual kinetic potencies (Fleck et al., 2012). Another way to conceptualize the kinetics of the antagonists is with respect to their residence time on the receptor, as reflected in the receptor dissociation rate. R121919 exhibits a slow off-rate (dissociation t1/2) of 130 min, an order longer than those for ONO-2333Ms, CP316,311 and pexacerfont (17, 4 and 14 min, respectively). SSR125543A, for which clinical results have not yet been reported, exhibits very favorable receptor residence kinetics (dissociation t1/2 = 430 min, kinetic Ki = 0.049 nM); results with SSR125543A may therefore provide discriminative information as to the importance of CRF1 receptor kinetics for clinical efficacy (Fleck et al., 2012).
5.3.3 Recent positive experimental results with CRF1 receptor antagonists in humans
Against the disappointing clinical outcomes to date, a few recent positive results may be instructive. As compared to placebo, oral emicerfont administration reduced blood oxygen level-dependent (BOLD) fMRI signal reductions in the hypothalamus, amygdala, hippocampus, insula, anterior cingulate, and orbitomedial prefrontal cortices during anticipation of pain in patients with irritable bowel syndrome (Hubbard et al., 2011). The inhibitory effects of emicerfont on hypothalamic activation were specific to patients who were experiencing (anticipatory) state anxiety. Furthermore, R317573/JNJ19567470/CRA5626 decreased regional glucose utilization in the amygdala of 12 healthy adults (Schmidt et al., 2010) and also decreased anxiety responses to 7.5% acute CO2 inhalation in a double-blind, placebo-controlled trial with healthy men (Bailey et al., 2011). The collective clinical findings are compatible with the revisionist hypothesis that CRF1 receptor antagonists may be differentially effective for conditions that involve dynamic, active responses to acute stressors, as opposed to low stress or chronic, sustained negative emotional states (Koob and Zorrilla, 2012).
5.3.4 Ongoing clinical trials of CRF1 receptor antagonists in addiction
In that context, and relevant to the reviewed stress-induced reinstatement preclinical literature, GlaxoSmithKline and NIH are collaboratively evaluating verucerfont for its ability to reduce stress-induced alcohol craving in anxious, stress-reactive alcoholic women (NCT01187511). Similarly, a comprehensive collaboration of Bristol Myers Squibb and NIH is currently testing oral daily pexacerfont for its efficacy to prevent: 1) stress-induced craving for palatable food in dieters (NCT01656577), 2) stress-induced craving for tobacco in smokers attempting to quit (NCT01557556), and 3) stress- or alcohol cue-induced craving in anxious, alcoholic women (NCT01227980). Even in the absence of a history of dependence or withdrawal state, CRF1 antagonism is effective to suppress alcohol self-administration in rats that show high innate levels of anxiety-like behavior (Hansson et al., 2006). Selecting for alcohol-dependent patients with high trait anxiety therefore may offer a strategy to enrich for subjects sensitive to CRF1 antagonism. This is critical for the ability of early human studies to detect a drug effect signal. Unselected populations of alcoholics diagnosed according to DSM-IV are heterogeneous, with many patients falling in an externalizing – impulsive cluster. Such patients are not necessarily expected to be sensitive to an anti-stress medication, and may therefore dilute a drug signal. Clinical assessments that distinguish trait from state anxiety are well validated and have successfully been used in prior studies that applied this type of enrichment strategy (George et al., 2008).
The inpatient study in anxious alcoholics involves a randomized, double-blind, placebo-controlled, parallel group design. Alcohol detoxification and associated withdrawal treatment is completed prior to inclusion in the experimental protocol. Participants are then randomized to 3 weeks of treatment (pexacerfont vs. placebo) in a 1:1 ratio. The oral dose in the pexacerfont trials involves a daily 300 mg/kg loading dose for 7 days, which results in ≥90% of subjects attaining a target circulating concentration of 0.5 μM within 5 days, followed by a 100 mg/day maintenance dose for 2 weeks. Co-primary outcomes include cravings induced by personalized, auditory scripts of stressful or alcohol-related guided imagery as compared to neutral imagery, as well as fMRI blood oxygenation level dependent (BOLD) responses to negative emotional stimuli. The stressful and alcohol-cue imagery used in the trial both induce alcohol craving, but only the stressful imagery also elevates state anxiety. This allows for testing the hypothesis that CRF1 antagonists have the potential to selectively target pathways to alcohol relapse that are associated with stressful triggers.
By using surrogate efficacy markers such as craving and fMRI BOLD responses, this experimental medicine based approach offers a high level of safety, and may accelerate development because it can go forward in the absence of drug – alcohol interaction data. If positive, it will provide a compelling rationale for such interaction data to be obtained and for full scale, outpatient relapse prevention studies to be carried out. While the predictive validity of animal models is frequently debated, less attention has been devoted to whether surrogate outcomes in human experimental models, such as stress-induced craving or brain responses to aversive stimuli, can predict clinical efficacy. This important issue is by no means settled, but some early observations give reason for measured optimism. Thus, both in alcohol and cocaine addiction, cravings in response to an experimental stressor in the laboratory have been shown to predict relapse during outpatient follow-up over three months (Sinha et al., 2011, 2006).
6. HETEROGENEITY OF ALCOHOLISM: A NEED FOR PERSONALIZED MEDICINE?
6.1 Heterogeneity of alcohol use disorders between and within individuals
The effectiveness of medications for alcohol use disorders may vary both across individuals and also within individuals at different times (Heilig et al., 2011, 2010b; Koob and Zorrilla, 2010; Logrip et al., 2011, 2012). For example, treatments may be differentially effective for different diagnoses because of the different underlying biological and psychological mechanisms, and, for similar reasons, during different stages of the addiction cycle. Based on the evidence described, CRF1 receptor antagonists may be more effective for alcoholism treatment after use has transitioned to primarily negatively-reinforced drinking (withdrawal/negative affect) or to protect against stress-induced relapse (stress-related craving). They may be less effective in preventing alcohol craving that is not related to negative emotional states or stressor precipitants, such as alcohol cue-induced relapse (Liu and Weiss, 2002). CRF1 receptor antagonists also may less effectively reduce reward-motivated, recreational binge drinking earlier in the addiction process (Heilig and Koob, 2007).
6.2 CRF system genetic variants and alcohol drinking phenotypes
6.2.1. Polymorphisms in animal models
The effectiveness of CRF1 receptor antagonists, as for other medicines, also may depend on genetic factors (Heilig et al., 2011; Sinha et al., 2011). Alcohol dependence has an estimated heritability of 50 – 60% (Goldman et al., 2005), with many susceptibility loci contributing individually to a small degree (Treutlein et al., 2009). Animal models support the hypothesis that variants in the genes that encode CRF system molecules, by altering CRF system activation under basal conditions or in response to stressful life events, may promote negatively-reinforced alcohol intake. For example, msP alcohol-preferring rats show two G-to-A polymorphisms in allelic identity with one another in the distal promoter of the Crhr1 gene, mutations that are not seen in other alcohol-preferring lines or outbred rats (Hansson et al., 2006); Logrip, Ciccocioppo, Walker, Koob and Zorrilla, unpublished observations). Perhaps as a result, the msP line exhibits increased CRF1 receptor expression in several stress-related brain regions, increased anxiety-like behavior and alcohol preference ratios, and increased sensitivity to the ability of CRF1 receptor antagonists to reduce alcohol self-administration and stress-induced reinstatement of alcohol seeking (Ciccocioppo et al., 2006; Gehlert et al., 2007; Hansson et al., 2006); Logrip, Ciccocioppo, Walker, Koob and Zorrilla, unpublished observations). Similarly, rhesus monkeys that carry a C-to-T single nucleotide polymorphism in the promoter of the Crh gene exhibit CRF peptide expression that is unrestrained by glucocorticoid feed-back inhibition. While without effect on voluntary alcohol intake in individuals with a normal life history, this is associated with a doubling in consumption in monkeys exposed to early life stress (Barr et al., 2009).
6.2.2 Polymorphisms in humans
Supporting the translational relevance of the genetic results in rats and monkeys, polymorphisms in human CRF system molecules also have been associated with alcohol use phenotypes. For example, variant Crhr1 haplotypes in adolescents predict binge drinking and lifetime prevalence of intoxication and alcohol dependence (Treutlein et al., 2006). Crhr1 single nucleotide polymorphisms (SNPs) also predicted greater alcohol consumption in already dependent individuals (Treutlein et al., 2006) and were associated with reduced P300 amplitude, an endophenotype seen in alcoholics (Chen et al., 2010). Adolescents homozygous for the C allele of the rs1876831 SNP of the Crhr1 gene exhibited greater future drinking (Blomeyer et al., 2008; Schmid et al., 2010) and an earlier onset of drinking in an interactive relation to stress history (Schmid et al., 2010). Conversely, adolescents homozygous for the H2 haplotype that contains the minor allele of rs1876831 are protected against early child abuse-associated increases in alcohol consumption and dependence (Nelson et al., 2010). Adolescent carriers of the A allele of the rs242938 Crhr1 SNP also reported greater alcohol drinking when exposed to stress in some (Schmid et al., 2010), but not other (Blomeyer et al., 2008), studies.
Genetic associations of CRF system polymorphisms to human alcohol phenotypes also have been seen for the CRF-binding protein (CRF-BP), which moderates the ability of CRF to interact with its receptors. For example, certain Crhbp gene SNPs are associated with decreased EEG alpha wave power, an endophenotype of alcoholism, and are more prevalent in alcohol use disorders (Enoch et al., 2008), including in alcoholics with comorbid anxiety disorders (Enoch et al., 1999). A Crhbp polymorphism (rs10055255) also has been associated with severity of stress imagery-induced alcohol craving and dysphoria (Ray, 2011). Recently, Crhbp (rs3811939) and Crhr1 SNPs (the widely-studied rs110402 polymorphism) were found to jointly predict comorbid alcohol use disorder in patients with schizophrenia (Ribbe et al., 2011). Elevated levels of CRF1 receptor mRNA relative to CRF-BP mRNA were seen in mononuclear blood cells from individuals carrying the dual polymorphism (Ribbe et al., 2011), supporting the hypothesis that that CRF-CRF1 receptor interactions predominate over CRF-CRF-BP interactions in those at risk for alcohol use disorders. Altogether, the human genetic findings further support the hypothesis that CRF1 receptor activation may contribute to the pathophysiology of alcohol use disorders. Furthermore, these findings suggest that pharmacogenomic profiling could identify genetically-vulnerable patients for whom CRF1 receptor antagonist pharmacotherapy may be especially useful to prevent alcohol relapse (Sinha et al., 2011).
7. CONCLUDING REMARKS
We briefly reviewed basic pharmacological and behavioral properties of small-molecule CRF1 receptor antagonists as well as their clinical trial status for psychiatric disorders. We discussed their potent effect in three main alcohol addiction-related behaviors in animal models: dependence-induced escalation of alcohol intake, negative emotional symptoms of acute and protracted withdrawal, and stress-induced relapse to alcohol seeking. These data provide a strong rationale for — translating these basic research findings to assess the efficacy of CRF1 receptor antagonists for the treatment of alcohol dependence and relapse. The future also holds great promise for discovering ways to individualize medications to suit the genetic profile of the individual. Genetic factors are likely to influence both the individual’s form of alcoholism and the pharmacokinetic and pharmacodynamic response to medications. In particular, CRF-related genes play an important role in alcohol dependence and relapse in humans. The outcome of clinical trials may depend on targeting the CRF1 receptor antagonists to a selected set of alcoholics for whom, due to experiential or genetic reasons, stress and negative reinforcement play a major role in the disease process. Personalized treatment, while holding promise for the long-term, poses a challenge for clinical development, because it requires studies to predict, identify and enrich for subjects likely to be responsive.
Acknowledgments
Role of Funding Source: The research was supported by National Institutes of Health grants AA006420, DA02812, DK026741 and DK070118 as well as travel support from the Behavior, Biology and Chemistry: Translational Research in Addiction 2012 symposium. Funding for the BBC conference was made possible, in part, by R13DA029347 from the National Institute on Drug Abuse. The research of YS and MH is supported by the Intramural Research Programs of NIDA and NIAAA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Health and Human Services, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse or the University of Texas at San Antonio Health Sciences Center. Mention of trade names, commercial practices, or organization does not imply endorsement by the U.S. Government. The funding sources had no role in writing the review or in the decision to submit the review for publication.
We thank Mary Gichuhi for administrative assistance. This is research publication number 21960 from The Scripps Research Institute.
Footnotes
Contributors: EPZ and YS wrote the first draft based on collective conceptual input of all authors at the symposium. All authors edited, substantively revised, are responsible for and approved the content of the submitted review.
Conflict of Interest: EPZ is co-inventor on a patent for the composition and use of non-peptide CRF1 receptor antagonists (US20100249138). MH, YS and HdW have no conflicts of interest with the content of this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology. 1996;15:288–295. doi: 10.1016/0893-133X(95)00212-V. [DOI] [PubMed] [Google Scholar]
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun. 2011;25(Suppl 1):S50–60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P. Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry. 2004;9:278–286. 224. doi: 10.1038/sj.mp.4001464. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Papadopoulos A, Diaper A, Phillips S, Schmidt M, van der Ark P, Dourish CT, Dawson GR, Nutt DJ. Preliminary evidence of anxiolytic effects of the CRF(1) receptor antagonist R317573 in the 7.5% CO(2) proof-of-concept experimental model of human anxiety. J Psychopharmacol. 2011;25:1199–1206. doi: 10.1177/0269881111400650. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci USA. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacol (Berl) 2005a;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005b;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brinkerhoff CJ, Choi JS, Linderman JJ. Diffusion-limited reactions in G-protein activation: unexpected consequences of antagonist and agonist competition. J Theor Biol. 2008;251:561–569. doi: 10.1016/j.jtbi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rice KC, Woods JH. Antalarmin, a putative CRH-RI antagonist, has transient reinforcing effects in rhesus monkeys. Psychopharmacol (Berl) 2002;164:268–276. doi: 10.1007/s00213-002-1187-y. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, Vinken P, Ashton D, Langlois X, Steckler T. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Chen AC, Manz N, Tang Y, Rangaswamy M, Almasy L, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Edenberg HJ, Schuckit MA, Tischfield J, Foroud T, Bierut LJ, Rohrbaugh J, Rice JP, Goate A, Hesselbrock V, Porjesz B. Single-nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event-related potential and alcohol dependence. Alcohol Clin Exp Res. 2010;34:988–996. doi: 10.1111/j.1530-0277.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Recent advances in small molecule antagonists of the corticotropin-releasing factor type-1 receptor-focus on pharmacology and pharmacokinetics. Curr Med Chem. 2006;13:1261–1282. doi: 10.2174/092986706776873014. [DOI] [PubMed] [Google Scholar]
- Chen YL, Mansbach RS, Winter SM, Brooks E, Collins J, Corman ML, Dunaiskis AR, Faraci WS, Gallaschun RJ, Schmidt A, Schulz DW. Synthesis and oral efficacy of a 4-(butylethylamino)pyrrolo[2,3-d]pyrimidine: a centrally active corticotropin-releasing factor1 receptor antagonist. J Med Chem. 1997;40:1749–1754. doi: 10.1021/jm960861b. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de Guglielmo G, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 2009;43:491–498. doi: 10.1016/j.alcohol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, Wu X, Gentile KA, Huang SP, Emison E, Delmonte T, D’Souza BB, Zimbroff DL, Grebb JA, Goddard AW, Stock EG. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27:417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- Coric V, Stock EG, Pultz J, Marcus R, Sheehan DV. Sheehan Suicidality Tracking Scale (Sheehan-STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont) 2009;6:26–31. [PMC free article] [PubMed] [Google Scholar]
- Di Fabio R, St-Denis Y, Sabbatini FM, Andreotti D, Arban R, Bernasconi G, Braggio S, Blaney FE, Capelli AM, Castiglioni E, Di Modugno E, Donati D, Fazzolari E, Ratti E, Feriani A, Contini S, Gentile G, Ghirlanda D, Provera S, Marchioro C, Roberts KL, Mingardi A, Mattioli M, Nalin A, Pavone F, Spada S, Trist DG, Worby A. Synthesis and pharmacological characterization of novel druglike corticotropin-releasing factor 1 antagonists. J Med Chem. 2008;51:7370–7379. doi: 10.1021/jm800744m. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:625–631. doi: 10.1016/S0278-5846(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, Albaugh B, Hodgkinson CA, Goldman D. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3:e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcohol Clin Exp Res. 1999;23:1312–1319. [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck BA, Hoare SR, Pick RR, Bradbury MJ, Grigoriadis DE. Binding kinetics redefine the antagonist pharmacology of the corticotropin-releasing factor type 1 receptor. J Pharmacol Exp Ther. 2012;341:518–531. doi: 10.1124/jpet.111.188714. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan PJ, Baldauf C, Cocuzza A, Chidester D, Zaczek R, Fitzgerald LW, McElroy J, Smith MA, Shen HS, Saye JA, Christ D, Trainor G, Robertson DW, Hartig P. The discovery of 4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5-a]-pyrimidine: a corticotropin-releasing factor (hCRF1) antagonist. Bioorg Med Chem. 2000;8:181–189. doi: 10.1016/s0968-0896(99)00271-0. [DOI] [PubMed] [Google Scholar]
- Gilligan PJ, Clarke T, He L, Lelas S, Li YW, Heman K, Fitzgerald L, Miller K, Zhang G, Marshall A, Krause C, McElroy JF, Ward K, Zeller K, Wong H, Bai S, Saye J, Grossman S, Zaczek R, Arneric SP, Hartig P, Robertson D, Trainor G. Synthesis and structure-activity relationships of 8-(pyrid-3-yl)pyrazolo[1,5-a]-1,3,5-triazines: potent, orally bioavailable corticotropin releasing factor receptor-1 (CRF1) antagonists. J Med Chem. 2009a;52:3084–3092. doi: 10.1021/jm900025h. [DOI] [PubMed] [Google Scholar]
- Gilligan PJ, He L, Clarke T, Tivitmahaisoon P, Lelas S, Li YW, Heman K, Fitzgerald L, Miller K, Zhang G, Marshall A, Krause C, McElroy J, Ward K, Shen H, Wong H, Grossman S, Nemeth G, Zaczek R, Arneric SP, Hartig P, Robertson DW, Trainor G. 8-(4-Methoxyphenyl)pyrazolo[1,5-a]-1,3,5-triazines: selective and centrally active corticotropin-releasing factor receptor-1 (CRF1) antagonists. J Med Chem. 2009b;52:3073–3083. doi: 10.1021/jm9000242. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GlaxoSmithKline Results Summary for CRI105626. [accessed on November 30 2012];A randomised, double blind, placebo controlled, crossover study to evaluate the efficacy and safety of the CRF1 receptor antagonist GW876008 in patients with irritable bowel ayndrome. 2008 http://download.gsk-clinicalstudyregister.com/files/20626.pdf.
- GlaxoSmithKline Results Summary for CRS106139. [accessed on November 30 2012];A Six-Week, Multicenter, randomized, double-blind, placebo-controlled, parallel-group study evaluating the efficacy, safety, and tolerability of GSK561679 compared to placebo in female subjects, diagnosed with major depressive disorder. 2010 http://download.gsk-clinicalstudyregister.com/files/ee490a01-8433-4cba-a027-9c486b866a67.
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylp henyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Liu XJ, Vaughn J, Palmer SF, True CD, Vale WW, Ling N, De Souza EB. 125I-Tyro-sauvagine: a novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2 alpha receptors. Mol Pharmacol. 1996;50:679–686. [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci USA. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci USA. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L. The relationship between dose and antihypertensive effect for different AT1-receptor blockers. Suppl. Blood Press; 2001. pp. 33–39. [DOI] [PubMed] [Google Scholar]
- Harro J, Tonissaar M, Eller M. The effects of CRA 1000, a non-peptide antagonist of corticotropin-releasing factor receptor type 1, on adaptive behaviour in the rat. Neuropeptides. 2001;35:100–109. doi: 10.1054/npep.2001.0851. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010a;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010b;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Held K, Kunzel H, Ising M, Schmid DA, Zobel A, Murck H, Holsboer F, Steiger A. Treatment with the CRH1-receptor-antagonist R121919 improves sleep-EEG in patients with depression. J Psychiatr Res. 2004;38:129–136. doi: 10.1016/s0022-3956(03)00076-1. [DOI] [PubMed] [Google Scholar]
- Hikichi T, Akiyoshi J, Yamamoto Y, Tsutsumi T, Isogawa K, Nagayama H. Suppression of conditioned fear by administration of CRF receptor antagonist CP-154,526. Pharmacopsychiatry. 2000;33:189–193. doi: 10.1055/s-2000-7587. [DOI] [PubMed] [Google Scholar]
- Hogan JB, Hodges DB, Jr, Lelas S, Gilligan PJ, McElroy JF, Lindner MD. Effects of CRF1 receptor antagonists and benzodiazepines in the Morris water maze and delayed non-matching to position tests. Psychopharmacol (Berl) 2005;178:410–419. doi: 10.1007/s00213-004-2028-y. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, Kelleher DL, Tillisch K, Naliboff BD, Mayer EA. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacol (Berl) 2005;180:215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, Coverdale S, McCloskey TC, Hoffman DC, Cassella JV. Effects of the CRF(1) receptor antagonist, CP 154,526, in the separation-induced vocalization anxiolytic test in rat pups. Neuropharmacology. 2000;39:1357–1367. doi: 10.1016/s0028-3908(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Takeuchi Y, Mori Y. Involvement of corticotropin-releasing factor in the retrieval process of fear-conditioned ultrasonic vocalization in rats. Physiol Behav. 2000;71:323–328. doi: 10.1016/s0031-9384(00)00352-8. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacology. 2012;37:308–309. doi: 10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kunzel HE, Ising M, Zobel AW, Nickel T, Ackl N, Sonntag A, Holsboer F, Uhr M. Treatment with a CRH-1-receptor antagonist (R121919) does not affect weight or plasma leptin concentration in patients with major depression. J Psychiatr Res. 2005;39:173–177. doi: 10.1016/j.jpsychires.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Kunzel HE, Zobel AW, Nickel T, Ackl N, Uhr M, Sonntag A, Ising M, Holsboer F. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res. 2003;37:525–533. doi: 10.1016/s0022-3956(03)00070-0. [DOI] [PubMed] [Google Scholar]
- Lacourciere Y, Asmar R. A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients: a placebo-controlled, forced titration study. Candesartan/Losartan study investigators. Am J Hypertens. 1999;12:1181–1187. doi: 10.1016/s0895-7061(99)00142-9. [DOI] [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00374.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe in relapse to alcohol seeking in rats. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotropin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lelas S, Wong H, Li YW, Heman KL, Ward KA, Zeller KL, Sieracki KK, Polino JL, Godonis HE, Ren SX, Yan XX, Arneric SP, Robertson DW, Hartig PR, Grossman S, Trainor GL, Taub RA, Zaczek R, Gilligan PJ, McElroy JF. Anxiolytic-like effects of the corticotropin-releasing factor1 (CRF1) antagonist DMP904 [4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5-a]-pyrimidine] administered acutely or chronically at doses occupying central CRF1 receptors in rats. J Pharmacol Exp Ther. 2004;309:293–302. doi: 10.1124/jpet.103.058784. [DOI] [PubMed] [Google Scholar]
- Li YW, Fitzgerald L, Wong H, Lelas S, Zhang G, Lindner MD, Wallace T, McElroy J, Lodge NJ, Gilligan P, Zaczek R. The pharmacology of DMP696 and DMP904, non-peptidergic CRF1 receptor antagonists. CNS Drug Rev. 2005;11:21–52. doi: 10.1111/j.1527-3458.2005.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Peprah D, Gershenfeld HK. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J Psychiatr Res. 2003;37:249–259. doi: 10.1016/s0022-3956(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience. 2003;117:243–247. doi: 10.1016/s0306-4522(02)00793-5. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs. 2011;25:271–287. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self-administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology. 2012;62:552–564. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol. 1997;323:21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF(1) receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Dorey G, Casara P, Dekeyne A. Anxiolytic properties of the selective, non-peptidergic CRF(1) antagonists, CP154,526 and DMP695: a comparison to other classes of anxiolytic agent. Neuropsychopharmacology. 2001;25:585–600. doi: 10.1016/S0893-133X(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Mossberg D, Liljeberg P, Borg S. Clinical conditions in alcoholics during long-term abstinence: a descriptive, longitudinal treatment study. Alcohol. 1985;2:551–553. doi: 10.1016/0741-8329(85)90133-8. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2010;15:1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurocrine Press Release April 5, 2000. [accessed on November 30 2012];Neurocrine Announces that Janssen Pharmaceutica Intends to Replace R121919 With A Back-Up Compound. http://www.thefreelibrary.com/Neurocrine%20Announces%20that%20Janssen%20Pharmaceutica%20Intends%20to%20Replace...-a061303934.
- Nielsen DM, Carey GJ, Gold LH. Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice. Eur J Pharmacol. 2004;499:135–146. doi: 10.1016/j.ejphar.2004.07.091. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22:243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Chaki S, Kawashima N, Suzuki Y, Ogawa S, Nakazato A, Kumagai T, Okubo T, Tomisawa K. Receptor binding, behavioral, and electrophysiological profiles of nonpeptide corticotropin-releasing factor subtype 1 receptor antagonists CRA1000 and CRA1001. J Pharmacol Exp Ther. 1999;289:926–935. [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Pharmaceutical Co Ltd. [accessed on November 30 2012];First Quarter (April 1 – June 30, 2008) Flash Report (unaudited) Three months ended June 30, 2008. 2008 http://www.ono.co.jp/eng/investor/pdf/fr/2009/fi0901.pdf.
- Oshima A, Flachskamm C, Reul JM, Holsboer F, Linthorst AC. Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology. 2003;28:2148–2159. doi: 10.1038/sj.npp.1300267. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of CRF1 receptor antagonist SSR125543 in an animal model of depression. Eur J Pharmacol. 2004;497:49–53. doi: 10.1016/j.ejphar.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur J Pharmacol. 2004a;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004b;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol Behav. 2011;104:149–156. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens RW, Hatsukami DK, Spicer JW, Svikis DS. Relapse by alcohol abusers. Alcohol Clin Exp Res. 1985;9:244–247. doi: 10.1111/j.1530-0277.1985.tb05744.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Ramsey SJ, Attkins NJ, Fish R, van der Graaf PH. Quantitative pharmacological analysis of antagonist binding kinetics at CRF1 receptors in vitro and in vivo. Br J Pharmacol. 2011;164:992–1007. doi: 10.1111/j.1476-5381.2011.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res. 2011;35:166–174. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Ribbe K, Ackermann V, Schwitulla J, Begemann M, Papiol S, Grube S, Sperling S, Friedrichs H, Jahn O, Sillaber I, Gefeller O, Krampe H, Ehrenreich H. Prediction of the risk of comorbid alcoholism in schizophrenia by interaction of common genetic variants in the corticotropin-releasing factor system. Arch Gen Psychiatry. 2011;68:1247–1256. doi: 10.1001/archgenpsychiatry.2011.100. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Rivier CL, Plotsky PM. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol. 1986;48:475–494. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacol (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacol (Berl) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Andrews RD, van der Ark P, Brown T, Mannaert E, Steckler T, de Hoon J, Van Laere K. Dose-dependent effects of the CRF(1) receptor antagonist R317573 on regional brain activity in healthy male subjects. Psychopharmacol (Berl) 2010;208:109–119. doi: 10.1007/s00213-009-1714-1. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, 3rd, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci USA. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Fitz SD, Nakazato A, Chaki S, Steckler T, Schmidt M. A selective, non-peptide CRF receptor 1 antagonist prevents sodium lactate-induced acute panic-like responses. Int J Neuropsychopharmacol. 2011;14:355–365. doi: 10.1017/S1461145710001355. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacol (Berl) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacol (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; New York: 1987. pp. 211–227. [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Sussman S, Lisha N, Griffiths M. Prevalence of the addictions: a problem of the majority or the minority? Eval Health Prof. 2011;34:3–56. doi: 10.1177/0163278710380124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, Tong G, Dockens R, Zinsmeister AR. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori K, Kawashima N, Chaki S, Nakazato A, Kameo K. Involvement of corticotropin-releasing factor subtype 1 receptor in the acquisition phase of learned helplessness in rats. Life Sci. 2001;69:1241–1248. doi: 10.1016/s0024-3205(01)01217-6. [DOI] [PubMed] [Google Scholar]
- Tellew JE, Lanier M, Moorjani M, Lin E, Luo Z, Slee DH, Zhang X, Hoare SR, Grigoriadis DE, St Denis Y, Di Fabio R, Di Modugno E, Saunders J, Williams JP. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20:7259–7264. doi: 10.1016/j.bmcl.2010.10.095. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Maier W, Moessner R, Gaebel W, Dahmen N, Fehr C, Scherbaum N, Steffens M, Ludwig KU, Frank J, Wichmann HE, Schreiber S, Dragano N, Sommer WH, Leonardi-Essmann F, Lourdusamy A, Gebicke-Haerter P, Wienker TF, Sullivan PF, Nothen MM, Kiefer F, Spanagel R, Mann K, Rietschel M. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tummino PJ, Copeland RA. Residence time of receptor-ligand complexes and its effect on biological function. Biochemistry. 2008;47:5481–5492. doi: 10.1021/bi8002023. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George TD, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;60:303–311. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vauquelin G, Fierens F, Van Liefde I. Long-lasting angiotensin type 1 receptor binding and protection by candesartan: comparison with other biphenyl-tetrazole sartans. J Hypertens Suppl. 2006;24:S23–30. doi: 10.1097/01.hjh.0000220403.61493.18. [DOI] [PubMed] [Google Scholar]
- Vauquelin G, Van Liefde I. Slow antagonist dissociation and long-lasting in vivo receptor protection. Trends Pharmacol Sci. 2006;27:356–359. doi: 10.1016/j.tips.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen I, Vanderheyden PM, De Backer JP, Vauquelin G. AT1 receptor antagonists. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:69–77. doi: 10.2174/1568016043477378. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano M, Yuki H, Yasuda S, Miyata K. Corticotropin-releasing hormone receptors mediate consensus interferon-alpha YM643-induced depression-like behavior in mice. J Pharmacol Exp Ther. 2000;292:181–187. [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007a;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007b;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Dockens RC, Liu-Kreyche P, Grossman SJ, Iyer RA. In vitro and in vivo metabolism and pharmacokinetics of BMS-562086, a potent and orally bioavailable corticotropin-releasing factor-1 receptor antagonist. Drug Metab Dispos. 2012;40:1093–1103. doi: 10.1124/dmd.111.043596. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla E, Fekete E, Mason BJ, Wirsching P, Janda KD, Koob GF. CRF1 receptor antagonists for anxiety. European Neuropsychopharmacology. 2003;13:s130–s131. [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Schulteis G, Ormsby A, Klaassen A, Ling N, McCarthy JR, Koob GF, De Souza EB. Urocortin shares the memory modulating effects of corticotropin-releasing factor (CRF): mediation by CRF1 receptors. Brain Res. 2002a;952:200–210. doi: 10.1016/s0006-8993(02)03345-0. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002b;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacol (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]