Abstract
Alzheimer’s disease (AD) brain is marked by severe neuronal death which has been partly attributed to increased oxidative stress. The pathophysiology accounting for this free radical injury is not well-delineated at this point, but one hypothesis is that a derangement in transition metal metabolism contributes to the process. We tested the hypothesis that peripheral derangement of transition metal metabolism is present early in the dementing process. We analyzed non-heme iron and copper levels in serum from subjects with normal cognition, mild cognitive impairment, and early stage senile dementia and followed these subjects over 5 years. An increase in the ratio of serum copper to non-heme iron levels predicted which subjects with mild cognitive impairment would progress to dementia versus those that would remain cognitively stable. This increase did not correlate with changes in expression of iron regulatory protein 2 or selected downstream targets in peripheral lymphocytes. A cDNA-based microarray (IronChip) containing genes relevant to iron and copper metabolism was used to assess transition metal metabolism in circulating lymphocytes from cognitively normal and demented subjects. No gene was identified as being dysregulated more than 2-fold, and verification using quantitative RT-PCR demonstrated no significant changes in expression for ALAS2, FOS, and CTR1. The increased ratio of serum copper to serum iron prior to dementia has potential as a biomarker for cognitive decline and mirrors other changes in serum previously reported by others, but iron and copper metabolism pathways appear to be broadly unaffected in peripheral blood in AD.
Keywords: ALAS, CTR1, FOS, IronChip, oxidative stress, biomarker, iron, copper, Alzheimer’s disease
INTRODUCTION
An impressive body of literature has emerged implicating oxidative stress as a factor in the profound neuronal death associated with Alzheimer’s disease (AD). The sources of this oxidative stress have been the subject of considerable debate and the subject remains contentious. Oxidative species originating outside of the central nervous system (CNS) may impact the cells of the CNS. While the literature on this subject is extensive and in many cases contradictory, several recent studies have demonstrated an increase in circulating levels of various peroxidized lipid species, and decreased anti-oxidant defense systems (particularly glutathione peroxidase and superoxide dismutase) in AD serum and serum from subjects characterized as mild cognitive impairment (MCI) [1–3]. Additionally, evidence of increased oxidative damage in serum was found in subjects with the APOE ε4 allele, a well-known risk-factor for AD [2]. Further supportive evidence was presented in a recent prospective study which demonstrated that markers of oxidative stress and reduced cellular anti-oxidative responses in the peripheral circulation predicted which subjects with MCI would progress to dementia versus those which would remain cognitively stable over time [4]. This issue is complicated by studies reporting contradictory findings including one which demonstrated significantly reduced levels of malondialdehyde, a marker of lipid peroxidation, in AD serum [5]. Furthermore, while it is possible that toxic oxidative species in the periphery are causally linked to the neuropathology of AD, these peripheral changes may in fact be an effect of CNS pathologies (or may be an epiphenomenon unrelated entirely). Nevertheless, identifying the potential source(s) of oxidative changes in the periphery is likely a key step in understanding how to effectively intervene in the process of neurodegeneration and may help explain the heterogeneity seen in levels of oxidative markers observed by various groups. Dysregulated transition metal metabolism in the periphery could conceptually account for the oxidative changes observed in blood in the setting of AD.
Increased total and free (non-protein bound) copper levels have been shown to correlate inversely with cognitive function in elderly patients [6, 7]. While the levels of copper reported in serum from AD patients are markedly heterogeneous between studies, a recent meta-analysis found that the data in aggregate suggest copper levels are increased [8]. Iron levels in serum (particularly non-heme iron levels) are less well-described. Nevertheless, changes in bulk metal levels and/or liganding could result in increased Fenton reactivity catalyzing free radical generation.
In this study, we evaluated the degree to which iron and copper homeostasis were altered in serum and lymphocytes from subjects with MCI and AD to determine if a generalized dysregulation of transition metal metabolism could be detected in blood. The subjects were followed for a period of five years enabling comparison between a group of MCI subjects that remained cognitively stable and a group that progressed from MCI to dementia of the Alzheimer’s type. We measured iron and copper parameters from both serum and whole blood, including serum metal levels and expression of a major iron/copper regulatory gene, IRP2, and selected downstream targets. Finally, a gene microarray analyzing 536 genes relevant to iron and copper metabolic pathways (IronChip) was used to broadly analyze any alteration in the handling of these transition metals between the disease states.
MATERIALS AND METHODS
The study design and manuscript preparation were done in accordance with the Code of Ethics of the World Medical Association and the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. Subjects participating in this study did so with informed consent (and/or with the informed consent of appropriate surrogates) and with the approval of the Loma Linda University Medical Center Institutional Review Board.
Serum iron and copper analysis
Subjects were enrolled in the study from a community-based recruitment as previously reported [9, 10]. Briefly, individuals with subjective memory complaints were screened and those classified as MCI or early AD were enrolled in the study. Patients with a history of smoking, diabetes, head trauma, known cerebrovascular disease, or uncontrolled hypertension were excluded from the study. The diagnosis of MCI was applied by Mayo Clinic criteria including confirmation of an isolated memory complaint on neuropsychological profiling (Clinical Dementia Rating (CDR) = 0.5) in subjects with otherwise normal cognition capable of performing normal activities of daily living. A cohort of (approximately age-matched) neurologic controls was also enrolled. Controls were without subjective memory complaints and within normal limits on neuropsychological testing consisting of a CDR score of 0. A sub-group of the larger cohort previously described was available to participate in this study; 60 total cases enrolled including 18 subjects with MCI, seven of whom progressed to dementia, 19 subjects with very early dementia (materials collected at the time of initial conversion from MCI to AD or within two years) and 19 approximately age and gender-matched neurologic control subjects (several controls were included who had a maximum of one neuropsychological assessment as MCI, but ended the trial as neurological controls). Materials were not available from all cases for each component of this study; cases included in individual analyses are detailed in Table 1. Additionally, one subject was included only in the longitudinal data (Fig. 1) who was enrolled as a neurologic control and progressed to MCI and later to dementia; this is the only control subject in the cohort to have progressed in this way. All participants consented to detailed, serial neuropsychological testing, blood donation, and neuroimaging (to assess for cerebral amyloid angiopathy by the Boston criteria using susceptibility weighted imaging at 3T field strength). Serum collection and cognitive evaluations were performed at approximately six month intervals and neuroimaging was conducted approximately yearly over the five year span of the study. Blood was collected from subjects at appointments for neuropsychological screening. For the isolation of serum, blood specimens were immediately placed on ice, allowed to clot overnight at 4°C, and centrifuged at 1800 g, 4°C for 10 min to remove cellular elements and the majority of clotting factors. For non-heme iron analysis, iron was extracted from serum as described before [11] using ethylenediaminetetraacetic acid (EDTA) and trichloroacetic acid (TCA). Specifically, 20 µL serum from each patient and time point was added to 360 µL 500 µM EDTA and 20% TCA and incubated at 90°C for 30 min. After cooling to room temperature for 10 min, 700 µL 500 µM EDTA was added, samples were vortexed and subsequently centrifuged at 13,000 g for 10 min at room temperature. The supernatant was collected as the non-heme iron component of serum and stored at −80°Cuntil analysis. Non-heme iron was measured to limit the impact of trace hemolysis during blood collection and clotting. Because of the substantial iron stores in hemoglobin and the relatively low levels of iron in serum, even trace hemolysis can result in distorted data (data not shown). For total copper analysis, specimens were wet ashed as previously described [12]. Briefly, 50 µL serum was added to 300 µL 30% nitric acid and allowed to incubate overnight. The resulting solution was heated to 80°C for 20 min, then allowed to cool to room temperature for 10 min. Hydrogen peroxide was then added to dissolve lipid components (300 µL of 10% solution). The samples were allowed to cool for 30 min at room temperature and were then heated to 70°C for 15 min and allowed to cool. Atomic absorption spectra were acquired on a Varian SpectrAA 220Z graphite furnace atomic absorption spectrometer. The standard curve was produced from 25, 50, 75, and 100 parts per billion solutions of standardized iron or copper in 1% nitric acid (Arcos Organics). The instrument was zeroed to a maximum of 0.005 mean absorbance. Samples were diluted 1 : 10 for iron analysis and 1 : 5 for copper. All values reported were acquired as the mean of two to four measurements.
Table 1.
Demographics
| Diagnosis |
n (Males/Females) |
Age (±SD) | Time to progression |
|---|---|---|---|
| Figure 1 | |||
| Control | 19 (10/9) | 75.6±4.0 | n/a |
| Stable MCI | 8 (4/4) | 78.7±6.2 | n/a |
| Progressive MCI | 7 (2/5) | 82.7±1.7 | 14.4 months |
| Early AD | 14 (5/9) | 80.6±2.7 | n/a |
| Figure 2 | |||
| Control | 20 (11/9) | 75.2±4.7 | n/a |
| Stable MCI | 10 (5/5) | 77.6±5.4 | n/a |
| Progressive MCI | 6 (2/4) | 82.8±3.9 | 10.3 months |
| Early AD | 15 (5/10) | 80.1±2.7 | n/a |
| Figure 3 | |||
| Control | 14 (7/7) | 76.4±4.8 | n/a |
| Early AD | 12 (6/6) | 81.7±4.8 | n/a |
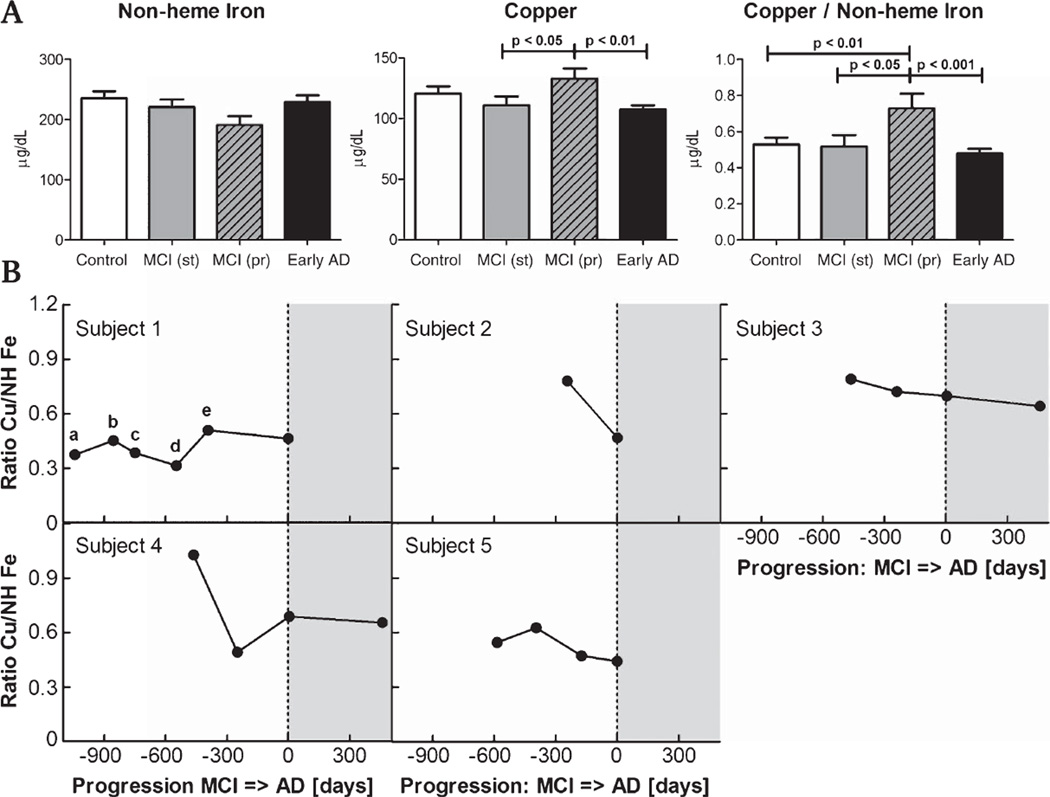
Fig. 1.
Serum non-heme iron and copper levels are altered in progressive MCI subjects. A) Copper levels are increased compared to early AD and MCI stable for at least five years (p < 0.01 and p < 0.05, respectively). The ratio of copper to non-heme iron levels in serum clearly separated progressive MCI subjects from all other groups, indicating a specific effect associated with cognitive decline (p < 0.01, p < 0.05, p < 0.005 for controls, stable MCI and AD, respectively; for n see Table 1). B) In five subjects who eventually developed AD (4 enrolled with MCI, case 5 enrolled as a neurological control), longitudinal data were collected. The maximum value of the copper/iron ratio occurred in the MCI stage for all subjects and significantly declined with cognitive decline (p < 0.05 between time of progression and 1 year prior). The peak in the copper/iron ratio appears to be transient. (gray areas = AD; subject 1 neurocognitive status: a control, b control, c MCI, d control, e MCI).
RNA extraction from whole blood
Blood was collected from subjects in tubes containing EDTA as an anticoagulant. Whole blood RNA was extracted using the QIAamp RNA Mini Kit (Qiagen) following the manufacturer’s protocol. The isolate was suspended in 60 µL ddH20 and stored at −80°C until analysis. Two micrograms of total RNA from each sample was allocated for microarray analysis, the remainder was retained for RT-PCR.
cDNA microarray–IronChip
A total 26 subjects were analyzed, including 12 early AD subjects and 14 age- and gender-matched neurological controls. A 2 µg sample of total RNA isolated from whole blood was allocated for microarray analysis from each subject. The cDNA-based microarray, IronChip, detects alterations in gene expression related to iron and copper metabolism. A total of 536 genes were analyzed concurrently with this technique, as previously described [13]. Because the IronChip is a two-color array, normal samples were pooled and analyzed against each AD case. Because this technique did not assess the variance in control samples, the variance for each gene in the AD cases was used as an estimate of variance for controls to enable statistical interpretation of the data. To be considered a gene of interest, a difference in mRNA expression had to be detected for 50% or more of the AD cases, with mean fold difference of 1.3 or greater.
Quantitative RT-PCR
The iron regulatory protein IRP2, and a downstream target DMT1 (IRE and non-IRE forms) were prospectively chosen for analysis by qRT-PCR. Additionally, CTR1, ALAS2, and FOS were selected from the IronChip microarray as genes of interest and their mRNA expression levels were verified by RT-PCR. The stability of a panel of six potential housekeeping genes was tested (beta actin, GAPDH, RPL32, HPRT1, HMBS, UBC) in control, MCI and AD patient samples using the BestKeeper software [14]. Based on this analysis, the expression levels of all target genes were normalized by the geometric mean of HPRT1, RPL32, and HMBS. Primers for each gene were designed and are shown in Table 2. Gene expression was determined using a fluorescent probe SYBR green and a BioRad thermocycler according to standard protocols.
Table 2.
Primer pairs
| RPL32 F: 5′-CAT CTC CTT CTC GGC ATC A-3′ R: 5′- AAC CCT GTT GTC AAT GCC TC -3′ |
| HPRT1 F: 5′-GAC CAG TCA ACA GGG GAC AT-3′ R: 5′- AAC ACT TCG TGG GGT CCT TTT C -3′ |
| HMBS F: 5′-ACC AAG GAG CTT GAA CAT GC-3′ R: 5′- GAA AGA CAA CAG CAT CAT GAG -3′ |
| IRP2 F: 5′-CAA AGC ACC TCA GGC AAG TAG G-3′ R: 5′- TGT CAA CAG GGA AAA AGC -3′ |
| DMT1 F: 5′-TCT ACT TGG GTT GGC AAT GTT T-3′ R: 5′- ACA CAC TGG CTC TGA TGG CTA C -3′ |
| DMT1-nonIRE F: 5′-GTG GTG GCT GCT GTG GTCA-3′ R: 5′- TCA ATC CCA GAT GGC ACG TAT -3′ |
| ALAS2 F: 5′-CTG CCA GGG TGC GAG ATT-3′ R: 5′- TTG GCT CCA CTG TTA CG -3′ |
| CTR1 F: 5′-TCA CCA TCA CCC AAC CAC TT-3′ R: 5′- TCT TAA AGC CAA AGT AGA AGG TCA -3′ |
| FOS F: 5′-GGC AAG GTG GAA CAG TTA TCT C-3′ R: 5′- CCG CTT GGA GTG TAT CAG TCA G -3′ |
Statistical analysis
Serum iron and copper data are presented as means ± SEM. Normality of the data was determined by the Shapiro-Wilk test. Following an ANOVA (non-parametric Kruskal-Wallis test for not normally distributed data) posthoc analysis of the statistically significant findings was performed using Student’s t-test. A p < 0.05 was considered as significant observation. For longitudinal data, datapoints nearest to one year prior to progression were compared to data from the time of progression by the paired Student’s t test. Quantitative RT-PCR results were normalized by the geometric mean of three housekeeping genes (HPRT1, RPL31, HMBS) and analyzed using the REST2009 software [15] using respective reaction efficiencies and 2000 iterations. Data are shown using whisker-box plots with the top and bottom error bars representing the upper and lower 25% of observations, the box representing the middle 50% of observations and the bar within the box representing the median. A p < 0.05, as estimated by the REST2009 software, was used to indicate significance of the findings.
RESULTS
Serum iron and copper levels
Because trace hemolysis is a significant source of contaminant iron in serum samples, non-heme iron was measured. No significant difference in serum non-heme iron levels was found between any of the sample groups (Fig. 1). Sub-group analysis for males and females separately also failed to demonstrate any significant differences between groups (not shown). Although the presence of cerebral amyloid angiopathy has previously been shown to correlate with increased iron levels in brain [12], no change in serum iron level was associated with the presence of radiological evidence of cerebral amyloid angiopathy (data not shown). Total copper levels were increased in MCI subjects who progressed to dementia compared to those that remained cognitively stable (p < 0.05) and early AD subjects (p < 0.01, Fig. 1). Because iron and copper metabolism are intricately linked [16], we also assessed the ratio of copper to non-heme iron. This analysis demonstrated that MCI subjects who progressed to dementia had a higher index of total copper to non-heme iron than the control group, the MCI subjects who were cognitively stable over five years, and subjects demonstrating early AD (p < 0.01, p < 0.05, p < 0.001, respectively; Fig. 1). Serum samples were available at multiple time-points from four progressive MCI cases which are shown in Fig. 1. Additionally, one case (case 5 in the figure) enrolled in the cohort as a control and progressed to dementia. These cases qualitatively demonstrate that the increase in the ratio of copper to non-heme iron is transient. The highest value of the ratio in each case occurred at MCI. Paired analysis between the time point closest to one year prior to decline and the measurement taken at the time subjects first met criteria of AD demonstrated a significant decrease (p < 0.05).
IRP2 and DMT1 expression in lymphocytes
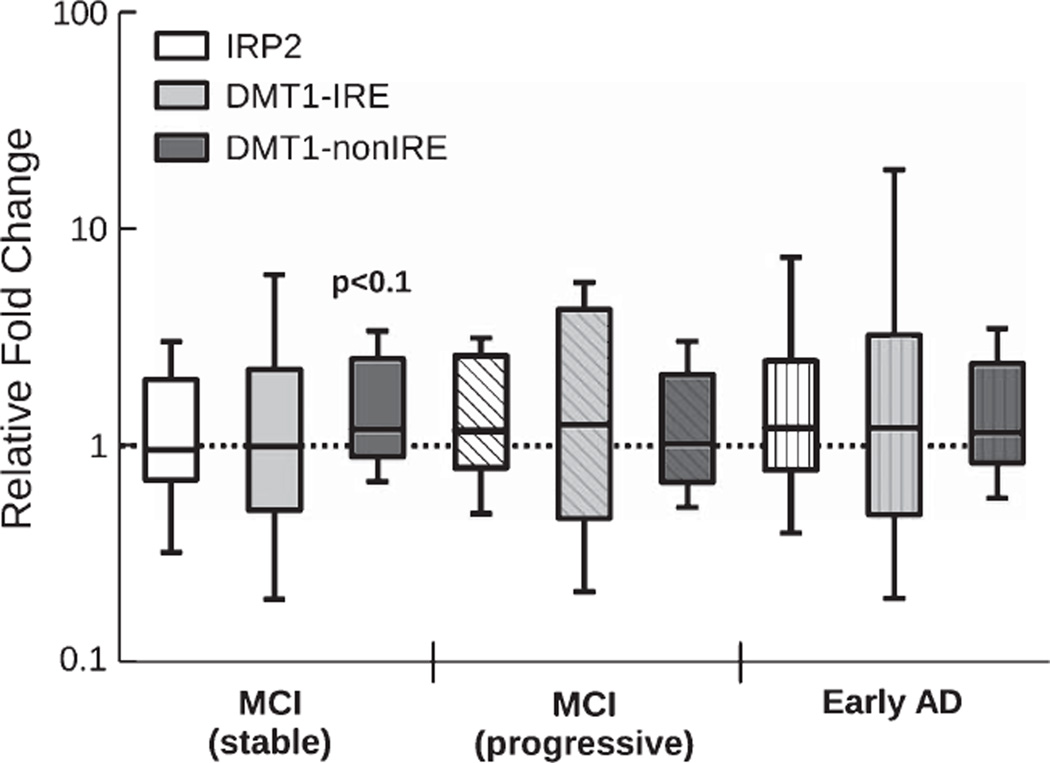
To assess the biological background to the observed alteration of serum iron and copper in progressive MCI subjects, mRNA expression of IRP2 and DMT1 was measured in circulating lymphocytes. DMT1 is a major transporter of divalent metal species and exists in two isoforms—one that contains an iron responsive element in the untranslated region and would be expected to be regulated by IRP2 (DMT1-IRE) and a second that lacks an IRE and should be regulated independently of IRP2 levels (DMT1-nonIRE). We only found a minor increase in expression of DMT1-nonIRE compared to normal controls (1.2 fold increase, p < 0.1; Fig. 2), while DMT1-IRE and IRP2 mRNA expression remained unchanged. This suggests that the extracellular alteration in the ratio of iron and copper does not induce significant alterations of intracellular iron/copper regulation when cells are directly exposed to this change in transition metal environment in the circulatory system.
Fig. 2.
IRP2 and DMT1 expression are largely unchanged in stable MCI, progressive MCI as well as early AD subjects. Only DMT1-nonIRE, which is not controlled by IRP2, is slightly elevated in MCI subjects that were cognitively stable for at least five years (1.2 fold increase, p < 0.1). Cumulatively, these data suggests that the iron regulatory axis remained largely intact in lymphocytes of pre-dementia and early dementia patients. (For n see Table 1).
IronChip microarray and gene validation
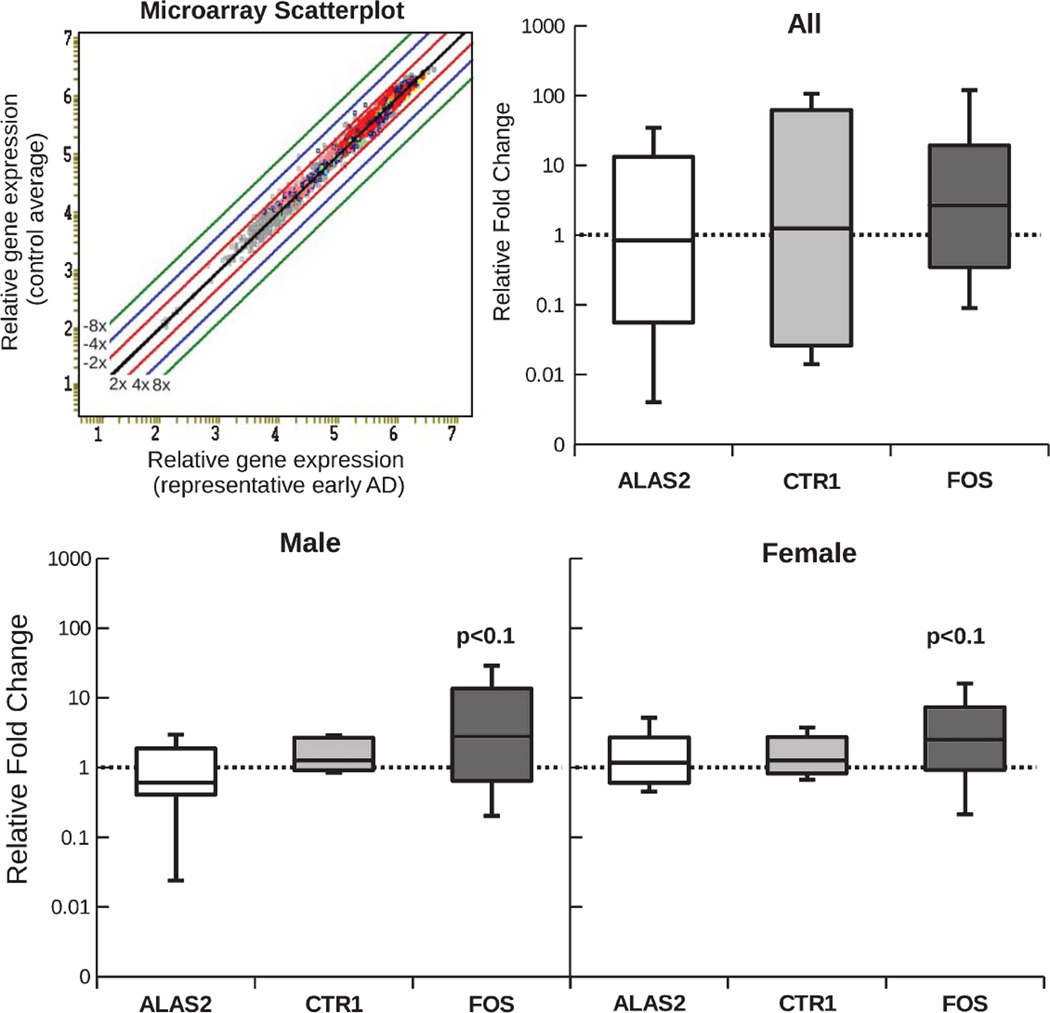
To further investigate the regulation of metal metabolism in peripheral lymphocytes during AD, we used a metal metabolism specific gene microarray (IronChip) that was designed to analyze genes related to iron and copper metabolism. Of these, 149 were found to be undetectable in the blood samples analyzed in both the control and AD groups. Representative images of the microarray and relative gene expression for one case are shown in Fig. 3. Three genes met our criteria for genes of interest (difference in expression for 50% or more of the AD cases, with a mean fold difference of at least 1.3): proto-oncogene c-Fos (FOS), 5-aminolevulinate synthase (ALAS2) and early growth response protein 1 (EGR1). No gene was regulated with a fold change in expression of ±2 or more. Average fold changes for the three genes of interest were: FOS = 1.5, ALAS2 = −1.8, and EGR1 = 1.7. We then chose to validate the expression of FOS and ALAS2 by quantitative RT-PCR because FOS expression was found to be altered in the highest number of AD cases (58%), while ALAS2 was exclusively downregulated in all males, with no regulation seen in any female subject. Although it was only regulated in 42% of subjects (average fold change = 1.4), we further added high affinity copper uptake protein 1 (CTR1) to the qRT-PCR analysis because of its direct involvement in copper transport.
Fig. 3.
The analysis of a representative microarray experiment is shown as a scatter-plot in the top row. It demonstrates that very few genes are differentially expressed in early AD (no fold-change >2). mRNA expression of three genes of interest was further validated using qRT-PCR: ALAS2, CTR1 and FOS. No significant difference in mRNA expression was found for any of the three genes evaluated. (For n see Table 1).
None of the three genes was found to be significantly altered in AD subjects after qRT-PCR analysis. Splitting samples into male and female resulted in a slight trend towards increased expression of FOS (p < 0.1; Fig. 3).
DISCUSSION
Over the last several decades a growing body of research has implicated oxidative stress and related tissue injury in the early stages of AD, but the source of this oxidative imbalance has not yet been clearly explained. An enticing hypothesis suggested that dysregulated copper and iron homeostasis resulted in iron and copper overload of the neocortex and these redoxactive species could catalyze the sorts of oxidative reactions that might initiate neurodegeneration. However, despite near-universal belief that brain iron levels were increased in AD neocortex, the majority of evidence suggests there is no alteration in bulk iron levels and copper levels are actually decreased [17]. An alternative explanation for neocortical oxidative injury is that metal dysregulation in the periphery produces oxidative species which could act centrally. The results of this study describe the levels and metabolic regulation of iron and copper in the circulatory system in MCI and AD subjects to determine whether this is consistent with that hypothesis.
A large body of evidence describing circulating markers of oxidative stress has been published, and results are somewhat heterogeneous. Thiobarbituric acid reactive substances (TBARS) are a general marker of lipid peroxidation and while several reports suggested levels in AD serum or erythrocytes were unchanged [18–20], multiple recent (larger) studies have shown very significant elevations [21–23]. Additionally, malondyaldehyde levels, another marker of lipid peroxidation, are thought to be increased in AD [24–26], although this finding has not been confirmed by every study [27, 28] and has recently been contradicted by Gironi et al. who found a very significant reduction in malondyaldehyde levels [5]. Evidence of oxidative injury to circulating proteins is also accumulating; although, again, results are inconsistent. Plasma carbonyl content has been used as a marker of broad protein oxidation, and levels have been reported to increase [29, 30], but several other studies were unable to reproduce these findings [31, 32]. Aldred and colleagues argue that oxidation of plasma proteins does not appear to represent a global increase in oxidative injury, but rather more specific oxidative changes [31]. This is a significant question in continuing research, as efforts to understand why oxidative changes are occurring in both the brain and peripheral sites in AD focus on explaining the mechanism(s) of injury.
Several potential mechanisms of oxidative injury have been evaluated. Total antioxidant capacity (TAC) in serum and plasma from AD patients was reported to be markedly decreased [33]. Since this report, the finding has been confirmed by several studies [34, 35] and questioned by others [5, 23, 27, 31]. TAC was found not to correlate with cognitive outcomes in a prospective study [36] and dietary supplementation with antioxidant vitamins was not found to produce any clinical benefit in AD subjects in several clinical trials [37, 38]. Additionally, a small clinical trial with d-penicillamine in AD patients demonstrated a significant reduction in circulating markers of oxidative stress, but this did not correlate with any delay in clinical progression of cognitive decline [39]. Key enzymes of cellular antioxidant pathways such as glutathione peroxidase and superoxide dismutase have also been extensively studied (in both serum and erythrocytes), and again results are strongly heterogeneous with a few studies showing significant increases [22–24] or decreases [1, 3] and most showing no change [19, 25, 29, 40].
Another mechanism that would account for broad oxidative injury is increased reactivity of transition metal stores, either by increasing total metal levels or altering the liganding of reactive species, enabling them to participate in Fenton and Haber-Weiss chemistry. A fairly common familial hemochromatosis allele, C282Y, has been associated with a slight genetic susceptibility to AD. Patients with both the C282Y allele and the C2 mutation in the transferrin gene have a substantially (reportedly up to 5 fold) increased risk of developing AD, which suggests that multiple “hits” in the iron metabolism cascade may confer meaningful risk [41]. These observations led to the hypothesis that dysregulation of peripheral iron and/or copper metabolism may be a major source of oxidative injury in AD patients. Iron levels reported in serum of AD patients are inconsistent, although no study to the best of our knowledge has specifically analyzed for nonheme (chelatable) iron [6, 24]. A recent meta-analysis revealed that copper levels are slightly increased in serum from AD patients [8]. We note, however, that the studies included in that meta-analysis were very heterogeneous with the bulk of the studies suggesting a positive association originating from the same laboratory. Also notable, several studies reporting no association or negative association of serum copper levels with AD were not considered in the analysis although the inclusion/exclusion criteria used would have supported their inclusion [42, 43]. In our opinion, there is currently limited evidence for increased levels of total copper in serum in AD, and the heterogeneity between reports is difficult to explain.
While neither non-heme iron, nor copper levels were altered in AD serum in the current study, copper was found to be significantly dysregulated in MCI subjects who subsequently progressed to dementia. In fact, the ratio of copper/non-heme iron as well as copper levels alone clearly distinguished progressive MCI subjects from cognitively stable MCI subjects. The necessity to identify the group at risk of progression at the MCI stage of cognitive decline is key for future treatment of AD and makes this a very useful potential biomarker. Moreover, this ratio mirrors the findings of a recent report describing the ratio of ceruloplasmin and transferrin (the major protein carriers of copper and iron, respectively) in serum from early AD cases: ceruloplasmin was increased while transferrin was decreased [7]. Notably, an older study confirms the significant increase in serum ceruloplasmin, but challenges the decrease in serum transferrin in AD [42].
While this finding may be valuable as a biomarker, it is not by itself clear evidence of global iron/copper dysregulation. Analysis of the expression of IRP2, a central regulator of intracellular iron metabolism and a player in copper metabolism, and a downstream target of IRP2, the IRE-containing gene DMT1, in peripheral lymphocytes failed to demonstrate any abnormalities. Moreover, microarray analysis of 536 genes associated with transition metal homeostasis failed to identify a single gene which was significantly dysregulated above a 2-fold expression difference in AD lymphocytes. Three genes identified in the microarray as having the potential for dysregulation (FOS, ALAS2, and CTR1) were subsequently found not to be significantly altered after qRT-PCR validation. A limitation of this study has been the small sample size with respect to the large biological variation seen in the expression of the genes evaluated. But these data could be viewed to argue that circulating iron and copper homeostasis remains essentially intact in AD.
On the other hand, it is well established that the liver is a key center for iron and copper metabolism outside of the brain and a major source for proteins involved in metal homeostasis. Changes in hepatic gene expression could therefore hold the key to our understanding of the transient alteration of peripheral iron/copper metabolism seen during the transition from MCI to AD.
Previously, measurements of oxidative injury at various stages of AD have not been found to correlate with disease severity [23]. This observation may be supported by the current study; changes in iron and copper metabolism peaked transiently at the preclinical stage (a stage of the disease not evaluated in the microarray experiment) and then resolved. This may suggest that oxidative injury occurs early in the disease process as an oxidative burst (of sorts), setting the stage for cognitive decline.
We draw several insights based on these findings and the larger context offered by the extensive literature on serum/plasma oxidative changes in AD. First of all, while a large number of studies suggest there are oxidative changes in blood in patient populations undergoing cognitive decline, no consistent pattern of changes has emerged. Sufficient unexplained heterogeneity is present between these studies that it may be reasonable to question whether global oxidative stress in blood is a feature of this disease. The findings from the current study suggest that global iron and/or copper dysmetabolism is transient and unlikely the major source of such a change. Alternatively, the observation that multiple genetic alterations in the iron metabolism pathway (C282Y and C2) together contribute substantial risk for AD while individually they are relatively benign could be extended to argue that multiple subtle pro-oxidative alterations could have significant effect, while remaining difficult to detect when analyzed in aggregate. Unfortunately, at present the available data are difficult to interpret with confidence and the failure of major clinical trials of antioxidant therapy raises meaningful questions as to whether peripheral oxidation status is a meaningful factor in the process of neurodegeneration.
Finally, we observed a significant elevation in the ratio of copper to iron in serum in MCI subjects who subsequently progressed to dementia. This elevation appears to be transient as subjects with early AD were nearly identical to controls and stable MCI subjects and longitudinal data show progressive MCI cases trend downward over time. The pathophysiological significance of this observation is unclear, but it may serve as a biomarker to identify subjects with subject memory complaints who are at risk of developing further cognitive decline. Further studies in a larger cohort and serial measurements to clarify the time course of the elevation in the ratio during the transition from normal cognition to AD are warranted to validate this potential biomarker of progressive MCI.
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health (AG20948).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1085).
REFERENCES
- 1.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 2.Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis. 2008;15:117–128. doi: 10.3233/jad-2008-15110. [DOI] [PubMed] [Google Scholar]
- 3.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoel R, Rodrigues A, Duro D, Oliveira CR. Oxidative damage and progression to Alzheimer’s disease in patients with mild cognitive impairment. J Alzheimers Dis. 2010;21:1165–1177. doi: 10.3233/jad-2010-091723. [DOI] [PubMed] [Google Scholar]
- 5.Gironi M, Bianchi A, Russo A, Alberoni M, Ceresa L, Angelini A, Cursano C, Mariani E, Nemni R, Kullmann C, Farina E, Martinelli BF. Oxidative imbalance in different neurodegenerative diseases with memory impairment. Neurodegener Dis. 2010;8:129–137. doi: 10.1159/000319452. [DOI] [PubMed] [Google Scholar]
- 6.Squitti R, Lupoi D, Pasqualetti P, Dal Forno G, Vernieri F, Chiovenda P, Rossi L, Cortesi M, Casseta E, Rossini PM. Elevation of serum copper levels in Alzheimer’s disease. Neurology. 2002;59:1153–1161. doi: 10.1212/wnl.59.8.1153. [DOI] [PubMed] [Google Scholar]
- 7.Squitti R, Salustri C, Siotto M, Ventriglia M, Vernieri F, Lupoi D, Cassetta E, Rossini PM. Ceruloplasmin/transferrin ratio changes in Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011 doi: 10.4061/2011/231595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucossi S, Ventriglia M, Panetta V, Salustri C, Pasqualetti P, Mariani S, Siotto M, Rossini PM, Squitti R. Copper in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. J Alzheimers Dis. 2010;24:175–185. doi: 10.3233/JAD-2010-101473. [DOI] [PubMed] [Google Scholar]
- 9.Britt WG, Hansen AM, Bhaskerrao S, Larsen JP, Petersen F, Dickson A, Dickson C, Kirsch WM. Mild cognitive impairment: Prodromal Alzheimer’s disease or something else? J Alzheimers Dis. 2011;27:543–551. doi: 10.3233/JAD-2011-110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirsch WM, McAuley G, Holshouser B, Petersen F, Ayaz M, Vinters HV, Dickson C, Haacke EM, Britt W, Larsen J, Kim I, Mueller C, Schrag M, Kido D. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimers Dis. 2009;17:599–609. doi: 10.3233/JAD-2009-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magaki S, Mueller C, Yellon SM, Fox J, Kim J, Snissarenko E, Chin V, Ghosh MC, Kirsch WM. Regional dissection and determination of loosely bound and non-heme iron in the developing mouse brain. Brain Res. 2007;1158:144–150. doi: 10.1016/j.brainres.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrag M, Crofton A, Zabel M, Jiffry A, Kirsch D, Dickson A, Mao XW, Vinters HV, Domaille DW, Chang CJ, Kirsch W. The effect of cerebral amyloid angiopathy on brain iron, copper and zinc in Alzheimer’s disease. J Alzheimers Dis. 2010;224:137–149. doi: 10.3233/JAD-2010-101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muckenthaler M, Richter A, Gunkel N, Riedel D, Polycarpou-Schwartz M, Hentze S, Falkenhahn M, Stremmel W, Ansorge W, Hentze MW. Relationships and distinctions in iron-regulatory networks responding to interrelated signals. Blood. 2003;101:3690–3698. doi: 10.1182/blood-2002-07-2140. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 15.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acid Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller C, Magaki S, Schrag M, Ghosh MC, Kirsch WM. Iron regulatory protein 2 is involved in copper homeostasis. J Alzheimers Dis. 2009;18:201–210. doi: 10.3233/JAD-2009-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrag M, Mueller C, Oyoyo U, Kirsch W. Iron, zinc and copper in the Alzheimer’s disease brain: A quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol. 2011;94:96–306. doi: 10.1016/j.pneurobio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bermejo P, Gomez-Serranillos P, Santos J, Pastor E, Gil P, Martin-Aragon S. Determination of malonaldehyde in Alzheimer’s disease: A comparative study of high-performance liquid chromatography and thiobarbituric acid test. Gerontology. 1997;43:218–222. doi: 10.1159/000213853. [DOI] [PubMed] [Google Scholar]
- 19.Ceballos-Picot I, Merad-Boudia M, Nicole A, Thevenin M, Hellier G, Legrain S, Berr C. Peripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer’s type – place of the extracellular glutathione peroxidase. Free Radic Biol Med. 1996;20:579–587. doi: 10.1016/0891-5849(95)02058-6. [DOI] [PubMed] [Google Scholar]
- 20.Galbusera C, Facheris M, Magni F, Galimberti G, Sala G, Tremolada L, Isella V, Guerini FR, Appollonio I, Galli-Kienle M, Ferrarese C. Increased susceptibility to plasma lipid peroxidation in Alzheimer disease patients. Curr Alzheimer Res. 2004;1:103–109. doi: 10.2174/1567205043332171. [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto EM, Munhoz CD, Glezer I, Bahia VS, Caramelli P, Nitrini R, Gorjao R, Curi R, Scavone C, Marcourakis T. Oxidative state in platelets and erythrocytes in aging and Alzheimer’s disease. Neurobiol Aging. 2005;26:857–864. doi: 10.1016/j.neurobiolaging.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Serra JA, Dominguez RO, Marschoff ER, Guareschi EM, Famulari AL, Boveris A. Systemic oxidative stress associated with the neurological diseases of aging. Neurochem Res. 2009;34:2122–2132. doi: 10.1007/s11064-009-9997-5. [DOI] [PubMed] [Google Scholar]
- 23.Zafrilla P, Mulero J, Xandri JM, Santo E, Caravaca G, Morillas JM. Oxidative stress in Alzheimer patients in different stages of the disease. Curr Med Chem. 2006;13:1075–1083. doi: 10.2174/092986706776360978. [DOI] [PubMed] [Google Scholar]
- 24.Ozcankaya R, Delibas N. Malondialdehyde, superoxide dismutase, melantonin, iron, copper, and zinc blood concentrations in patients with Alzheimer disease: Cross-sectional study. Croat Med J. 2002;43:28–32. [PubMed] [Google Scholar]
- 25.Martin-Aragon S, Bermejo-Bescos P, Benedi J, Felici E, Gil P, Ribera JM, Villar AM. Metalloproteinase’s activity and oxidative stress in mild cognitive impairment and Alzheimer’s disease. Neurochem Res. 2009;34:373–378. doi: 10.1007/s11064-008-9789-3. [DOI] [PubMed] [Google Scholar]
- 26.Gustaw-Rothenberg K, Kowalczuk K, Stryjecka-Zimmer M. Lipid peroxidation markers in Alzheimer’s disease and vascular dementia. Ger Geron Int. 2010;10:161–166. doi: 10.1111/j.1447-0594.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 27.Sekler A, Jimenez JM, Rojo L, Pastene E, Fuentes P, Slachevsky A, Maccioni RB. Cognitive impairment and Alzheimer’s disease: Links with oxidative stress and cholesterol metabolism. Neuropsychiatr Dis Treat. 2008;4:715–722. doi: 10.2147/ndt.s3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cito A, Porcelli B, Coppola MG, Mangiavacchi P, Cortelazzo A, Terzuoli L. Analysis of serum levels of homocysteine and oxidative stress markers in patients with Alzheimer disease. Biomed Pharmacother. 2010 doi: 10.1016/j.biopha.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, Vallar AM. Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from mild cognitive impairment. Free Radic Res. 2008;42:162–170. doi: 10.1080/10715760701861373. [DOI] [PubMed] [Google Scholar]
- 30.Greilberger J, Fuchs D, Leblhuber F, Greilberger M, Wintersteiger R, Tafeit E. Carbonyl proteins as a clinical marker in Alzheimer’s disease and its relation to tryptophan degradation and immune activation. Clin Lab. 2010;56:441–448. [PubMed] [Google Scholar]
- 31.Aldred S, Bennett S, Mecocci P. Increased low-density lipoprotein oxidation, but not plasma protein oxidation, in Alzheimer’s disease. Clin Biochem. 2010;43:267–271. doi: 10.1016/j.clinbiochem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Sinem F, Dildar K, Gokhan E, Melda B, Orhan Y, Filiz M. The serum protein and lipid oxidation marker levels in Alzheimer’s disease and effects of cholinesterase inhibitors and antipsychotic drugs therapy. Curr Alzheimer Res. 2010;7:463–469. doi: 10.2174/156720510791383822. [DOI] [PubMed] [Google Scholar]
- 33.De Leo ME, Borrello S, Passantino M, Palazzotti B, Mordente A, Daniele A, Filippini V, Galeotti T, Masullo C. Oxidative stress and overexpression of manganese superoxide dismutase in patients with Alzheimer’s disease. Neurosci Lett. 1998;250:173–176. doi: 10.1016/s0304-3940(98)00469-8. [DOI] [PubMed] [Google Scholar]
- 34.Repetto MG, Reides CG, Evelson P, Kohan S, de Lustig ES, Llesuy SF. Peripheral markers of oxidative stress in probable Alzheimer’s patients. Eur J Clin Invest. 1999;29:643–649. doi: 10.1046/j.1365-2362.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- 35.Serra JA, Marschoff ER, Dominguez RO, Guareschi EM, Famulari AL, Pagani MA, de Lustig ES. Collaborative Group for the Study of the Oxidative Stress, Oxidative stress in Alzheimer’s and vascular dementias: Masking of the antioxidant profiles by a concomitant type II diabetes mellitus condition. J Neurol Sci. 2004;218:17–24. doi: 10.1016/j.jns.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Squitti R, Bressi F, Pasqualetti P, Bonomini C, Ghidoni R, Binetti G, Cassetta E, Moffa F, Ventriglia M, Vernieri F, Rossini PM. Longitudinal prognostic value of serum “free” copper in patients with Alzheimer disease. Neurology. 2009;72:50–55. doi: 10.1212/01.wnl.0000338568.28960.3f. [DOI] [PubMed] [Google Scholar]
- 37.Peterson R, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, Van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 38.Isaac M, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD002854.pub2. CD002854. [DOI] [PubMed] [Google Scholar]
- 39.Squitti R, Rossini PM, Cassetta E, Moffa F, Pasqualetti P, Cortesi A, Rossi L, Finazzi-Agro A. D-penicillamine reduces serum oxidative stress sin Alzheimer’s disease patients. Eur J Clin Invest. 2002;32:51–59. doi: 10.1046/j.1365-2362.2002.00933.x. [DOI] [PubMed] [Google Scholar]
- 40.Bourdel-Marchasson I, Delmas-Beauvieux MC, Peuchant E, Richard-Harston S, Decamps A, Reignier B, Emeriau JP, Rainfray M. Antioxidant defenses and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing. 2006;30:235–241. doi: 10.1093/ageing/30.3.235. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann DJ, Schuur M, Warden DR, Hammond N, Belbin O, Kolsch H, Lehmann MG, Wilcock GK, Brown K, Kehoe PG, Morris CM, Barker R, Coto E, Alvarez V, Deloukas P, Mateo I, Gwillian R, Combarros O, Arias-Vasquez A, Aulchenko YS, Ikram MA, Breteler MM, van Duijn CM, Oulhaj A, Heun R, Cortina-Borja M, Morgan K, Robson K, Smith AD. Transferrin and HFE genes interact in Alzheimer’s disease risk: The Epistasis Project. Neurobiol Aging. 2012;33:202.e1–202.e13. doi: 10.1016/j.neurobiolaging.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Molaschi M, Ponzetto M, Bertacna B, Berrino E, Ferrario E. Determination of selected trace elements in patients affected by dementia. Arch Gerontol Geriatr. 1996;22(supp1):39–42. doi: 10.1016/0167-4943(96)86910-X. [DOI] [PubMed] [Google Scholar]
- 43.Shore D, Henkin RI, Nelson NR, Agarwal RP, Wyatt RJ. Hair and serum copper, zinc, calcium and magnesium concentrations in Alzheimer-type dementia. J AmGeriatr Soc. 1984;32:892–895. doi: 10.1111/j.1532-5415.1984.tb00889.x. [DOI] [PubMed] [Google Scholar]