Abstract
PURPOSE
We hypothesized that antibiotic overuse for acute cough illness (ACI) is in part due to a mismatch between patients’ expectations and the natural history of ACI.
METHODS
We performed a population-based random digit dialing survey of 493 adults in Georgia to determine their expectations regarding the duration of ACI. We also performed a systematic review of observational studies and the placebo or untreated control groups of randomized controlled trials to determine the duration of ACI from the published medical literature. We included studies of otherwise healthy adults with undifferentiated ACI, no clear bacterial cause, data on at least 1 cough outcome, and at least 1 week of follow-up.
RESULTS
The mean duration of cough in the published literature was 17.8 days. Survey respondents reported a median duration of 5 to 7 days and a mean duration of 7.2 to 9.3 days depending on the specific scenario. Patients expecting a longer duration of illness were more likely to be white, female, and have self-reported asthma or chronic lung disease. Independent predictors of the belief that antibiotics are always helpful included nonwhite race (OR = 1.82, 95% CI, 1.14–2.92), some college education or less (OR = 2.08, 95% CI, 1.26–3.45), and previous antibiotics for ACI (OR = 2.20, 95% CI, 1.34–3.55).
CONCLUSIONS
There is a mismatch between patients’ expectations regarding the duration of ACI and the actual duration based on the best available evidence. Efforts to reduce inappropriate antibiotic use should target this discrepancy.
Keywords: anti-bacterial agents, drug resistance, bacterial, bronchitis, cough, natural history
INTRODUCTION
Acute cough illness (ACI) is one of the most common reasons that patients seek care in the outpatient primary care setting. In 2006 there were more than 3 million outpatient visits in the United States for a chief complaint of cough and more than 4.5 million outpatient visits with a final diagnosis of “acute bronchitis or bronchiolitis.”1 Overall, ACI accounts for approximately 2% to 3% of visits to outpatient physicians.1
Although typically a self-limited condition caused by viruses, many patients seek care and request antibiotics for ACI. Even though the rate of antibiotic prescribing for ACI is decreasing (from 65% of visits for ACI in 1996 to 50% in 2006), it remains too high.2 In addition, an increasing proportion of prescriptions are for broad-spectrum antibiotics, such as azithromycin and respiratory quinolones.2
We hypothesized that patients may request antibiotics in part because of a mismatch between their expectations regarding the duration of ACI and the natural history of the condition. A single study in children aged less than 4 years found that parents underestimated the duration of cough in their children.3 Although a previous systematic review examined the natural history of ACI in children,4 we were unable to identify a previous systematic review of the natural history of ACI in otherwise healthy adults. We were also unable to identify any published descriptions of patients’ expectations regarding the duration of ACI in adults. We undertook the current study to explore this issue further, specifically to determine whether such a mismatch in fact exists and to better understand predictors of a belief in the efficacy of antibiotics for ACI. A better understanding of these issues could help clinicians develop educational and public health strategies to reduce inappropriate demand for antibiotics.
This study was approved by the Human Subjects Committee of the University of Georgia.
METHODS
Systematic Review of the Natural History of ACI
We used the following strategy to search the MED-LINE database on February 1, 2011, with subsequent monthly searches to identify any additional studies through November 2011. The strategy is similar to that used by Hay and colleagues in their study of the natural history of cough in children4:
(“acute bronchitis”[Text Word] OR “acute bronchitis” [MeSH Terms] OR “respiratory tract infections”[MeSH Terms] OR “respiratory tract infection”[Text Word] OR “chest cold”[Text Word] OR “chest cold”[MeSH Terms])
This search yielded 258,693 abstracts. Adding limits of English or German, abstract, and human reduced the number to 84,536. Limiting the search to adults resulted in 39,006 abstracts. Because the focus of our study was the natural history of undifferentiated acute respiratory tract infection with cough in otherwise healthy adults, we added terms to exclude studies of sinusitis, pneumonia, tuberculosis, vaccinations, asthma, allergies, anthrax, chronic lung disease, or cystic fibrosis. The final search strategy returned 478 abstracts.
Additionally, we performed the following search: ((“duration”[Title/Abstract]) AND (“acute cough”[Title/ Abstract] OR “acute bronchitis”[Title/Abstract]). So doing yielded 26 studies, 6 of which were potentially relevant and had not been found previously by the above search strategy, and 2 of which were ultimately included. We also retrieved all clinical trials identified in the reference list of the most recent Cochrane review of antibiotics for acute bronchitis, because the untreated placebo group in a clinical trial may be relevant to our study. Abstracts and unpublished studies were not used.
We reviewed each abstract in tandem and, where necessary, performed a detailed review of the full text to identify studies that met our inclusion criteria. All studies were reviewed by the principal investigator, an experienced researcher and family physician (M.H.E.); each study was also reviewed by one of the other investigators (one of whom was also a physician). Specifically, we included prospective cohort studies and controlled trials (where there was an untreated group of patients) that studied community-dwelling adult outpatients without serious pulmonary comorbidity. We selected only those studies that predominantly recruited patients with undifferentiated ACI, as well as studies of “acute bronchitis.” Included studies had to gather data on the presence or severity of individual ACI symptoms or a validated cough severity score for at least 1 week.
We excluded studies set in the hospital, studies of adults with serious chronic respiratory illness, and studies of patients for whom there was a clearly suspected cause of cough (eg, pneumonia, sinusitis, anthrax, or influenza), because our goal was to study undifferentiated ACI. We also excluded studies in which patients were inoculated with a particular virus or of patients whose cough was induced pharmacologically, as well as studies of specific groups (ie, swimmers, mountain climbers, postoperative patients, postintubation patients, smokers). Finally, we excluded studies of prevention of acute respiratory infection, studies in which the predominant symptom was sore throat or rhinitis, and studies of postinfectious or chronic cough.
Data regarding study design and study findings were abstracted in tandem by 2 of the investigators. Discrepancies were resolved by consensus. In clinical trials, we used data for only those patients who did not receive antibiotic, herbal, or antiviral therapy (ie, the control group). The total days with cough were directly reported by some studies and determined from examination of survival curves in others. Some studies reported the mean days of cough before and after enrollment in the study. If a study did not report the mean days of cough before enrollment but did specify a maximum number of days of symptoms before enrollment (ie, “acute cough for 7 or fewer days”), we used one-half of that number as an estimate of the mean days cough before enrollment. For example, 1 day if up to 2 days were specified, 4 days if up to 7 days were specified, and 7 days if up to 14 days were specified.
Regarding quality assessment of included studies, we included only studies that gathered data prospectively for at least 1 week and had at least 80% follow-up (confirmed by independent review of 2 researchers). Quality of included studies was not otherwise assessed; such study design characteristics as allocation concealment, masking, and randomization method were not relevant because we included only patients from the placebo group.
Survey of the General Population Regarding ACI
We used the Georgia Poll, a random digit dialing survey of approximately 500 Georgia residents aged 18 years and older that is conducted twice each year by the Survey Research Center of the University of Georgia . Participants were asked about 1 of 6 randomly selected versions of the following scenario:
Suppose that you get sick and the main symptom is a cough. You are coughing up yellow mucus and have a slight fever (100.5 degrees). You are not taking any medicine for the cough. About how long do you expect that it will take from the time you first feel sick until the time where you feel well and the cough is gone?
We created the 6 scenarios by altering the 3 types of cough (yellow sputum, green sputum, or dry cough) and whether the patient had a fever. We then asked participants the extent to which they believed that antibiotics were effective for the ACI described in the scenario using the following question: “Which of the following best describes how you feel about antibiotics for this illness?” Possible responses included the following: “antibiotics would always be helpful and would always reduce how long I am sick”; “antibiotics would usually be helpful and would probably reduce how long I am sick”; “antibiotics would sometimes be helpful and might reduce how long I am sick”; “antibiotics would rarely be helpful and would be unlikely to reduce how long I am sick”; “antibiotics would never be helpful, and would not reduce how long I am sick.”
Finally, participants were asked whether they had ever been prescribed an antibiotic for an acute cough illness, whether they had asthma or chronic lung disease, and whether they had any chronic medical conditions. Basic demographic data (age, educational attainment, marital status, race, residence, income) were also obtained.
Analysis
Results are primarily descriptive; weighted means were calculated for mean days of cough and mean days of productive cough. Ranges are reported; standard deviation was not provided by 2 studies, making DerSimonian Laird random effects meta-analysis or a formal measure of heterogeneity such as the I2 statistic impossible to calculate.
To analyze the survey findings, we used a Pearson χ2 test for bivariate analysis of categorical variables, Student’s t test for comparison of 2 means, and analysis of variance (ANOVA) for comparisons of more than 2 means. Logistic regression was used to identify independent predictors of belief in the efficacy of antibiotics, and linear regression was used to identify variables independently associated with the predicted duration of acute cough illness. Stata 11.1 (StataCorp, LP) was used for statistical analyses.
RESULTS
Systematic Review of the Natural History of ACI
We identified 19 studies with between 23 and 1,230 patients that met our inclusion criteria. Characteristics of included studies are summarized in Table 1. Follow-up ranged from 7 days to 2 months. Most studies explicitly excluded patients who were pregnant, had a clear indication for antibiotics (ie, pneumonia), had chronic lung disease, or had another serious chronic condition. When reported, the mean age ranged from 29.7 to 44.0 years. Eight studies took place in Europe, 7 in the United States, 3 in Russia, and 1 in Kenya. Outcomes reported by at least 2 studies included clinical cure, percentage coughing, percentage complete remission, and percentage unable to work; mean days of any cough, daytime cough, nighttime cough, and productive cough, and cough severity using the 20-point Bronchitis Severity Score. The Bronchitis Severity Score is a validated measure of symptoms of acute cough illness that rates the severity of 5 symptoms (cough, sputum, rales/rhonchi, chest pain during coughing, and dyspnea) from 0 to 4 points each, with higher scores indicating greater illness severity.
Table 1.
Characteristics of Studies Included in the Systematic Review
| First Author, Year | Duration of Follow-up | Setting | Inclusion Criteria | Mean Age, y | Male % | Country |
|---|---|---|---|---|---|---|
| Stott, 19765 | 13 d | 3 Group practices | Aged >14 y with cough and purulent sputum for ≤1 wk | NR | NR | Wales |
| Williamson, 19846 | 2 mo | 2 Academic family medical centers, University of Missouri | Adults aged 21 to 65 y with cough and sputum as prominent complaints, who also had concurrent URTI, rhonchi, or history of fever | 35 | 34 | United States |
| Brickfield, 19867 | 7 d | Academic family medicine center, Washington, DC | Adults aged 18 to 65 y with clinically diagnosed acute bronchitis (lower respiratory infection with sputum production) of <2 wk | 32.5 | 58 | United States |
| Dunlay, 19878 | 14 d | Academic family medicine center, Chelsea, Mchigan | Adults with productive cough | 44 | 42 | United States |
| Scherl, 19879 | 2 wk | Outpatient medical clinic, University of Kentucky | Patients aged ≥12 y with cough and purulent sputum for <2 wk | 31 | 26 | Unite d States |
| Verheij, 199410 | 11 d | 22 General practices | Adult with cough and purulent sputum | 41 | 42 | Netherlands |
| Hueston, 199411 | 7 d | 2 Family practice centers, Kentucky and Wisconson | Adults aged 18 to 65 y with productive cough <30 d and no signs pneumonia | 36.9 | 30 | United States |
| King, 199612 | 14–18 d | 3 Academic family medicine centers, North Carolina | Aged >8 y, cough and sputum production, and onset within 2 wk | 38.2 | 36 | United States |
| Littenberg, 199613 | 7 d | Dartmouth Hitchcock Medical Center | Adults with nonspecific bronchitis or acute cough <4 wk | 33.7 | 39 | United States |
| Matthys, 200014 | 4 wk | 40 Outpatient clinics | Adults with acute bronchitis (<5 d, ≥4 nightly awakenings due to cough); otherwise in good mental and physical condition | 39 | 46 | Poland |
| Matthys, 200315 | 7 d | Outpatient clinics, totaling 36 physicians | Adults with clinically diagnosed acute bronchitis, <4 8 h of symptoms, and BSS ≥5 | 39.9 | 32 | Germany |
| Chuchalin, 200516 | 7 d | Urban primary care outpatient clinics | Adults with clinically diagnosed acute bronchitis <48 h and BSS ≥5a | 35.9 | 37 | Russia |
| Little, 200517 | 21 d | 37 General practice physicians | Patients aged ≥3 y (83% >6 y) with primary symptom of cough and ≥1 other lower respiratory symptoms | 38 | NA | England |
| Kemmerich, 200718 | 11 d | 23 Outpatient clinics | Adults with clinical diagnosis of acute bronchitis and onset of bronchial mucus production in past days, as well as ≥10 coughing f ts on previous day | 43.5 | 38 | Germany |
| Matthys, 200719 | 7 d | 3 Academic polyclinics, Moscow | Adults aged 18 to 65 y with clinically diagnosed acute bronchitis, <4 8 h, and BSS >5 | 37.4 | 21 | Russia |
| Nduba, 200820 | 14 d | Public clinic, Nairobi | Adult with productive cough <2 wk | 29.7 | 38 | Kenya |
| Matthys, 200821 | 7 d | 6 Outpatient clinics, Moscow | Adults with acute bronchitis, BSS ≥5, symptoms <48 h | 37 | 21 | Russia |
| Matthys, 201022 | 7 d | 18 Centers | Adults with acute bronchitis <48 h, BSS ≥5 | 38.5 | NR | Ukraine |
| Butler, 201023 | 28 d | 387 General practitioners | Patients aged ≥18 y, with acute or worsened cough as predominant symptom or clinical signs of lower respiratory tract infection, duration up to 28 d | 36 | 13 European countries |
BSS = Bronchitis Severity Score; NR = not reported; URTI = upper respiratory tract infection.
Scores range from 0–20, with higher scores indicating greater severity of illness.
Results of the meta-analysis for the mean duration of any cough and subsets of cough are shown in Table 2. The weighted mean duration of any cough was 17.8 days (range = 15.3 to 28.6 days), and for productive cough was 13.9 days (range = 13.3 to 17.4 days).
Table 2.
Mean Days of Any Cough, Daytime Cough, Nighttime Cough, and Productive Cough
| Outcome First Author, Year | Patients Studied | Days of Cough Mean (SD) |
|---|---|---|
| Any cough | 17.8a | |
| Williamson, 1984 | 32 | 28.6 |
| Nduba, 2008 | 275 | 15.3 (4.3) |
| Scherl, 1987 | 15 | 17.8 |
| Little, 2005 | 269 | 21.3 (5.8) |
| Butler, 2010 | 1,230 | 17.3 (6.6) |
| Daytime cough | 12.7a | |
| Stott, 1976 | 103 | 10.3 (3) |
| Verheij, 1994 | 69 | 16.2 (3.2) |
| Nighttime cough | 10.4a | |
| Stott, 1976 | 84 | 8.9 (3.1) |
| Verheij, 1994 | 69 | 12.2 (2.7) |
| Productive cough | 13.9a | |
| Williamson, 1984 | 32 | 13.7 |
| Scherl, 1987 | 15 | 17.4 |
| Verheij, 1994 | 69 | 13.3 (3) |
Note: standard deviation shown only if reported by the original study.
Weighted mean.
The range for the percentage of patients who were clinically cured 8 days after the onset of symptoms was 20% to 73%.15,16,21 Because the outcome of clinical cure was defined differently by different studies, we did not attempt to pool these data. The percentage with cough 8 days after onset was more consistent (86.4% to 95.0%), likely because the 3 studies reporting this outcome were funded by the same sponsor, and thus used similar outcome definitions.15,16,19 The percentage with cough at the end of the study was 91% at 16 days in 1 study,11 73% at 17 days in a second,13 and 82% at 21 days in a third.8 Finally, 8 days after the onset of symptoms, 3 studies reported that the percentage of patients unable to work as a result of their ACI ranged from 33% to 52%.15,21,22
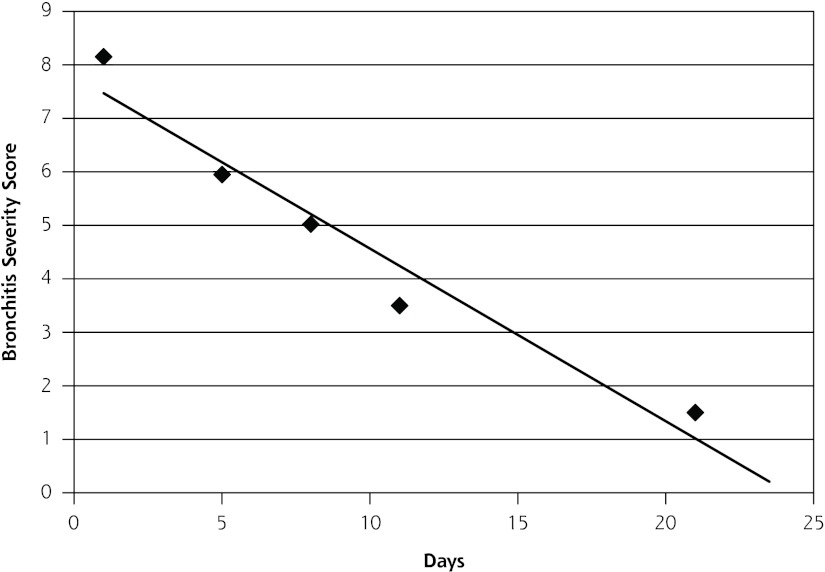
The Bronchitis Severity Score at different points at the onset of cough is shown in Supplemental Table 1, available at http://annfammed.org/content/11/1/5/suppl/DC1. Figure 1 graphs the weighted mean \ Bronchitis Severity Score score against days from onset of symptoms. A simple linear regression shows that the score would equal 0 approximately 24 days after the onset of symptoms and approximately 1 at 18 days after onset of symptoms, consistent with the data regarding the mean duration of cough (17.8 days).
Figure 1.
Weighted mean of the Bronchitis Severity Score by the number of days from the onset of symptoms.
Survey of the General Population Regarding ACI
Of 1,131 eligible persons contacted by the Georgia Poll, 493 responded to the survey questionnaire (43.6%). The final sample was 36.9% male; 68.0% were white, 27.6% African-American, 1.4% Hispanic, and the remainder multiracial or other. Regarding education, 44.0% were college graduates, and all but 6.5% were high school graduates. Regarding age, 26.7% were aged between 18 and 44 years, 45.7% were between 45 and 64 years, and 27.6% were 65 years or older. Based on a comparison with 2010 census figures for Georgia, the respondents were somewhat older, better educated, and more likely to be female than the general population but had a similar racial distribution. We believe they reflect a typical population seeking care for ACI in the United States.
Approximately 20% of respondents to the Georgia Poll did not respond to the question regarding expected duration of cough in the scenario. Respondents to this question were younger (54 vs 63 years, P <.001) and more likely to be female (24% vs 16%, P = .03). The predicted mean duration of ACI among all respondents (n = 395) ranged from 6.9 to 9.3 days depending on the scenario, and from 6.5 to 9.2 days for respondents who had no self-reported history of asthma or chronic lung disease (n = 352). A histogram of the distribution of estimates by patients without chronic respiratory disease is shown in Figure 2. The median estimated duration of an episode was 5 to 7 days, depending on the scenario. There was a trend to ascribe a longer duration of illness to episodes characterized by green sputum rather than yellow sputum or dry cough (8.7 vs 7.6 days, P = .06) and by episodes without fever (7.5 vs 8.5 days, P = .08).
Figure 2.
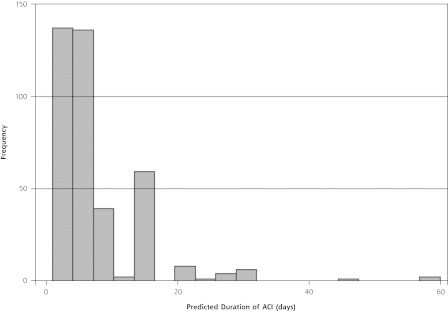
Predicted duration of acute cough illness (ACI) in days by a random sample of Georgia adults.
In the bivariate analysis, a longer predicted duration of ACI was associated with previous use of antibiotics for ACI, self-reported chronic lung disease, female sex, white race, and lower educational attainment (Table 3). A linear regression identified white race, self-reported asthma or chronic lung disease, and female sex as independent predictors of a longer predicted duration of ACI.
Table 3.
Predicted Days of Duration of Acute Cough Illness by Vignette, Illness Characteristics, and Patient Characteristics
| Characteristics | No. | Predicted Duration Mean (95% CI) | P Value |
|---|---|---|---|
| Vignettes | |||
| Yellow sputum and fever | 58 | 7.2 (5.8–8.6) | .45 |
| Yellow sputum and no fever | 66 | 7.9 (6.1–9.7) | |
| Green sputum and fever | 69 | 8.3 (6.8–9.8) | |
| Green sputum and no fever | 53 | 9.3 (7.5–11.1) | |
| Dry cough and fever | 80 | 6.9 (5.6–8.2) | |
| Dry cough and no fever | 69 | 8.4 (6.0–10.7) | |
| ACI characteristics | |||
| Green sputum | 122 | 8.7 (7.6–9.9) | .06 |
| Other | 273 | 7.6 (6.7–8.4) | |
| Yellow sputum | 124 | 7.6 (6.4–8.7) | .25 |
| Other | 271 | 8.1 (7.2–9.0) | |
| Dry cough | 149 | 7.6 (6.3–8.9) | .21 |
| Other | 246 | 8.1 (7.3–9.0) | |
| Fever | 207 | 7.5 (6.6–8.3) | .08 |
| No fever | 188 | 8.5 (7.3–9.6) | |
| Self-reported previous use of antibiotics for ACI | |||
| Yes | 246 | 8.7 (7.7–9.7) | .004 |
| No | 149 | 6.7 (5.9–7.6) | |
| Self-reported asthma or chronic lung disease | |||
| Yes | 43 | 10.9 (7.1–14.6) | .002 |
| No | 352 | 7.6 (6.9–8.2) | |
| Annual income | |||
| $75,000 or higher | 174 | 7.7 (6.9–8.6) | .31 |
| Less than $75,000 | 221 | 8.1 7.0–9.1) | |
| Sex | |||
| Male | 136 | 6.4 (5.5–7.4) | .001 |
| Female | 259 | 8.7 (7.8–9.6) | |
| Race | |||
| White | 262 | 8.7 (7.8–9.7) | .001 |
| Nonwhite | 133 | 6.4 (5.5–7.2) | |
| Educational attainment | |||
| College or professional | 157 | 7.1 (6.3–7.9) | .03 |
| Some college or less | 238 | 8 .5 (7.4–9.5) |
ACI = acute cough illness.
We also examined the association between patient characteristics and belief in antibiotic efficacy, classified as either “antibiotics are always helpful” or “antibiotics are always or usually helpful.” Bivariate predictors of the belief that antibiotics are “always helpful” for acute respiratory tract infections included lower income, less education, not being married, non-white race, a self-reported history of asthma or chronic lung disease, and previous prescriptions of antibiotics for ACI. Bivariate predictors of the belief that antibiotics are “always or usually helpful” included less education, nonwhite race, and previous prescriptions of antibiotics for ACI (Table 4). In the logistic regression (Table 5), independent predictors of the belief that antibiotics are “always helpful” included nonwhite race (OR = 1.82, 95% CI, 1.14–2.94), some college education or less (OR = 2.08, 95% CI, 1.26–3.45), and previous antibiotics for ACI (OR = 2.2, 95% CI, 1.34–3.55).
Table 4.
Bivariate Analysis for Perceived Usefulness of Antibiotics
| Variable | Antibiotics Always Helpful No. (%) | PValue | Antibiotics Always or Usually Helpful No. (%) | P Value |
|---|---|---|---|---|
| Education | ||||
| College or professional (n= 191) | 29 (15.2) | 73 (38.2) | ||
| Other (n = 302) | 83 (27.5) | .001 | 143 (47.4) | .047 |
| Income | ||||
| ≥$75,000 (n = 208) | 39 (18.7) | 81 (38.9) | ||
| <$75,000 (n = 285) | 73 (25.6) | .072 | 135 (47.4) | .063 |
| Marital status | ||||
| Married (n = 297) | 56 (18.9) | 124 (41.7) | ||
| Other (n = 196) | 56 (28.6) | .012 | 92 (46.9) | .256 |
| Race | ||||
| White (n = 330) | 64 (19.4) | 129 (39.1) | ||
| Other (n = 163) | 48 (29.5) | .012 | 87 (53.4) | .003 |
| Sex | ||||
| Male (n = 182) | 45 (24.7) | 84 (46.1) | ||
| Female (n = 311) | 67 (21.5) | .416 | 132 (42.4) | .423 |
| Residence | ||||
| Rural (n = 122) | 35 (28.7) | 55 (45.1) | ||
| Other (n = 371) | 77 (20.7) | .07 | 161 (43.4) | .745 |
| Self-reported asthma or chronic lung disease | ||||
| Yes (n = 57) | 21 (36.8) | 29 (50.9) | ||
| No (n = 436) | 91 (20.9) | .007 | 187 (42.9) | .253 |
| Self-reported any chronic disease | ||||
| Yes (n =209) | 47 (22.5) | 93 (44.5) | ||
| No (n = 284) | 65 (22.9) | .917 | 123 (43.3) | .793 |
| Previous antibiotic use for respiratory tract infection | ||||
| Yes (n.= 287) | 78 (27.2) | 144 (50.2) | ||
| No (n = 206) | 34 (16.5) | .005 | 72 (34.9) | .001 |
| Age, y | 54.6 (alwayshelpful) vs 56.6(other) | .14 | 55.5 (always orusually helpful) vs56.6 (other) | .23 |
Table 5.
Logistic Regression With Antibiotics Always Effective and Antibiotics Always or Usually Effective as Dependent Variables
| Variable | Antibiotics Always Effective OR (95% CI) | Antibiotic Always or Usually Effective OR (95% CI) |
|---|---|---|
| Age | 0.99 (0.98–1.01) | 1.00 (0.99–1.01) |
| Married | 0.66 (0.41–1.07) | 0.93 (0.62–1.40) |
| Nonwhite | 1.82 (1.14–2.94)a | 2.04 (1.35–3.12)a |
| Male | 1.52 (0.96–2.40) | 1.37 (0.93–2.02) |
| Some college or less education | 2.08 (1.26–3.45)a | 1.45 (0.9 8–2.17) |
| Income ≥$75,000/y | 0.90 (0.55–1.48) | 0.78 (0.52–1.16) |
| Self-reported asthma or chronic lung disease | 1.94 (1.00–3.76) | 1.11 (0.60–2.04) |
| Any chronic medical condition | 0.77 (0.47–1.27) | 0.96 (0.64–1.44) |
| Self-reported previous antibiotic use for ACI | 2.20 (1.34–3.55)a | 2.22 (1.49–3.30)a |
ACI = acute cough illness; OR = odds ratio.
P <. 0 5.
DISCUSSION
We found a significant mismatch between expectations of community-dwelling adults regarding the mean duration of a typical ACI (6.5 to 9.2 days) and the actual duration of ACI from the medical literature (approximately 18 days). The largest and best single study to date, a European collaborative study of 1,230 patients,23 reported results similar not only to those of our study regarding mean duration of ACI (17.3 days) but also to the other 4 studies reporting this outcome in the literature (range 15.3 to 28.6 days).6,9,17,20 Furthermore, studies using the validated Bronchitis Severity Score also found that symptoms persisted beyond 2 weeks for many patients (Figure 1).
The specific scenario (yellow sputum vs green sputum vs dry cough, and fever or no fever) had relatively little influence on the predicted duration of symptoms, with the difference between scenarios about 1 day. A shorter duration was predicted by men and by nonwhite respondents, whereas a longer duration was reported by those with self-reported asthma or chronic lung disease. This finding is not surprising, as patients with an acute exacerbation of asthma or chronic lung disease complicated by an acute respiratory tract infection often have a more protracted course. They also may be more aware of their symptoms and may have more episodes, thus providing a more accurate estimate of duration.
The mismatch between patients’ expectations and reality for the natural history of acute cough illness has important implications for antibiotic prescribing. If a patient expects that an episode of ACI should last about 6 or 7 days, it makes sense that they might seek care for that episode and request an antibiotic after 5 or 6 days. Furthermore, if they begin taking an antibiotic 7 days after the onset of symptoms, they may begin to feel better 3 or 4 days later, with the episode fully resolving 10 days later. Although this outcome may reinforce the mistaken idea that the antibiotic worked, it is merely a reflection of the natural history of ACI.
Other researchers have found that previous experiences with antibiotics, having a chronic medical condition, and believing antibiotics are helpful for viruses predict a belief that antibiotics are helpful for ACI.24 Our results are consistent with these previous findings, in particular the belief of patients with a chronic respiratory illness and among those who have previously received antibiotics in the efficacy of antibiotics. A Dutch study found that longer perceived duration of illness was associated with a greater perceived need for antibiotics, which supports our supposition that a better understanding of the typical duration of ACI could reduce demands for antibiotics.25
Efforts to prevent inappropriate use of antibiotics for acute respiratory tract infection in general and ACI in particular have focused on labeling ACI as a chest cold rather than acute bronchitis,26 providing delayed prescriptions,27 using patient education leaflets,28 and emphasizing the distinction between bacteria and viruses (the CDC Get Smart: Know When Antibiotics Work campaign).29 We suggest that setting appropriate expectations should be an additional component of these efforts.
It is therefore important that physicians emphasize the natural history of ACI with patients when they seek care for an episode of acute cough. Patients should be told that it is normal to still be coughing 2 or even 3 weeks after onset, and that they should only seek care if they are worsening or if an alarm symptom, such as high fever, bloody or rusty sputum, or shortness of breath, occurs. Our survey confirmed that previous prescriptions of antibiotics increase the belief in their efficacy, creating the potential for a cycle of expectation and prescription.
We are unaware of data regarding physician knowledge of the natural history of ACI, an area for future research. If physicians also underestimate its duration, they may require continuing professional education to correct these beliefs. Finally, sources that provide advice for patients regarding ACI should be updated to emphasize an expected duration of 2 to 3 weeks until symptoms are completely resolved, sometimes longer.
Limitations of the current study include those of any systematic review: our conclusions are only as valid as the available literature and the completeness and accuracy of study reports in the published literature. In particular, some studies did not report standard deviation for means, making it impossible to perform a more statistically appropriate random effects meta-analysis. The consistency of the mean duration of ACI in the included studies is reassuring, though, with none reporting a mean duration of less than 15 days. Because most of the included studies were from countries in temperate climates, the findings may not apply to tropical countries. The population-based survey is limited to adults in the state of Georgia and by the response rate; thus, it may not reflect beliefs or expectations regarding ACI and antibiotic use in other parts of the United States or in other countries. We are reassured by the diversity of our population in terms of age, race, sex, income, and educational attainment, as well as the relatively good response rate for this kind of survey. In the survey we asked about the duration of acute cough illness; asking about the duration of the symptom of cough alone may have generated a different answer. Finally, that we found no statistically significant difference between expected durations of illness for the 6 vignettes may have been due to small sample size.
We found a large mismatch between patients’ expectations regarding the duration of acute cough illness and its actual natural history based on a systematic review of the best available published evidence. We believe that education of the general public, the media, and physicians should emphasize appropriate expectations regarding the natural history of ACI in order to reduce inappropriate demands for antibiotics.
Funding support: The survey was supported by the Institute for Evidence-Based Health Professions Education at the University of Georgia, Athens, Georgia.
Footnotes
AJCAnnals Journal Club selection; see inside back cover or http://www.annfammed.org/AJC/
Conflicts of interest: authors report none
To read or post commentaries in response to this article, see it online at http://www.annfammed.org/content/11/1/5.
References
- 1.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat. 2011;13(169) [PubMed] [Google Scholar]
- 2.Grijalva CG, Nuorti J P,, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay AD, Wilson A, Fahey T, Peters TJ. The duration of acute cough in preschool children presenting to primary care: a prospective cohort study. Fam Pract. 2003;20(6):696–705 [DOI] [PubMed] [Google Scholar]
- 4.Hay AD, Wilson AD. The natural history of acute cough in children aged 0 to 4 years in primary care: a systematic review. Br J Gen Pract. 2002;52(478):401–409 [PMC free article] [PubMed] [Google Scholar]
- 5.Stott NCH, West RR. Randomised controlled trial of antibiotics in patients with cough and purulent sputum. Br Med J. 1976;2(6035):556–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson HA., JrA randomized, controlled trial of doxycycline in the treatment of acute bronchitis. J Fam Pract. 1984;19(4):481–486 [PubMed] [Google Scholar]
- 7.Brickfield FX, Carter WH, Johnson RE. Erythromycin in the treatment of acute bronchitis in a community practice. J Fam Pract. 1986;23(2):119–122 [PubMed] [Google Scholar]
- 8.Dunlay J, Reinhardt R, Roi LDA. A placebo-controlled, double-blind trial of erythromycin in adults with acute bronchitis. J Fam Pract. 1987;25(2):137–141 [PubMed] [Google Scholar]
- 9.Scherl ER, Riegler SL, Cooper JK. Doxycycline in acute bronchitis: a randomized double-blind trial. J Ky Med Assoc. 1987;85(9):539–541 [PubMed] [Google Scholar]
- 10.Verheij TJM, Hermans J, Mulder JD. Effects of doxycycline in patients with acute cough and purulent sputum: a double blind placebo controlled trial. Br J Gen Pract. 1994;44(386):400–404 [PMC free article] [PubMed] [Google Scholar]
- 11.Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract. 1994;39(5):437–440 [PubMed] [Google Scholar]
- 12.King DE, Williams WC, Bishop L, Shechter A. Effectiveness of erythromycin in the treatment of acute bronchitis. J Fam Pract. 1996;42(6):601–605 [PubMed] [Google Scholar]
- 13.Littenberg B, Wheeler M, Smith DS. A randomized controlled trial of oral albuterol in acute cough. J Fam Pract. 1996;42(1):49–53 [PubMed] [Google Scholar]
- 14.Matthys H, de Mey C, Carls C, Rys A, Geib A, Wittig T. Effiacy and tolerability of myrtol standardized in acute bronchitis: a multi-centre, randomised, double-blind, placebo-controlled parallel group trial vs cefuroxime and ambroxol. Arzneimmittelforschung. 2000;50(8):700–711 [DOI] [PubMed] [Google Scholar]
- 15.Matthys H, Eisebitt R, Seith B, Heger M. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis. A randomised, double-blind, placebo-controlled trial. Phytomedicine. 2003;10(Suppl 4):7–17 [DOI] [PubMed] [Google Scholar]
- 16.Chuchalin AG, Berman B, Lehmacher W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPs 7630): a randomized, double-blind, placebo-controlled trial. Explore (NY). 2005;1(6):437–445 [DOI] [PubMed] [Google Scholar]
- 17.Little P, Rumsby K, Kelly J, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial. JAMA. 2005;293(24):3029–3035 [DOI] [PubMed] [Google Scholar]
- 18.Kemmerich B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of thyme herb and primrose root in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled multicentre clinical trial. [Drug Research]. Arzneimittelforschung. 2007;57(9):607–615 [DOI] [PubMed] [Google Scholar]
- 19.Matthys H, Heger M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): a randomised, double-blind, placebo-controlled, multicentre study. Curr Med Res Opin. 2007;23(2):323–331 [DOI] [PubMed] [Google Scholar]
- 20.Nduba VN, Mwachari CW, Magaret AS, et al. Placebo found equivalent to amoxicillin for treatment of acute bronchitis in Nairobi, Kenya: a triple blind, randomised, equivalence trial. Thorax. 2008;63(11):999–1005 [DOI] [PubMed] [Google Scholar]
- 21.Matthys H, Funk P. EPs 7630 improves acute bronchitic symptoms and shortens time to remission. Results of a randomised, double-blind, placebo-controlled, multicentre trial. Planta Med. 2008;74(6):686–692 [DOI] [PubMed] [Google Scholar]
- 22.Matthys H, Lizogub VG, Malek FA, Kieser M. Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: a randomised, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides. Curr Med Res Opin. 2010;26(6):1413–1422 [DOI] [PubMed] [Google Scholar]
- 23.Butler CC, Hood K, Kelly MJ, et al. Treatment of acute cough/lower respiratory tract infection by antibiotic class and associated outcomes: a 13 European country observational study in primary care. J Antimicrob Chemother. 2010;65(11):2472–2478 [DOI] [PubMed] [Google Scholar]
- 24.Wilson AA, Crane LA, Barrett PH, Gonzales R. Public beliefs and use of antibiotics for acute respiratory illness. J Gen Intern Med. 1999;14(11):658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cals JWL, Boumans D, Lardinois RJM, et al. Public beliefs on antibiotics and respiratory tract infections: an internet-based questionnaire study. Br J Gen Pract. 2007;57(545):942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips TG, Hickner J. Calling acute bronchitis a chest cold may improve patient satisfaction with appropriate antibiotic use. J Am Board Fam Pract. 2005;18(6):459–463 [DOI] [PubMed] [Google Scholar]
- 27.Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce the use of antibiotics for the common cold? A single-blind controlled trial. J Fam Pract. 2002;51(4):324–328 [PubMed] [Google Scholar]
- 28.Macfarlane J, Holmes W, Gard P, Thornhill D, Macfarlane R, Hub-bard R. Reducing antibiotic use for acute bronchitis in primary care: blinded, randomised controlled trial of patient information leaflet. BMJ. 2002;324(7329):91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Get Smart campaign Web site. http://www.cdc.gov/getsmart/ Accessed Oct 30, 2011