Abstract
Exercise affects neuroplasticity and neurotransmission including dopamine (DA), which modulates drug-taking behavior. Previous research in rodents has shown that exercise may attenuate the rewarding effects of drugs of abuse. The present study examined the effects of high and low exercise on cocaine responses in male Wistar rats that had been trained to self-administer and were compared to a group of sedentary rats. High exercise rats (HE) ran daily on a treadmill for 2 h and low exercise (LE) ran daily for 1 h. After 6 weeks of this exercise regimen, rats were tested over 2 days for reinstatement (day 1: cue-induced reinstatement; day 2: cocaine-primed reinstatement). During cue-induced reinstatement, the sedentary rats showed the expected increase in active lever responses when compared to maintenance, whereas these increased responses were inhibited in the exercised rats (HE and LE). During cocaine-primed reinstatement, however, there was a significant increase in active lever presses when compared to maintenance only in the HE group. This data suggests that chronic exercise during abstinence attenuates the cue-induced reinstatement seen in the sedentary rats by 26% (LE) and 21% (HE). In contrast, only the high exercise rats exhibited sensitized cocaine-seeking behavior (active lever presses) following cocaine-primed reinstatement. Finally, while sedentary rats increased locomotor activity during cocaine-primed reinstatement over that seen with cocaine during maintenance, this was not observed in the exercised rats, suggesting that exercise may interfere with the sensitized locomotor response during cocaine reinstatement.
Keywords: Dopamine, Exercise, Cocaine, Self-administration, Relapse, Abstinence
1. Introduction
Physical exercise is important to neuroplasticity and neuronal health [1]. Although exercise induces plasticity in many brain regions [2,3], the area of greatest study has been the striatum. There, cerebral glucose uptake increases during exercise [4], and exercise produces a long term increase in dopamine type 2 receptor levels in mature and old rats [5,6]. Exercise has also been shown to have effects on the DA metabolite DOPAC in the accumbens and caudate nucleus [7,8] and to be neuroprotective of DA neurons in models of Parkinson’s disease [9]. Treadmill exercise has been shown to facilitate behavioral recovery after MPTP lesioning of the basal ganglia, and this appeared to be mediated through effects on dopamine transporters (DAT) and D2 mRNA levels [10]. Thus, these studies show that forced exercise can alter the striatal dopaminergic system.
The DA system modulates the rewarding responses to drugs of abuse and is involved in the neuroadaptations that occur with addiction [11]. Thus, it is hypothesized that exercise, via its effects on the striatal DA system, would affect responses to drugs of abuse. Indeed, survey studies in adolescents suggest a relationship between lack of physical activity and high-risk behaviors, such as taking drugs, smoking cigarettes, and consuming alcohol [12,13]. Additionally, adolescents who exercise on a daily basis are 50% and 40% less likely to smoke cigarettes and marijuana, respectively [14]. Preclinical studies provide strong evidence for this as well, as exercise inhibited cocaine conditioned place preference (CPP) in male Lewis rats and attenuated it in females (six weeks of daily treadmill running) [15], reduced cocaine self-administration (0.2 mg/ml) in female, but not male, Sprague Dawley rats (running wheel for 2 weeks) [16], and decreased cocaine seeking [break point for a Progressive Ratio schedule] in female Long-Evans rats (running wheel for 6 weeks) [17].
Current research is also exploring the ability of exercise to prevent relapse. Already, it has been shown that women enrolled in a smoking-cessation program were twice as likely to quit successfully if they engaged in regular exercise [18]. Exercise has also been shown to prevent relapse of drug-seeking behavior in rodents. Specifically, in female Wistar rats, cocaine-primed reinstatement was decreased during the experimental phase (extinction or reinstatement) when rats had access to a running wheel and the effect disappeared upon removal of the running wheel [19], and male Sprague Dawley rats with access to a running wheel exhibited decreased cocaine-seeking behavior during both extinction and cue-paired reinstatement sessions [20].
Here, we assess the effect of long-term exercise (six weeks) following cocaine discontinuation on cocaine relapse in rats using a cocaine-primed and a cue-paired reinstatement model [21-23]. Forced daily exercise (motorized treadmill) was used instead of voluntary wheel running in order to control the level of exercise (time of onset, duration, and amount), since we wanted to assess the effects of low versus high exercise exposure. Furthermore, it has been theorized that forced exercise is a better model for humans, as it is performed for a restricted period of time, and forced running also models most humans’ attitude toward exercise; whereas voluntary exercise would more accurately model athletic individuals that would choose to engage in prolonged physical activity (i.e. endurance athletes) [24,25]. In light of this, forced daily exercise is a better model for studying the effects of exercise in recovering addicted subjects who are being encouraged to run as part of the activities of a substance abuse treatment program.
The abstinence phase of the experiment was six weeks long, since we wanted to study the long-term effects of exercise, as had been shown by previous studies [15,17]. To our knowledge, this is the longest exercise exposure tested during a post-cocaine self-administration phase (prior studies limited exercise exposures to 2 weeks) [19].
Reinstatement was examined using both cue- and cocaine-primed reinstatement [1]. Cue-induced reinstatement was used as previously described, where rats were exposed to environmental cues previously paired with cocaine [26]. During cocaine-induced reinstatement, rats received a cocaine priming injection (15 mg/kg i.p.) along with cocaine-paired cues as previously described [21]. This paradigm allowed us to examine the effect of exercise on both pharmacological and environmental factors that contribute to cocaine reinstatement.
2. Materials and methods
2.1. Animals
Male Wistar rats (Taconic, Hudson, NY) at 8 weeks of age were individually housed under standard laboratory conditions at 22.0 ± 2 °C with a 12 h reverse light/dark cycle (lights off: 08:00–20:00 h). Food and water were available ad libitum for the duration of the study, with the exception of the night prior to food training, during which subjects were denied food. All subjects were handled daily. The experiment was conducted in accordance with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals (1996) and Brookhaven National Laboratory Institutional Animal Care and Use Committee.
2.2. Drugs
Cocaine hydrochloride was prepared by dissolving cocaine in 0.9% saline to the desired concentration. Rats received 0.5 mg/kg/infusion intravenously during cocaine training and self-administration periods. For cocaine-primed reinstatement, rats received a 15 mg/kg i.p. cocaine injection.
2.3. Apparatus
2.3.1. Self-administration chambers
Self-administration chambers (Coulbourn Instruments, Allentown, PA) were placed inside sound attenuation cubicles. Each operant chamber (30 cm × 25 cm × 30 cm) consisted of a floor composed of thin metal bars, two metal side walls, two clear plastic front and back walls, and a metal ceiling. On one of the metal walls, there were two levers with a food receptacle in between them and a cue light directly above each lever. The left lever was the active (reinforced) lever and the right the inactive (non-reinforced) lever during the food and drug administration phases. Each box was equipped with infrared locomotor sensors that measured beam breaks (locomotor activity) during the runs. A house light on the side of the operant chamber faced the levers and the food receptacle remained lit throughout the sessions. The cocaine infusion pump was set at a fixed rate of 0.025 ml/s for a 4 s duration. An infusion line attached to a swivel arm entered through the top of the chamber and connected directly to the rat’s catheter. The experimental protocols were programmed and data was collected using Graphic State v3.01 software (Coulbourn Instruments, Allentown, PA).
2.3.2. Treadmill
A custom-made motorized treadmill divided into eight lanes by Plexiglas walls and by a sheet of metal at its end to keep the rats enclosed on the treadmill was used. The dimensions of the running lanes were 56 cm long × 9 cm wide × 31 cm high.
2.4. Procedures
2.4.1. Food training
Prior to surgery, all animals were food-restricted overnight and trained to lever press for food pellets using a fixed ratio (FR1) schedule (Fig. 1). Pressing the active lever resulted in activation of its associated lever light for 2 s and the delivery of a single 40 mg food pellet into the food receptacle. After food delivery, the cue light ceased to illuminate, and a 30 s timeout period followed during which lever presses and locomotor activity were recorded, but no reinforcement was delivered upon pressing the active lever. The inactive lever had no programmed response. Rats were said to have achieved food training criteria if they showed a 95% or greater preference for the active lever in the overnight food training session. All rats achieved this criterion in one session.
Fig. 1.
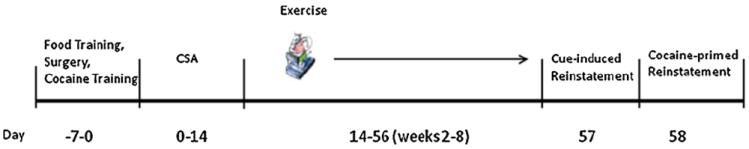
A timeline outlining the sequence of procedures during the study.
2.4.2. Jugular vein catheterization surgery
After reaching food criteria, jugular vein catheterization surgery was performed using a previously described method [27]. Briefly, rats were anesthetized with a 100/10 mg/kg ketamine/xylazine mixture, and an indwelling catheter was implanted in the right external jugular vein. Ketorolac (5 mg/kg) was used as a post-operative analgesic immediately following surgery. Rats recovered for a minimum of 3 days, and a glycerol–heparin solution with cefazolin was infused daily to prevent clotting and infection. Catheter patency was tested 72 h post-operatively using a 10:1 mg/kg ketamine:xylazine solution. Catheters were deemed patent if the rats lost the righting reflex within 3 s. Patency was checked twice weekly during the cocaine self-administration period. Rats that lost patency before completing the self-administration phase of the experiment were excluded from the study.
2.4.3. Cocaine training
After recovery from surgery, rats underwent cocaine training using an FR1 schedule of reinforcement (Fig. 1). Cocaine training sessions were 1 h in duration, during which a single press on the active lever resulted in a 0.5 mg/kg i.v. infusion of cocaine. The cocaine infusion was accompanied by illumination of the lever light, which served as a visual cue, and the sound of the infusion pump, which served as an auditory cue. Each cocaine infusion was followed by a 30 s timeout period, during which pressing the active lever did not infuse cocaine. Once a rat met criteria, it moved on to the cocaine self-administration phase. The criteria was set such that the subject had to press the active lever at least twice as many times as the inactive lever during the session, or was on cocaine training for a maximum of 7 days.
2.4.4. Cocaine self-administration
Following cocaine training, rats underwent a 14-day cocaine self-administration period (Fig. 1). Daily sessions were 6 h in duration and under the same FR1 schedule as in cocaine training.
2.4.5. Cocaine abstinence and exercise regimen
Following cocaine self-administration, all rats were subjected to a six-week abstinence period, during which access to cocaine and cocaine-associated cues were removed (Fig. 1). At this point, rats were randomly assigned to one of the following groups: Low Exercise (LE; n = 13), High Exercise (HE; n = 11) or Sedentary (S; n = 13) group. The LE and HE rats were placed on the treadmill (10 m/min) five days a week for the six-week period. The first day of exercise lasted 10 min and was increased by 10 min daily, until the set duration was reached; 1 h for LE and 2 h for HE. A 10-min break followed every half hour of exercise. In the course of the 6 weeks, the HE rats ran a total of approximately 29.4 km, and the LE rats ran a total of approximately 16.5 km. During this period, the sedentary (S) group was restricted to their cages and received no exercise other than normal cage ambulation.
2.4.6. Reinstatement
Following the cocaine abstinence period, during which exercise was performed (except for the sedentary group), all rats began reinstatement (Fig. 1). Cocaine reinstatement consisted of two 6-h runs in the self-administration apparatus on subsequent days. On the first day of reinstatement (cue-induced reinstatement), rats were placed in the operant chamber as in the past. A single press on the active lever did not result in delivery of cocaine but did deliver the visual (lever light on) and auditory (infusion pump sound) cues. The next day (cocaine-primed reinstatement) was identical to the previous day, except that before the rats were placed in the chamber, they received a 15 mg/kg i.p. cocaine-priming injection.
2.4.7. Data analysis
A series of one-way ANOVAs were performed comparing the three treatment groups for active and inactive lever presses, as well as locomotor activity, on both cue-induced and cocaine-induced reinstatement as compared to the mean of the last three days of cocaine self-administration (maintenance). When appropriate, follow-up pairwise multiple comparisons tests were performed using the Holm–Sidak method (α = 0.05). Pearson Product Moment correlations were performed to assess relationships between cue-induced and cocaine-primed active lever presses during reinstatement, as well as between cue-induced reinstatement active lever presses and locomotor activity during the cocaine-primed reinstatement period. Statistics were performed with the SigmaStat 3.5 statistics program.
3. Results
3.1. Cue-induced reinstatement
3.1.1. Active lever presses
The one-way ANOVA comparing active lever presses by the three treatment groups during cue-induced reinstatement and the maintenance levels was significant [F(3,73) = 7.39; p < 0.001]. Pairwise comparisons found that the increase in lever presses from maintenance to reinstatement levels were significant in the sedentary group (t = 4.32; p < 0.001; Fig. 2), but did not reach significance in LE or HE rats (Fig. 2). However, active lever during cue-induced reinstatement did not differ between the 3 treatment groups, which indicates that while exercise attenuated the increases in lever presses seen in reinstatement over those in maintenance (HE and LE), the effect was not large enough as to show a significant effect when compared with that in the S group.
Fig. 2.
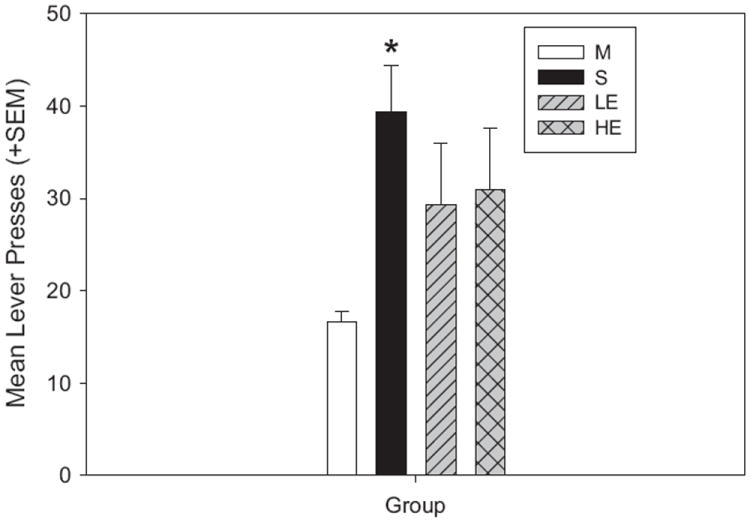
Cue-induced reinstatement: active lever presses vs. maintenance (M). The sedentary group shows a significant increase in active lever presses when compared to M (*p < 0.001). There is no significant increase from maintenance for either of the exercise groups.
3.1.2. Inactive lever presses
A one-way ANOVA revealed no differences between inactive lever presses for each treatment group during cue-induced reinstatement and maintenance levels [F(3,73) = 1.496; p > 0.05], nor between the treatment groups during reinstatement.
3.1.3. Locomotor activity
Similarly, no significant differences in locomotor activity were observed between treatment groups, nor compared to maintenance [F(3,73) = 0.483; p > 0.05)].
3.2. Cocaine-primed reinstatement
3.2.1. Active lever presses
The one-way ANOVA comparing active lever presses by the three treatment groups during cocaine-primed reinstatement and the maintenance levels was significant [F(3,73) = 3.793; p = 0.01; Fig. 3]. Pairwise comparisons revealed that the increase from maintenance to reinstatement was significant only in HE rats (t = 3.291; p < 0.01), not in LE or S rats. Comparisons between groups showed that during reinstatement, HE rats responded more than LE rats (t = 2.681; p < 0.01) but the comparison with the S rats were not significant. There were no differences between LE and S rats.
Fig. 3.
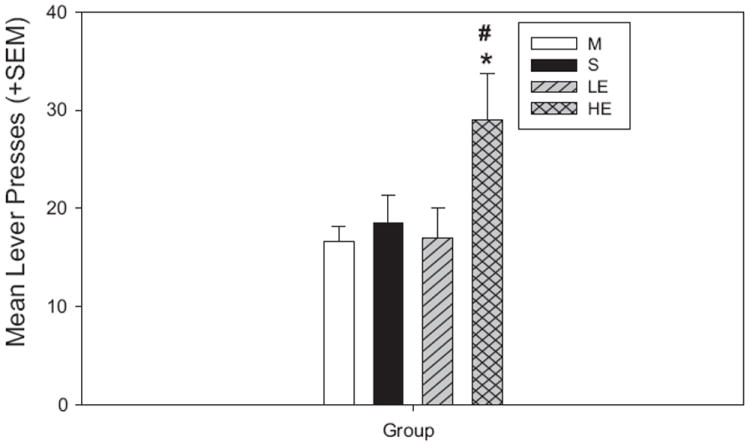
Cocaine-primed reinstatement: active lever presses vs. M. HE rats lever pressed more than during M (*p < 0.01), and also exhibited significantly increased lever pressing compared to LE rats during reinstatement (#p < 0.01).
3.2.2. Inactive lever presses
A one-way ANOVA found no significant differences in inactive lever presses across treatment and compared to maintenance [F(3,73) = 2.546; p > 0.05].
3.2.3. Locomotor activity
A one-way ANOVA showed a significant difference among treatment groups during cocaine-induced reinstatement compared to maintenance [F(3,73) = 5.873; p = 0.001; Fig. 4]. Pairwise comparisons showed that the increase in locomotor activity was significant in S rats (t = 3.861; p < 0.001; Fig. 4), but not in HE and LE rats. However, comparisons between the groups showed no significant differences in locomotor activity during cocaine-induced reinstatement.
Fig. 4.
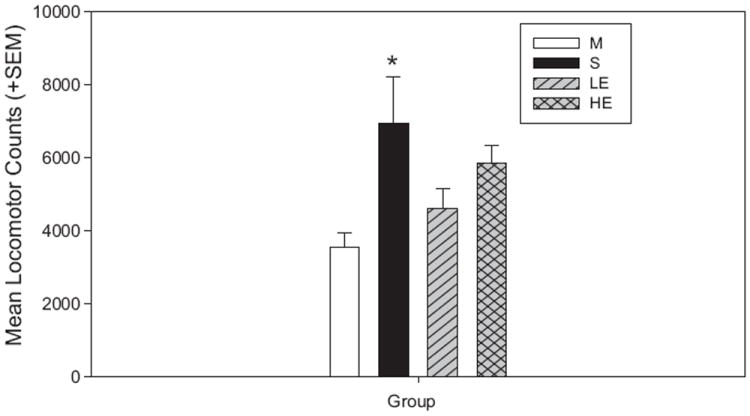
Locomotor activity during cocaine-primed reinstatement. There was a significant increase in locomotor activity for only the S group during reinstatement compared to M (*p < 0.001).
3.3. Correlation analyses
A Pearson Correlation analysis was performed comparing active lever presses during cue-induced and cocaine-primed reinstatement (Fig. 5). The positive significant correlation between these measures approached significance when analyzed across all treatment groups (r = 0.279; p = 0.09), and reached statistical significance within the S (r = 0.555; p = 0.05) and HE (r = 0.779; p < 0.01) groups but not in the LE rats (r = −0.296; p > 0.05).
Fig. 5.
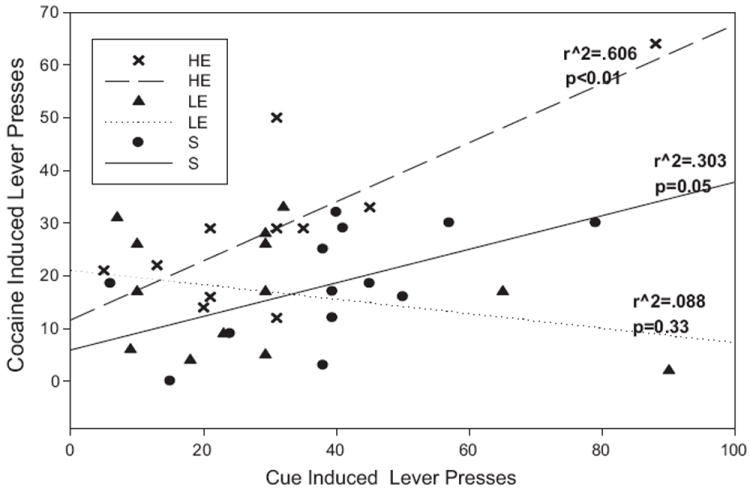
Correlations between number of cue induced active lever presses on the first reinstatement test with cocaine induced active lever presses on the second reinstatement test. There is a significant correlation between cocaine induced and cue induced active lever presses in the HE (p < 0.01) and S (p = 0.05) groups. There was no significant correlation between active lever presses for the two reinstatement tests in the LE group (p = 0.33).
Since both levels of exercise reduced active lever pressing during cue-induced reinstatement and behavioral sensitization (locomotor activity during cocaine-primed reinstatement), a Pearson correlation tested whether there was a relationship between these two measures, which was not significant (p > 0.05).
4. Discussion
Relapse into drug-taking behavior can be triggered by drug-priming, environmental cues, and stress, and these reflect differential involvement of specific brain regions [28-30]. Here, we examined the effects of forced exercise during the abstinence period and show what appeared as opposite effects for cue-induced (attenuated in LE and HE) versus cocaine-primed (HE facilitated) reinstatement.
During cue-induced reinstatement, sedentary rats showed active lever presses that were significantly increased over those seen in maintenance. Whereas lever presses were also increased over maintenance in HE and LE rats, this increase was not significant. Hence, it appears as if exercise may have attenuated the increase in active lever presses to a similar extent in the high and the low exercise treated rats. These results are consistent with prior findings, which found that exercise decreased cue-paired cocaine reinstatement [20], and that voluntary exercise beginning in adolescence and continuing throughout cocaine self-administration, extinction, and reinstatement decreased cue-induced reinstatement [31].
During cocaine-primed reinstatement, sedentary and LE rats did not show a difference in active lever presses compared to maintenance levels. As was seen for cue-induced reinstatement, one might also expect an increase in lever presses from maintenance during cocaine-primed reinstatement due to cocaine incubation [32]. While this lack of incubation might be explained in the LE group as an effect of exercise treatment, one would still expect to see an increase in the S group. We hypothesize that the cue-induced reinstatement session that occurred on the day prior may have interfered with the subsequent drug-primed reinstatement. Previous studies that tested cue-induced and drug-primed reinstatement in the same rats exposed subjects to extinction sessions prior to each type of reinstatement [22,31], one of which also re-established cocaine self-administration before the second reinstatement test [31].
Contrastingly, HE rats showed a significant increase in active lever responses compared to maintenance levels and to LE rats during this reinstatement session, suggesting that this relatively high level of exercise facilitates cocaine-primed relapse. This effect is opposite of that seen with cue-induced reinstatement, which is likely to reflect the differential effects of exercise on unique neuro-biological substrates that underlie cue versus drug-induced relapse [26,33-36]. Additionally, there was a positive correlation between cocaine-induced and cue-induced active lever presses in the HE and S groups. This may point to a shared or parallel mechanism in the two types of reinstatement in these groups. On the other hand, this result was not shared in the LE group; which implies that that the low exercise dose may disrupt this mechanism in some way.
It is generally accepted that pathways of both cue-induced and cocaine-primed reinstatement converge on the dorsolateral pre-frontal cortex (dPFC), particularly the anterior cingulate cortex, which sends glutamatergic projections to the nucleus accumbens [36]. These two modes of reinstatement diverge, however, in their innervation of the anterior cingulate cortex, which may explain the differential effects of exercise that we observed. Cue-induced reinstatement, but not cocaine-primed reinstatement, is mediated by dopaminergic innervation of the basolateral amygdala (acting on D1 receptors) from the VTA, which then subsequently signals to the anterior cingulate [35,36]. It has been found that a similar treadmill running regimen (4 weeks, 5 days per week, 60 min/day, at a speed from 9 to 16 m/min) increases dendritic arborization, spine density, and expression of proteins involved in neurotransmission and synaptic plasticity (TrkB, SNAP-25, Syt I, and Syt IV) in the basolateral amygdala compared to sedentary rats [34]. Therefore, further investigation is warranted into the possible role of exercise-induced changes in the basolateral amygdala on the inhibition of cue-paired cocaine reinstatement.
Rats show individual variability in the incentive motivation responses to cues previously paired with reward [37-39], that predicts their propensity toward addictive behavior [37]. Cocaine-paired cues increased reinstatement in a subset of animals [37]. Recently, it was shown that in some rats, a reward (food) associated cue can become desirable in and of itself (“sign trackers”), whereas in other rats (“goal trackers”), the cue by itself is not desirable, and these rats go directly to the location where the reward is to be dispensed [39]. DA in nucleus accumbens was associated with the acquisition of sign tracking but not goal tracking [39]. Since there are known effects of exercise on DA [5,6,9,10], it is therefore possible that these DA changes may contribute to the exercise-mediated attenuation of cue-induced reinstatement.
The pathway of drug-primed cocaine reinstatement, on the other hand, involves the VTA sending dopaminergic projections directly to the dPFC (anterior cingulate and NAc shell). It has also been shown that glutamate activating DA neurons in the VTA is an important factor in cocaine primed reinstatement [40]. It is known that exercise has a strong effect on dopaminergic neurons in the VTA. It has been shown that six weeks of access to a running wheel increases levels of tyrosine hydroxylase mRNA in the VTA [41]. It has also been shown that forced exercise facilitates recovery of DA neurons in mice that were subjected to MPTP lesioning [42] The precise effect of exercise on glutamate in the VTA is to our knowledge unexplored and is an interesting topic for future research.
Both levels of exercise not only inhibited cue-induced reinstatement, but also sensitization to cocaine, as exercise blocked the increase in locomotor activity from maintenance to cocaine-primed reinstatement that was observed in sedentary animals. It should be noted that exercise did not affect cocaine-induced locomotion per se, as activity levels during cocaine-primed reinstatement were comparable to those exhibited during the maintenance period of cocaine self-administration. To our knowledge, this is the first evidence that exercise attenuates the sensitized locomotor-activating response of acute cocaine administration following abstinence. This raises the question of whether there may be a shared mechanism modulating both of these effects. One possible mechanism involves the adrenergic system, aspects of which have been shown to mediate cocaine reinstatement [33,43], as well as the development and expression of cocaine sensitization [44,45]. Clonidine, an alpha2 adrenergic agonist, blocks cue-induced reinstatement (but not cocaine-primed reinstatement) [33,43], retards the development of cocaine sensitization, and inhibits the expression of cocaine sensitization during reinstatement [44]. Additionally, yohimbine, an alpha2 adrenergic antagonist, facilitates and potentiates cue-induced cocaine reinstatement, but does not block sensitization [46,47]. Chronic forced exercise was shown to increase: noradrenalin levels [48,49]; in the pons and medulla [50]; nor-adrenaline turnover (which would lead to noradrenaline receptor down-regulation) [51]; and endothelial alpha-2adrenergic receptor binding affinity and response to clonidine [52]. Furthermore, chronic treadmill exercise also decreased affinity of α2 adrenoreceptors while increasing affinity of vasopressin receptors in the nucleus tractus solitarius [53]. The effect of treadmill exercise on α2 adrenergic receptors in relevant brain regions remains to be explored, as does this possible link to blocking cue-induced reinstatement and cocaine sensitization. There was not, however, a significant correlation between active lever presses during cue-induced reinstatement and locomotor activity during cocaine-primed reinstatement, suggesting that these two effects may reflect distinct mechanisms.
In comparing the findings from previous studies that evaluated the effects of exercise on cocaine reinstatement, there are important methodological differences that are likely to affect the results obtained such as type of exercise (voluntary or forced), timing and duration of the exercise, and the reinstatement paradigm used.
4.1. Forced vs. voluntary exercise
Prior studies have mostly used a voluntary wheel running exercise in the rats’ home environment [19,20], whereas the present study utilized a forced treadmill exercise paradigm. These two exercise paradigms are significantly different and may render different results [25]. Leasure and Jones compared forced exercise with voluntary exercise and reported that rats exposed to a very high level of forced exercise (20 m/min for 150 min/day) showed increased anxiety, as measured by an open-field test and increased defecation; however, the paradigm used was of greater intensity than the one used in the present study in time (120 min) and speed (10 m/min) [25]. Furthermore, Leasure and Jones also reported that there was no difference between voluntary and forced exercise groups as measured by a fecal corticosterone assay which is a standard physiological measure of stress [25].
An advantage of the treadmill exercise method is the ability to precisely regulate the intensity and duration of the exercise exposure. In our study, this allowed us to gauge the effect of two different exercise levels on cocaine reinstatement. In contrast, wheel running produces great inter subject variability in levels of exercise and is affected by differences in motivation.
Finally, as stated previously, forced exercise is preferable for this particular experimental design because it would be a more accurate representation of the sort of exercise effect that would be expected from aerobic exercise that is utilized in a cocaine rehabilitation program. Cocaine addicts tend to be non-athletic individuals who would not engage in physical exercise without some form of coercion. It is postulated that forced exercise is a better model for non-athletic individuals who are being made to exercise; where as voluntary exercise is a more accurate model for athletic individuals who take pleasure in prolonged physical activity [25] (Leasure, 2008 #19778).
4.2. Exercise design
Previous studies have treated rats with voluntary exercise before cocaine self- administration [43], during cocaine self-administration [43], during extinction [19,43] or abstinence [20], and during reinstatement [19,20,31]. Also, the duration of exercise varies greatly among these studies. Lynch et al. (2010) used 2 weeks of voluntary exercise during extinction; Smith et al. (2011) used 12 weeks of voluntary exercise throughout the course of the whole study; and Zlebnik et al. (2010) used 2–3 weeks of voluntary exercise. The present study treated the rats to treadmill forced exercise only during the 6 weeks of abstinence. This was done to model the clinical scenario where most addicts do not typically exercise while consuming drugs, and the initiation of exercised is linked with their participation in a treatment program during abstinence.
4.3. Reinstatement paradigm
Studies differ in the reinstatement paradigm used. Some tested exercise effects on cocaine cue-induced reinstatement [20,31], while others examined exercise effects on cocaine-primed reinstatement [19,31]. The present study examined both, thereby allowing us to identify a differential pattern within rats. In the same groups, we found that HE increased lever pressing during cocaine-induced reinstatement, whereas both LE and HE attenuated the increases in lever pressing during cue-induced reinstatement.
In conclusion, the present study showed that moderate (1 h/day) treadmill exercise may be protective against cue-induced cocaine reinstatement and attenuates cocaine-induced locomotor activation. On the other hand, vigorous (2 h/day) exercise can lead to increases in cocaine induced reinstatement. These findings provide evidence of the complex nature of cocaine addiction and the effects of physical exercise on reinstatement. These and other data are encouraging and point to the potential benefits of aerobic exercise (although care is advisable in its application), while also pointing to the need for further research into the specific mechanisms of action of exercise with respect to cocaine relapse.
HIGHLIGHTS.
-
►
Daily treadmill exercise may be protective against cue-induced cocaine reinstatement.
-
►
Daily treadmill exercise attenuates cocaine-induced locomotor activation.
-
►
Potential benefits of aerobic exercise with respect to cocaine relapse.
Acknowledgments
This work was supported by the NIAAA (AA 11034 and AA07574, AA07611). We also thank the SULI and IRTA programs for partial support of LSR.
References
- 1.Anker JJ, Carroll ME. Adolescent nicotine exposure sensitizes cue-induced reinstatement of cocaine seeking in rats bred for high and low saccharin intake. Drug and Alcohol Dependence. 2011;118(1):68–72. doi: 10.1016/j.drugalcdep.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 2.McCloskey DP, Adamo DS, Anderson BJ. Exercise increases metabolic capacity in the motor cortex and striatum, but not in the hippocampus. Brain Research. 2001;891(1–2):168–75. doi: 10.1016/s0006-8993(00)03200-5. [DOI] [PubMed] [Google Scholar]
- 3.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. Journal of Cerebral Blood Flow and Metabolism. 1992;12(1):110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 4.Vissing J, Andersen M, Diemer NH. Exercise-induced changes in local cerebral glucose utilization in the rat. Journal of Cerebral Blood Flow and Metabolism. 1996;16(4):729–36. doi: 10.1097/00004647-199607000-00025. [DOI] [PubMed] [Google Scholar]
- 5.MacRae PG, Spirduso WW, Cartee GD, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neuroscience Letters. 1987;79(1–2):138–44. doi: 10.1016/0304-3940(87)90686-0. [DOI] [PubMed] [Google Scholar]
- 6.MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology. 1987;92(2):236–40. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- 7.Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Research Bulletin. 1994;35(1):41–9. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 8.Freed CR, Yamamoto BK. Regional brain dopamine metabolism: a marker for the speed, direction, and posture of moving animals. Science. 1985;229(4708):62–5. doi: 10.1126/science.4012312. [DOI] [PubMed] [Google Scholar]
- 9.Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, et al. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Research. 2010;1310:200–7. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 10.Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. Journal of Neuroscience Research. 2004;77(3):378–90. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Archives of Neurology. 2007;64(11):1575–9. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 12.Hair EC, Park MJ, Ling TJ, Moore KA. Risky behaviors in late adolescence: co-occurrence, predictors, and consequences. Journal of Adolescent Health. 2009;45(3):253–61. doi: 10.1016/j.jadohealth.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Zweig JM, Phillips SD, Lindberg LD. Predicting adolescent profiles of risk: looking beyond demographics. Journal of Adolescent Health. 2002;31(4):343–53. doi: 10.1016/s1054-139x(02)00371-3. [DOI] [PubMed] [Google Scholar]
- 14.Johnston L. Lower rates of cigarette and marijuana smoking among exercising teens. National Network. 2009;22(4) [Google Scholar]
- 15.Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, et al. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behavioural Brain Research. 2010;215(1):77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacology Biochemistry and Behavior. 2002;73(3):663–71. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 17.Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug and Alcohol Dependence. 2008;98(1–2):129–35. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine and Tobacco Research. 2005;7(6):871–80. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- 19.Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209(1):113–25. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biological Psychiatry. 2010;68(8):774–7. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Featherstone RE, Burton CL, Coppa-Hopman R, Rizos Z, Sinyard J, Kapur S, et al. Gestational treatment with methylazoxymethanol (MAM) that disrupts hippocampal-dependent memory does not alter behavioural response to cocaine. Pharmacology Biochemistry and Behavior. 2009;93(4):382–90. doi: 10.1016/j.pbb.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, et al. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behavioural Brain Research. 2009;202(2):238–44. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson EB, Carroll ME. Wheel running as a predictor of cocaine self-administration and reinstatement in female rats. Pharmacology Biochemistry and Behavior. 2005;82(3):590–600. doi: 10.1016/j.pbb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Research. 2006;1104(1):64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 25.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–65. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Smith RJ, Aston-Jones G. alpha(2)Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biological Psychiatry. 2011;70(8):712–9. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attentuates cocaine self administration in rats. Synapse. 2008;62(5):481–6. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. Journal of Psychiatry and Neuroscience. 2000;25(2):125–36. [PMC free article] [PubMed] [Google Scholar]
- 29.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neuroscience and Biobehavioral Reviews. 2003;27(5):457–91. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 30.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 31.Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug and Alcohol Dependence. 2011;121(1–2):54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in Neurosciences. 2011;34(8):411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35(11):2165–78. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin TW, Chen SJ, Huang TY, Chang CY, Chuang JI, Wu FS, et al. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiology of Learning and Memory. 2012;97(1):140–7. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 35.See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology. 2001;154(3):301–10. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- 36.Kalivas PW, M K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 37.Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biological Psychiatry. 2010;67(8):730–6. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behavioural Brain Research. 2011;220(1):238–43. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177(3):315–23. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- 41.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural Brain Research. 2011;217(2):354–62. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad SO, Park JH, Stenho-Bittel L, Lau YS. Effects of endurance exercise on ventral tegmental area neurons in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid-treated mice. Neuroscience Letters. 2009;450(2):102–5. doi: 10.1016/j.neulet.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 43.Smith RJ, Aston-Jones G. alpha(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biological Psychiatry. 2011;70(8):712–9. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimenez-Rivera CA, Feliu-Mojer M, Vazquez-Torres R. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Annals of the New York Academy of Sciences. 2006;1074(1):390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- 45.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, et al. a1b-Adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. The Journal of Neuroscience. 2001;7(22):2873–84. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology. 2011;216(1):53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioural Brain Research. 2006;174(1):1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 48.Brown BS, Payne T, Kim C, Moore G, Krebs P, Martin W. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. Journal of Applied Physiology. 1979;46(1):19–23. doi: 10.1152/jappl.1979.46.1.19. [DOI] [PubMed] [Google Scholar]
- 49.Chaouloff F, Laude D, Elghozi JL. PHysical exercise: evidence for differential consequences of tryptophan on 5-HT synthesis and metabolism in central serotonergic cell bodies and terminals. Journal of Neural Transmission. 1989;78(2):121–30. doi: 10.1007/BF01252498. [DOI] [PubMed] [Google Scholar]
- 50.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Medicine and Science in Sports and Exercise. 1997;29(1):63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Kastello GM, Sothmann MS. Brain norepinephrine changes with simulated weightlessness and relation to exercise training. Physiology and Behavior. 1999;66(5):885–91. doi: 10.1016/s0031-9384(99)00030-x. [DOI] [PubMed] [Google Scholar]
- 52.Chen HI, Cheng SY, Jen CJ. Chronic exercise enhances vascular responses to clonidine in rats by increasing endothelial alpha2-adrenergic receptor affinity. Chinese Journal of Physiology. 1999;42(2):61–6. [PubMed] [Google Scholar]
- 53.De Souza CG, Michelini LC, Fior-Chadi DR. Receptor changes in the nucleus tractus solitarii of the rat after exercise training. Medicine and Science in Sports and Exercise. 2001;33(9):1471–6. doi: 10.1097/00005768-200109000-00008. [DOI] [PubMed] [Google Scholar]


