Summary
Background
For women with oestrogen receptor (ER)-positive early breast cancer, treatment with tamoxifen for 5 years substantially reduces the breast cancer mortality rate throughout the first 15 years after diagnosis. We aimed to assess the further effects of continuing tamoxifen to 10 years instead of stopping at 5 years.
Methods
In the worldwide Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial, 12 894 women with early breast cancer who had completed 5 years of treatment with tamoxifen were randomly allocated to continue tamoxifen to 10 years or stop at 5 years (open control). Allocation (1:1) was by central computer, using minimisation. After entry (between 1996 and 2005), yearly follow-up forms recorded any recurrence, second cancer, hospital admission, or death. We report effects on breast cancer outcomes among the 6846 women with ER-positive disease, and side-effects among all women (with positive, negative, or unknown ER status). Long-term follow-up still continues. This study is registered, number ISRCTN19652633.
Findings
Among women with ER-positive disease, allocation to continue tamoxifen reduced the risk of breast cancer recurrence (617 recurrences in 3428 women allocated to continue vs 711 in 3418 controls, p=0·002), reduced breast cancer mortality (331 deaths vs 397 deaths, p=0·01), and reduced overall mortality (639 deaths vs 722 deaths, p=0·01). The reductions in adverse breast cancer outcomes appeared to be less extreme before than after year 10 (recurrence rate ratio [RR] 0·90 [95% CI 0·79–1·02] during years 5–9 and 0·75 [0·62–0·90] in later years; breast cancer mortality RR 0·97 [0·79–1·18] during years 5–9 and 0·71 [0·58–0·88] in later years). The cumulative risk of recurrence during years 5–14 was 21·4% for women allocated to continue versus 25·1% for controls; breast cancer mortality during years 5–14 was 12·2% for women allocated to continue versus 15·0% for controls (absolute mortality reduction 2·8%). Treatment allocation seemed to have no effect on breast cancer outcome among 1248 women with ER-negative disease, and an intermediate effect among 4800 women with unknown ER status. Among all 12 894 women, mortality without recurrence from causes other than breast cancer was little affected (691 deaths without recurrence in 6454 women allocated to continue versus 679 deaths in 6440 controls; RR 0·99 [0·89–1·10]; p=0·84). For the incidence (hospitalisation or death) rates of specific diseases, RRs were as follows: pulmonary embolus 1·87 (95% CI 1·13–3·07, p=0·01 [including 0·2% mortality in both treatment groups]), stroke 1·06 (0·83–1·36), ischaemic heart disease 0·76 (0·60–0·95, p=0·02), and endometrial cancer 1·74 (1·30–2·34, p=0·0002). The cumulative risk of endometrial cancer during years 5–14 was 3·1% (mortality 0·4%) for women allocated to continue versus 1·6% (mortality 0·2%) for controls (absolute mortality increase 0·2%).
Interpretation
For women with ER-positive disease, continuing tamoxifen to 10 years rather than stopping at 5 years produces a further reduction in recurrence and mortality, particularly after year 10. These results, taken together with results from previous trials of 5 years of tamoxifen treatment versus none, suggest that 10 years of tamoxifen treatment can approximately halve breast cancer mortality during the second decade after diagnosis.
Funding
Cancer Research UK, UK Medical Research Council, AstraZeneca UK, US Army, EU-Biomed.
Introduction
For women with oestrogen receptor (ER)-positive breast cancer, treatment for 5 years with adjuvant tamoxifen substantially reduces the rate of recurrence not only during the treatment period but throughout the first decade, and reduces breast cancer mortality by about a third throughout the first 15 years (including years 10–14), with little net effect on other mortality.1 Although 5 years of tamoxifen is more effective than is 1–2 years of treatment,1,2 whether 10 years of treatment would have an even greater effect on breast cancer recurrence and mortality in ER-positive disease is not known.3,4 Conversely, treatment with 5 years of tamoxifen can cause side-effects such as endometrial cancer and thromboembolic disease,1,5 and continuing tamoxifen for an additional 5 years is likely to increase these side-effects.
Early trials of continuing adjuvant tamoxifen to 10 years versus stopping tamoxifen at 5 years6–8 recruited relatively few patients. Although some of these studies had adverse early results,9 the small numbers of patients meant that these adverse results could have been due to the play of chance, so larger trials were needed.3,4,10
Moreover, as 5 years of tamoxifen has a prolonged carryover effect after treatment ends, with a substantial reduction in mortality throughout the first 15 years, trials of continuing beyond 5 years of tamoxifen should eventually be followed up to beyond 15 years.4 The UK adjuvant Tamoxifen—To offer more? (aTTom) trial randomly allocated 7000 women, most with unknown ER status, to continue tamoxifen to 10 years or stop at 5 years, but has yet to report long-term findings.11–13 We report results from the global Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial, which randomly allocated 12 894 women to continue tamoxifen to 10 years or stop at 5 years. Our main analyses of breast cancer outcomes involve only the 6846 women with ER-positive disease (sensitivity analyses shown in the appendix include the other women); side-effect analyses include all 12 894 women, regardless of whether the ER status of their disease was positive, negative or unknown.
Methods
Study design and participants
ATLAS is an international trial of continuation of adjuvant tamoxifen for an extra 5 years. Women were eligible for randomisation if they had had early breast cancer (in which all detected disease could be removed); they had subsequently received tamoxifen for some years and were still on it (or had stopped in the past year and could resume treatment with little interruption); they appeared clinically free of disease (with any local recurrence removed and no distant recurrence detected); follow-up seemed practicable; and substantial uncertainty was shared by the woman and her doctor as to whether to stop tamoxifen or continue for about 5 more years. No restrictions were placed on age, type of initial surgery or histology, hormone receptor status, nodal status, or other treatments.
Any contraindications to continuation of tamoxifen precluded entry; clinicians were responsible for assessment of these contraindications, which were not protocol-defined. However, the protocol did suggest clinicians consider pregnancy, breastfeeding, retinopathy, need for coagulation therapy, endometrial hyperplasia, other serious toxicity attributed to tamoxifen, negligibly low risk of death from breast cancer, or presence of another life-threatening disease as possible contraindications.
ATLAS recruited patients from 36 countries or regions during 1996–2005. In general, each country or region had a coordinator on the international steering committee who liaised with collaborators at local hospitals to help ensure eligible patients were considered and, if entered, treated and followed up appropriately. Ethical approval was provided by the Oxford Tropical Research Ethics Committee, national and regional ethics committees where applicable, and local ethics committees in each hospital. Patients were offered information leaflets in local languages. After patients provided signed consent, baseline characteristics were recorded and patients were entered into the study by post, telephone, or fax to the Oxford Clinical Trial Service Unit (CTSU) or to four national or regional centres (listed at the end of the paper). Forms were stored in the CTSU, with copies at local hospitals.
When ATLAS began in 1996, 2 years and 5 years of tamoxifen were both standard treatment options,14 so patients were eligible irrespective of previous duration of tamoxifen treatment. Shortly afterwards, a consensus emerged that 5 years of treatment was better than 2 years of treatment.2,10 Entry with less than 4 years of previous tamoxifen (and, in most countries, of women with ER-negative disease) was therefore stopped in 2000, before ATLAS had meaningful results. The women who had entered ATLAS after less than 4 years of tamoxifen will be reported separately within an update of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analyses2 of longer treatment versus about 2 years of treatment (figure 1). This report is of the remaining women; as they had completed a median of 5 (IQR 4·8–5·2) years of tamoxifen, each patient's entry date counts as year 5 (ignoring exact prior durations).
Figure 1.
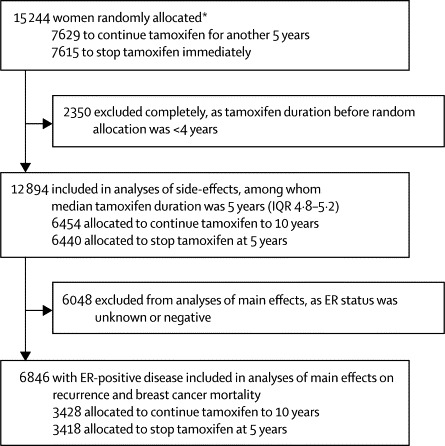
Trial profile, showing the different populations analysed to assess the side-effects and the main effects of continuing tamoxifen to 10 years versus stopping tamoxifen at 5 years
ER=oestrogen receptor. *39 patients were allocated twice in error, but stayed on their original allocation. Excludes 18 patients entered in error (17 with distant recurrence and one without ethics approval).
Randomisation
The CTSU or the four regional or national centres used CTSU software to allocate eligible women randomly to continue tamoxifen to 10 years (stopping only if a definite contraindication emerged) or to stop tamoxifen immediately at 5 years (open control). No placebo treatment was used among controls, and tamoxifen was restarted only if a definite indication was thought to have emerged. Randomisation was minimised 1:1 to balance treatment allocation by country or region and by major prognostic factors (age group, node negativity, tumour diameter, and ER status).
Procedures
Other than duration of tamoxifen treatment, patient management was at the responsible clinician's discretion. The tamoxifen regimen before and during ATLAS was almost always Nolvadex 20 mg per day; where tamoxifen was not affordable, free Nolvadex was provided by the study. Patients were not required to make any extra visits, and no extra investigations were required. Central organisers sent forms to responsible clinicians every year asking about use of tamoxifen or other adjuvant endocrine treatments, breast cancer recurrence, new primary cancer incidence (particularly endometrial cancer), reasons for any hospital admissions (particularly hysterectomy and myocardial infarction), and, if the patients had died, underlying cause of death (asking particularly about breast cancer, endometrial cancer and myocardial infarction); death certificates were also sought. Recurrence was defined as first recurrence after ATLAS entry of any breast cancer (new or same tumour, distant [including unspecified or multiple sites], locoregional, or contralateral). Yearly follow-up forms might report only the worst new endpoint, so if no recurrence had been recorded before a death from breast cancer (or if only contralateral or locoregional recurrence had been recorded before a death), we assumed distant recurrence just preceded death. Deaths from unknown causes without any recorded recurrence were regarded as unrelated to breast cancer. Events were coded, generally in ignorance of (but not masked to) treatment allocation, by the ATLAS principal investigator (CD) according to the 10th International Classification of Diseases (ICD-10).15 Data errors were sought centrally by extensive manual or computer checks, and investigated.
Although 6846 (53%) of 12 894 women had ER-positive disease, 4800 (37%) had unknown ER status. A retrospective project sought missing ER values, but the project was soon abandoned (because sample retrieval and assays differed between women still attending hospital for continued tamoxifen and those who had stopped treatment). The retrospective assay results from this project are not included in the main analyses, although the assays that were done supported reports16 that ER-positivity is substantially less common in Asia than in Europe. (Many ER-untested samples were from Asia, suggesting that only about 60% of all ER-untested samples would, if tested, have been ER-positive; appendix p 26).
Until mid-2010, when the last trial treatment ended, yearly interim analyses (split by ER status) of recurrence, death with recurrence, and cause-specific mortality before recurrence were sent to an independent data monitoring committee.
Statistical analysis
The protocol stated that 20 000 patients would need to be randomised in ATLAS and the other trials of tamoxifen duration to detect reliably an absolute difference of 2–3% in mortality. Entry to ATLAS was halted in 2005 (with 12 894 patients, including 6846 with ER-positive disease) because the MA.17 trial17 showed benefit from continued endocrine treatment after 5 years of tamoxifen. We report the dataset of Aug 31, 2012, because in September, 2012 this dataset was supplied to help update the periodic EBCTCG metaanalyses of all tamoxifen duration trials,2 which will eventually become public. After this preliminary report, further follow-up of ATLAS will continue.
Intention-to-treat log-rank analyses, using in-house programs, yield the event rate ratio (RR; also known as risk ratio) and its standard error, the CI, and the two-sided p value.1,2,18 If a log-rank statistic (observed – expected [O – E]) from a main or sensitivity analysis has variance V, then RR = exp([O – E] / V). Kaplan-Meier graphs show absolute risks during years 5–14. Analyses (of the first relevant event since entry) were of: recurrence (censored at death from other causes), side-effects (censored at recurrence), breast cancer mortality, and overall mortality. Breast cancer mortality analyses subtracted the log-rank statistics for death without recurrence from those for overall mortality1,2 (without assuming all recurrences are equally life-threatening).
We used data for all patients with ER-positive, ER-negative, or ER-untested disease to assess side-effects, but data for patients with ER-negative or ER-untested disease cannot contribute directly to assessment of effects in ER-positive disease. Therefore, the main emphasis in this report is on non-breast-cancer outcomes in all 12 894 women, but on recurrence and breast cancer mortality only in the 6846 women with ER-positive disease (as in the recent EBCTCG meta-analyses of tamoxifen trials1).
In addition, sensitivity analyses (appendix pp 14–18) combine results in ER-positive and ER-untested disease (taking the effect in ER-untested to be 60% of that in ER-positive disease; appendix p 26) by adding 0·6 times the log-rank (O – E) statistic for ER-untested disease to that for ER-positive disease, changing the variance V accordingly, then again using RR = exp([O – E] / V).
The protocol-defined main analysis (appendix pp 27–49) was of all-cause mortality in all women, irrespective of ER status or previous tamoxifen duration; this analysis is provided. The questions that still need answering about tamoxifen duration have, however, changed since ATLAS began, and the main analyses in the present paper are in line with those changes. Negative ER measurements are known to identify reliably patients with little or nothing to gain from tamoxifen. Moreover, for patients with ER-positive disease, 5 years of tamoxifen is known to be better than 2 years of tamoxifen (although the full benefits take at least 15 years to emerge), and 5 years of tamoxifen has little net effect on mortality not caused by breast cancer (despite specific side-effects such as endometrial cancer).1,2
Therefore, the main issue is how, in ER-positive disease, 10 years of treatment compares with 5 years of tamoxifen in terms of main effects on recurrence and breast cancer mortality, and how the specific side-effects of 10 years and 5 years of tamoxifen differ.1,2,10
If the aim is to assess effects on breast cancer outcomes in ER-positive disease, analyses need to be based either on the findings in patients known to have ER-positive disease (which are straightforward to present and are provided in full) or on a combination of the findings in ER-positive and ER-untested disease (which are provided as sensitivity analyses). Emphasis on breast cancer outcomes only in ER-positive disease was proposed by the data monitoring committee statistician (RP) who knew the ATLAS results, but the results from the main and sensitivity analyses were much the same: appendix pp 14–18.
This study is registered, number ISRCTN19652633.
Role of the funding source
Oxford University (Oxford, UK) was the trial sponsor. The study was designed, conducted, analysed, interpreted and reported by the investigators independently of all funding bodies (who saw the manuscript only after acceptance). CD, HP, JG, RG, and RP had full access to all data; all authors had final responsibility for the decision to submit for publication.
Results
Figure 1 describes the different populations that were analysed to assess the side-effects and the main effects of continuing tamoxifen to 10 years versus stopping tamoxifen at 5 years. After exclusion of 18 women who had been entered in error and 2350 women who had completed a median of only 2·4 years (IQR 2·0–3·1) of adjuvant tamoxifen, 12 894 women remained who had completed a median of 5·0 years (4·8–5·2) of adjuvant tamoxifen. All were included in the analyses of side-effects, regardless of ER status.
After exclusion of a further 6048 women with ER status unknown or with ER-negative disease, 6846 women with ER-positive disease remained for the main analyses of the effects on breast cancer recurrence and breast cancer mortality. Table 1 shows the characteristics of the included patients.
Table 1.
Characteristics of patients at diagnosis and at ATLAS trial entry (∼5 years later)
|
Any ER status |
ER-positive |
||||
|---|---|---|---|---|---|
| Continue tamoxifen to 10 years (n=6454) | Stop tamoxifen at 5 years (n=6440) | Continue tamoxifen to 10 years (n=3428) | Stop tamoxifen at 5 years (n=3418) | ||
| Status at diagnosis | |||||
| ER status | |||||
| ER-positive | 3428 (53%) | 3418 (53%) | .. | .. | |
| ER-negative | 625 (10%) | 623 (10%) | .. | .. | |
| ER-unknown | 2401 (37%) | 2399 (37%) | .. | .. | |
| Age, years | |||||
| <45 (median 40) | 1246 (19%) | 1236 (19%) | 640 (19%) | 630 (18%) | |
| 45–54 (median 49) | 2070 (32%) | 2076 (32%) | 1090 (32%) | 1099 (32%) | |
| 55–69 (median 61) | 2557 (40%) | 2567 (40%) | 1373 (40%) | 1357 (40%) | |
| ≥70 (median 73) | 581 (9%) | 561 (9%) | 325 (9%) | 332 (10%) | |
| Nodal status | |||||
| Node-negative | 3360 (52%) | 3354 (52%) | 1832 (53%) | 1845 (54%) | |
| N1–3 | 1667 (26%) | 1621 (25%) | 938 (27%) | 893 (26%) | |
| N4 or more | 968 (15%) | 965 (15%) | 536 (16%) | 534 (16%) | |
| Unknown | 459 (7%) | 500 (8%) | 122 (4%) | 146 (4%) | |
| Tumour diameter | |||||
| 1–20 mm | 2462 (38%) | 2463 (38%) | 1660 (48%) | 1620 (47%) | |
| 21–50 mm | 2749 (43%) | 2727 (42%) | 1309 (38%) | 1328 (39%) | |
| >50 mm | 620 (10%) | 628 (10%) | 251 (7%) | 252 (7%) | |
| Unknown | 623 (10%) | 622 (10%) | 208 (6%) | 218 (6%) | |
| Status at ATLAS trial entry | |||||
| Year of entry | |||||
| 1995–99 | 1538 (24%) | 1541 (24%) | 521 (15%) | 527 (15%) | |
| 2000–02 | 2755 (43%) | 2752 (43%) | 1415 (41%) | 1403 (41%) | |
| 2003–05 | 2161 (33%) | 2147 (33%) | 1492 (44%) | 1488 (44%) | |
| Previous duration of tamoxifen, years | |||||
| 4–4·9 | 2149 (33%) | 2129 (33%) | 1095 (32%) | 1081 (32%) | |
| 5–5·9 | 3690 (57%) | 3702 (57%) | 2103 (61%) | 2105 (62%) | |
| ≥6 | 615 (10%) | 609 (9%) | 230 (7%) | 232 (7%) | |
| Local recurrence before entry | |||||
| Yes (successfully managed) | 128 (2%) | 121 (2%) | 38 (1%) | 37 (1%) | |
| No | 6316 (98%) | 6307 (98%) | 3382 (99%) | 3373 (99%) | |
| Unknown | 10 (<1%) | 12 (<1%) | 8 (<1%) | 8 (<1%) | |
| Ever any contralateral primary | |||||
| Yes | 151 (2%) | 157 (2%) | 75 (2%) | 80 (2%) | |
| No | 6297 (98%) | 6276 (97%) | 3350 (98%) | 3332 (97%) | |
| Unknown | 6 (<1%) | 7 (<1%) | 3 (<1%) | 6 (<1%) | |
| Entire breast ever removed | |||||
| Yes | 4634 (72%) | 4563 (71%) | 2230 (65%) | 2162 (63%) | |
| No | 1819 (28%) | 1874 (29%) | 1198 (35%) | 1255 (37%) | |
| Unknown | 1 (<1%) | 3 (<1%) | 0 | 1 (<1%) | |
| Hysterectomy | |||||
| Yes | 1066 (17%) | 1160 (18%) | 620 (18%) | 679 (20%) | |
| No | 5359 (83%) | 5254 (82%) | 2792 (81%) | 2728 (80%) | |
| Unknown | 29 (<1%) | 26 (<1%) | 16 (<1%) | 11 (<1%) | |
| Menopausal status | |||||
| Premenopausal | 537 (8%) | 521 (8%) | 326 (10%) | 304 (9%) | |
| Postmenopausal* | 5778 (90%) | 5784 (90%) | 3035 (89%) | 3044 (89%) | |
| Perimenopausal or unknown | 139 (2%) | 135 (2%) | 67 (2%) | 70 (2%) | |
| Geographical distribution | |||||
| Europe, Australia, New Zealand, USA, and South Africa† | 2515 (39%) | 2529 (39%) | 1595 (47%) | 1599 (47%) | |
| Latin America‡ | 1759 (27%) | 1771 (28%) | 982 (29%) | 971 (28%) | |
| Asia and Middle East§ | 2180 (34%) | 2140 (33%) | 851 (25%) | 848 (25%) | |
ER=oestrogen receptor. ATLAS=Adjuvant Tamoxifen: Longer Against Shorter.
Artificial or natural menopause.
Predominantly of European origin.
Argentina, Brazil, Chile, Colombia, Cuba, Mexico, and Paraguay.
India, China, other Asia or the Middle East.
Figure 2 shows compliance with the trial treatment allocation. Among women who were without recurrence 2 years after entry (ie, at year 7 after diagnosis), 84% of those allocated to continue were still on tamoxifen compared with 4% of controls, a difference of 80%. Fewer than 1% of women were receiving any adjuvant endocrine treatment other than tamoxifen.
Figure 2.
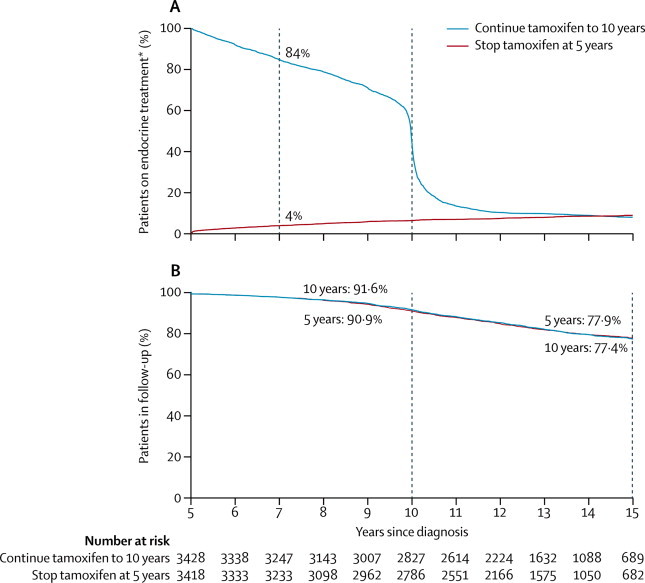
Treatment compliance (A) and proportion of patients in follow-up (B) by year since randomisation for 6846 women with ER-positive disease (54% node-negative)
*>99% tamoxifen.
Figure 2 also shows that the completeness of follow-up was similar in both treatment groups. In each group 91% of the survivors were still being followed up 10 years after diagnosis and 77% were still being followed up 15 years after diagnosis; these proportions will increase as more follow-up accumulates. Some incompleteness was due to a few centres withdrawing and stopping all follow-up in both treatment groups. Nine patients allocated to continue tamoxifen and ten controls withdrew consent to further follow-up, but their earlier follow-up is included. Log-rank analyses by allocated treatment, censored at the last available record, allow for any incompleteness of follow-up, yielding treatment comparisons with no material bias (especially as incompleteness did not differ between the two treatment groups).
We recorded 1328 recurrences (899 during years 5–9, 379 during years 10–14, and 50 after reaching year 15). Among women with ER-positive disease, allocation to continue tamoxifen reduced the risk of recurrence (617 recurrences in 3428 women allocated to continue vs 711 in 3418 controls; RR 0·84, 95% CI 0·76–0·94; p=0·002), reduced breast cancer mortality (331 deaths with recurrence in women allocated to continue vs 397 in controls, p=0·01) and reduced overall mortality (639 deaths vs 722 deaths, p=0·01). The risk of recurrence during years 5–14 was 21·4% for women allocated to continue versus 25·1% for controls (absolute recurrence reduction 3·7%); figure 3. There was no evidence of a rebound increase in the recurrence rate when tamoxifen treatment ended. Breast cancer mortality during years 5–14 was 12·2% for women allocated to continue versus 15·0% for controls (absolute mortality reduction 2·8%).
Figure 3.
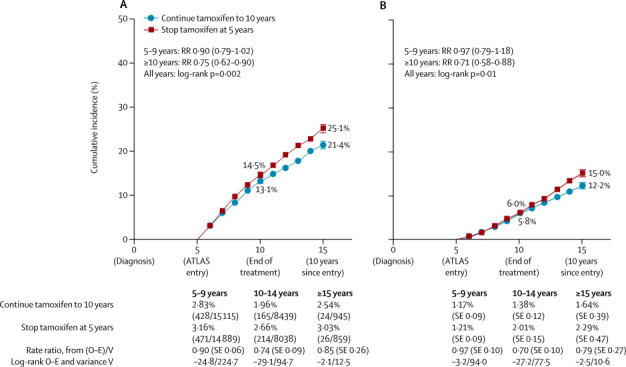
Recurrence (A) and breast cancer mortality (B) by treatment allocation for 6846 women with ER-positive disease
Bars show SE. Recurrence rates are percentage per year (events/patient-years of follow-up). Death rates (overall rate – rate in women without recurrence) are percentage per year (SE). ATLAS=Adjuvant Tamoxifen: Longer Against Shorter.
The main effects on recurrence and, particularly, on breast cancer mortality became apparent only during the second decade after diagnosis (figure 3). For recurrence, the RR was less extreme during years 5–9 (0·90, 95% CI 0·79–1·02; p=0·10) than after reaching year 10 (0·75, 0·62–0·90; p=0·003), but the heterogeneity between the RRs in the two time periods was not significant (p=0·10). For breast cancer mortality the RR was again less extreme during years 5–9 (0·97, 0·79–1·18; p=0·74) than after reaching year 10 (0·71, 0·58–0·88; p=0·0016), and the heterogeneity between the mortality RRs in the two time periods was significant (p=0·04).
To date, twice as many recurrences have been recorded during the first 5 years as during the second 5 years after randomisation, so taking all time periods together the overall recurrence RR in ER-positive disease is dominated by the first 5 years. Figure 4 shows various subgroup analyses for this overall result, with no significant heterogeneity of the proportional risk reduction with respect to patient or tumour characteristics or site of first recurrence. The reduction in distant recurrence is not separately significant in figure 4 (p=0·12 for distant recurrence as first event, but p=0·05 for distant recurrence at any time; appendix p 20).
Figure 4.
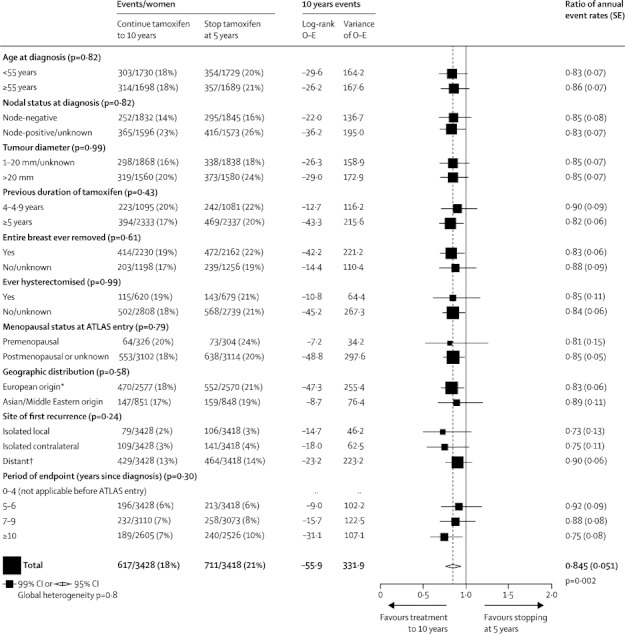
Recurrence by treatment allocation for 6846 women with ER-positive disease, subdivided by patient or tumour characteristics and location or time of first recurrence
*Europe, Australia, New Zealand, USA, Latin America, and South Africa (all predominantly of European origin). †Including multiple and unspecified sites.
Table 2 subdivides by ER status the effects of the treatment allocation on mortality with and without recurrence. For death with recurrence, the effect of the treatment allocation in ER-unknown disease seems to be intermediate between the significant effect already described in ER-positive disease and the lack of apparent effect in ER-negative disease.
Table 2.
Effects of allocation (continue tamoxifen to 10 years vs stop at 5 years) on mortality with and without previous recurrence in each category of ER status at entry, and on various outcomes without previous recurrence in all women of any ER status
|
Number of events |
Log-rank O – E | Variance of O – E | Event rate ratio (95% CI) | p value* | ||||
|---|---|---|---|---|---|---|---|---|
| Continue tamoxifen to 10 years | Stop tamoxifen at 5 years | |||||||
| Mortality analyses, by ER status | ||||||||
| ER-positive (3428 vs 3418)† | ||||||||
| Any death | 639 | 722 | −47·7 | 340·2 | 0·87 (0·78–0·97) | 0·01 | ||
| Death with recurrence | 331 | 397 | −32·9 | 182·0 | 0·83 (0·72–0·96) | 0·01 | ||
| Death without recurrence | 308 | 325 | −14·8 | 158·1 | 0·91 (0·78–1·06) | 0·24 | ||
| ER unknown (2401 vs 2399)† | ||||||||
| Any death | 625 | 635 | −10·5 | 314·6 | 0·97 (0·87–1·08) | 0·55 | ||
| Death with recurrence | 302 | 334 | −18·4 | 158·7 | 0·89 (0·76–1·04) | 0·15 | ||
| Death without recurrence | 323 | 301 | 7·9 | 155·9 | 1·05 (0·90–1·23) | 0·53 | ||
| ER-negative (625 vs 623)† | ||||||||
| Any death | 123 | 116 | 3·5 | 59·7 | 1·06 (0·82–1·37) | 0·66 | ||
| Death with recurrence | 63 | 63 | 0·0 | 31·5 | 1·00 (0·71–1·42) | 0·99 | ||
| Death without recurrence | 60 | 53 | 3·4 | 28·2 | 1·13 (0·78–1·63) | 0·52 | ||
| Any ER status (6454 vs 6440)† | ||||||||
| Any death | 1387 | 1473 | −54·7 | 714·5 | 0·93 (0·86–1·00) | 0·04 | ||
| Death with recurrence | 696 | 794 | −50·9 | 372·2 | 0·87 (0·79–0·97) | 0·008 | ||
| Death without recurrence‡ | 691 | 679 | −3·8 | 342·3 | 0·99 (0·89–1·10) | 0·84 | ||
| Analyses of events without prior recurrence‡, any ER status | ||||||||
| Death without recurrence | ||||||||
| Vascular death | ||||||||
| Stroke | 62 | 59 | 0·8 | 30·2 | 1·03 (0·72–1·46) | 0·89 | ||
| Pulmonary embolus | 10 | 8 | 0·8 | 4·5 | 1·21 (0·48–3·04) | 0·69 | ||
| Heart disease§ | 178 | 205 | −16·1 | 95·7 | 0·85 (0·69–1·03) | 0·10 | ||
| Neoplastic death | ||||||||
| Endometrial cancer¶ | 17 | 11 | 2·8 | 7·0 | 1·49 (0·71–3·13) | 0·29 | ||
| Other neoplastic disease | 78 | 75 | 0·4 | 38·2 | 1·01 (0·74–1·39) | 0·94 | ||
| Other death | ||||||||
| Specified cause | 171 | 161 | 2·3 | 82·9 | 1·03 (0·83–1·28) | 0·80 | ||
| Unspecified cause | 175 | 160 | 5·1 | 83·7 | 1·06 (0·86–1·32) | 0·58 | ||
| Second cancer incidence | ||||||||
| Contralateral breast cancer | 419 | 467 | −28·9 | 221·5 | 0·88 (0·77–1·00) | 0·05 | ||
| Endometrial cancer¶ | 116 | 63 | 24·8 | 44·8 | 1·74 (1·30–2·34) | 0·0002 | ||
| Primary liver cancer | 3 | 3 | −0·0 | 1·5 | 0·99 (0·20–4·90) | 0·99 | ||
| Colorectal cancer | 46 | 52 | −3·8 | 24·5 | 0·86 (0·58–1·27) | 0·44 | ||
| Unspecified site | 254 | 251 | −1·3 | 126·2 | 0·99 (0·83–1·18) | 0·91 | ||
| Non-neoplastic disease (ever hospitalised or died) | ||||||||
| Stroke | 130 | 119 | 3·8 | 62·2 | 1·06 (0·83–1·36) | 0·63 | ||
| Pulmonary embolus | 41 | 21 | 9·7 | 15·5 | 1·87 (1·13–3·07) | 0·01 | ||
| Ischaemic heart disease | 127 | 63 | −20·2 | 72·5 | 0·76 (0·60–0·95) | 0·02 | ||
| Gallstones | 75 | 66 | 3·7 | 35·2 | 1·11 (0·80–1·54) | 0·54 | ||
| Cataract | 72 | 63 | 3·5 | 33·7 | 1·11 (0·79–1·56) | 0·54 | ||
| Bone fracture | 62 | 70 | −4·9 | 33·0 | 0·86 (0·61–1·21) | 0·39 | ||
The log-rank analyses of death with recurrence are done by subtraction of the log-rank analyses of death without recurrence from those of any death. If O – E is negative, its value is about half the number of events prevented; if V is its variance, event rate ratio is exp([O – E] / V). ER=oestrogen receptor.
Two-sided.
In parentheses: number of women allocated to continue tamoxifen vs number allocated to control.
Delay of recurrence by continuation of tamoxifen increases woman-years at risk before recurrence by about 3% in ER-positive disease; the log-rank analyses allow for this, but crude comparisons of total numbers of events before recurrence do not.
Mainly heart disease, but includes all vascular causes apart from stroke and pulmonary embolus.
Mainly endometrial adenocarcinoma, but includes all other uterine tumours apart from cervical cancer; analyses of uterine tumour incidence exclude women with hysterectomy recorded at trial entry.
Sensitivity analyses that use the intermediate treatment effects in ER-unknown disease to help estimate the treatment effects in ER-positive disease did not materially alter the estimated RRs for breast cancer outcomes in all time periods (appendix pp 14–18), but made the p values for them somewhat more extreme (recurrence p=0·0009, distant recurrence at any time p=0·02, breast cancer mortality p=0·004, and all-cause mortality p=0·011).
For death without recurrence in all 12 894 women, irrespective of ER status, there was no significant effect of the treatment allocation, either overall or for death from any particular cause (table 2). In the hypothetical absence of any breast cancer mortality, the probability of dying from another cause during years 5–14 after breast cancer diagnosis would be 5% for women younger than 60 years at entry and 20% for older women.
Table 2 also describes various other events without previous recurrence in all women. For the incidence (hospitalisation or death) rates of specific diseases, RRs were: pulmonary embolus 1·87 (95% CI 1·13–3·07, p=0·01 [including 0·2% mortality in both treatment groups]), stroke 1·06 (0·83–1·36, p=0·63), ischaemic heart disease 0·76 (0·60–0·95, p=0·02), and endometrial cancer 1·74 (1·30–2·34, p=0·0002 [including all non-cervical uterine cancers]). The cumulative risk of endometrial cancer during years 5–14 was 3·1% (mortality 0·4%) for women allocated to continue tamoxifen versus 1·6% (mortality 0·2%) for controls (absolute mortality increase 0·2%).
Discussion
Previous trials have shown that, for women with ER-positive early breast cancer, 5 years of adjuvant tamoxifen substantially reduces recurrence rates throughout the first 10 years after diagnosis and substantially reduces breast cancer mortality throughout the first 15 years.1 Thus, the effects of 5 years of treatment with tamoxifen on annual rates of mortality persist for at least a decade after treatment ends. Because of this carryover benefit after only 5 years of tamoxifen, it was already recognised4 when ATLAS began that there could well be little additional benefit during the first few years of additional treatment, even if worthwhile benefit would emerge later. With an average of 7·6 woman-years of further follow-up after entry at 5 years, the findings thus far available conform with these expectations. ATLAS has now shown that, compared with stopping after only 5 years of tamoxifen, continuing for another 5 years (to 10 years) provides further protection against recurrence and breast cancer mortality, particularly after reaching 10 years.
Table 2 includes the prespecified protocol analysis of overall mortality in all women in ATLAS irrespective of ER status, but this is less informative than are the main analyses in the present report of breast cancer outcomes in women with ER-positive disease. Inferences about the effects of tamoxifen on recurrence and on breast cancer mortality in ER-positive disease can be made either from these main analyses or from the sensitivity analyses involving ER-untested disease (appendix pp 14–18), although these sensitivity analyses are somewhat dependent on the assumed proportion that would have been ER-positive if tested. Irrespective of whether the main or the sensitivity analyses are preferred, the results are similar. Moreover, as follow-up was equally thorough in both treatment groups, ascertainment of mortality (particularly after treatment ends) is not materially biased by controls not having been given placebo tablets.
By combining results from the previous trials1 with the new results from ATLAS, we can estimate what would be seen in trials of 10 years of adjuvant tamoxifen compared with no treatment. Table 3 provides, by time since diagnosis, the recurrence RRs from the trials of 5 years of tamoxifen versus no tamoxifen and from the ATLAS trial of continuing tamoxifen to 10 years versus stopping at 5 years, and multiplies these RRs together to estimate what would be seen in trials of 10 years of tamoxifen versus no tamoxifen. Table 3 also provides similar estimates for breast cancer mortality. The recurrence and, particularly, the mortality findings are remarkable, and suggest that in trials of 10 years of tamoxifen versus no tamoxifen, breast cancer mortality rates during the second decade after diagnosis would be almost halved, although the real finding is not the point estimate but the CI (which shows that the reduction could be as little as a third rather than a half).
Table 3.
Event rate ratios (95% CIs) in ER-positive disease, by time period from diagnosis
| A: effects in meta-analyses of the trials of 5 years of tamoxifen vs none1(n=10 645) | B: effects in the ATLAS trial of continuing tamoxifen to 10 years vs stopping at 5 years (n=6846) | C: estimated effects in a trial of 10 years of tamoxifen vs none (product of A and B) | |
|---|---|---|---|
| Recurrence | |||
| 0–4 years | 0·53 (0·48–0·57)* | 1 | 0·53 (0·48–0·57)* |
| 5–9 years | 0·68 (0·60–0·78)* | 0·90 (0·79–1·02) | 0·61 (0·51–0·73)* |
| ≥10 years | 0·94 (0·79–1·12) | 0·75 (0·62–0·90)† | 0·70 (0·54–0·91)† |
| Breast cancer mortality | |||
| 0–4 years | 0·71 (0·62–0·80)* | 1 | 0·71 (0·62–0·80)* |
| 5–9 years | 0·66 (0·58–0·75)* | 0·97 (0·79–1·18) | 0·64 (0·50–0·82)‡ |
| ≥10 years | 0·73 (0·62–0·86)‡ | 0·71 (0·58–0·88)§ | 0·52 (0·40–0·68)* |
(A) Trials of 5 years of tamoxifen (n=10 645; ∼80% complied). (B) ATLAS trial of 10 years vs 5 years of tamoxifen (n=6846; ∼80% difference in tamoxifen use [figure 2]). (C) Hypothetical trial of 10 years of tamoxifen vs none (with ∼80% compliance). Two-sided p values in this table relate to particular time periods; values elsewhere combine all time periods. ER=oestrogen receptor.
p<0·00001.
p<0·01.
p=0·0001.
p=0·0016.
However, both in trials of 5 years of tamoxifen1 and in ATLAS, there was a difference of only about 80% between the prevalence of tamoxifen use in the two treatment groups, so the estimates in table 3 are likewise of what would be seen in trials of 10 years of tamoxifen compared with no tamoxifen that had only about 80% compliance. The risk reduction achievable by full compliance with 10 years of tamoxifen should, therefore, be appreciably greater, strengthening the conclusion that breast cancer mortality during the second decade after diagnosis (or at least during years 10–14) can be approximately halved. Thus, good evidence now exists that 10 years of tamoxifen in ER-positive disease produces substantial reductions in rates of recurrence and in breast cancer mortality not only during the first decade (while treatment continues) but also during the second decade (after it ends).
Continued follow-up of ATLAS will eventually yield further evidence about effects on breast cancer outcomes during the second decade after diagnosis. Before then, substantial additional information about events during the second decade will have been contributed by the other trials of continuing tamoxifen to 10 years versus stopping at 5 years (particularly aTTom, the UK counterpart of ATLAS, which reported little benefit during years 5–9 but has not yet reported on outcomes during the second decade13). EBCTCG meta-analyses of ATLAS, aTTom, and the smaller trials will eventually clarify the effects on breast cancer outcomes 10–14 years after diagnosis (panel).
Panel. Research in context.
Systematic review
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) periodically reviews trials of adjuvant tamoxifen versus no tamoxifen in early breast cancer and of longer versus shorter tamoxifen durations. The EBCTCG's meta-analyses1,2 show that, in oestrogen receptor (ER)-positive disease, 5 years of adjuvant tamoxifen substantially decreases breast cancer recurrence, breast cancer mortality, and overall mortality (despite small absolute increases in endometrial cancer and pulmonary embolus). Previous trials have not, however, answered the question of how 10 years of tamoxifen compares with only 5 years. Because the decrease in breast cancer mortality produced by 5 years of tamoxifen continues to be substantial for a decade after treatment ends (ie, throughout the first 15 years after diagnosis), trials of 10 years versus 5 years of tamoxifen will need to be followed up for at least 15 years from diagnosis.
Interpretation
The Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial, with a mean of 7·6 years of further follow-up after entry at year 5, shows that recurrence and breast cancer mortality during the second decade after diagnosis are reduced more effectively by 10 years of adjuvant tamoxifen than by 5 years. Although known side-effects were increased (at least in postmenopausal women) by longer treatment, the absolute reduction in breast cancer mortality was an order of magnitude greater than the absolute increase in mortality due to these side-effects. Taken together with the results from trials of 5 years of tamoxifen versus none, the results from ATLAS show that 10 years of effective endocrine therapy can approximately halve breast cancer mortality during years 10–14 after diagnosis. Longer follow-up of ATLAS (and a meta-analysis of all such trials) will be needed to assess the full benefits and hazards throughout the second decade.
Tamoxifen produces favourable lipid profile changes19–21 and the ATLAS results do suggest some protection against ischaemic heart disease. Because, however, no significant protection against heart disease was seen in trials of tamoxifen versus no tamoxifen,1,2 the apparent reduction in ATLAS might be largely a chance finding (especially since the apparent protection was against events after the treatment period, and long-term follow-up of trials of cholesterol-lowering statin treatment find little further benefit after treatment ends22). Conversely, although the US Food and Drug Administration lists stroke as a possible side-effect,5,23 no apparent increase in stroke incidence or mortality was seen during the treatment period either in ATLAS or in the trials of 5 years of tamoxifen.1
However, definite long-term side-effects of tamoxifen do exist, which require longer follow-up and meta-analyses of all relevant trials for final assessment. In both ATLAS and the trials of 5 years of tamoxifen versus no treatment,1,2 tamoxifen increases the incidence of endometrial cancer in postmenopausal women who had not had a hysterectomy before trial entry. Although there is little risk in premenopausal women, life-table calculations for older women (together with allowance for the imperfect compliance with treatment allocations in trials) suggest that actual use of 5 years of adjuvant tamoxifen would produce an absolute 15 year endometrial cancer risk of about 2–3%,1 and that use of 10 years rather than 5 years of tamoxifen would produce an additional risk by year 15 of about 2%.
The death rate from endometrial cancer was, however, only about a tenth of the incidence rate, suggesting that full compliance with 10 years of tamoxifen in postmenopausal women would produce a 15 year risk of a few per thousand of eventually dying from the excess of uterine cancer. This risk is greatly outweighed in ER-positive disease by the decrease in breast cancer mortality.
For tamoxifen, 10 years of treatment has greater protective effects against ER-positive breast cancer than does 5 years of treatment, so the same might well be true for any comparably effective endocrine treatment, either with another selective oestrogen receptor modifier or, in postmenopausal women, with an aromatase inhibitor.24 In both cases, 10 years of treatment should be expected to have a greater protective effect than 5 years of treatment would have, although other endocrine treatments can, like tamoxifen, also have long-term side-effects.
For premenopausal women with continued ovarian activity, however (among whom aromatase inhibitors are not an alternative to tamoxifen), there is little risk of tamoxifen causing uterine cancer or vascular side-effects to counterbalance the large absolute reduction in breast cancer mortality. Hence, our results are particularly relevent to premenopausal women with ER-positive disease—and, young women protected by 10 years of tamoxifen from death from breast cancer gain several decades of life expectancy.
This online publication has been corrected. The corrected version first appeared at thelancet.com on February 11, 2013
Acknowledgments
Acknowledgments
Our chief acknowledgment is to the women who participated in the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) study, the collaborating doctors, nurses, and data managers in many institutions in many countries, the international co-ordinating centre in the Oxford Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) and the CTSU randomisation service and infrastructure. Ian Jackson (deceased) greatly facilitated establishment of the study.
Contributors
CD, RG, RC, JG, MC, and RP designed the trial. AD, HP, JG, and YW (for China) did the data analyses. MA, VHA, RA, AB, XB, JB, SRD, JFF, PH, M-FH, MI, HK, JK, W-HK, BSM, BM, AN, OP, FP, LP, TP, VR, MTR, ST, GU, and MV (alphabetic) coordinated more than 200 women per country or region. BR was the top randomiser. CD, HP, JG, RG, RC, and RP performed statistical analyses, interpretation, and report writing. All authors revised the report.
ATLAS coordinating centre and CTSU supporting staff (1995–2012)
Principal investigator: Christina Davies. Administrative office: Jenny Sayer, Valerie Collett. Central randomisation: Jill Crowther, Angela Radley. Analysts/programmers: Antonella Delmestri, Jon Godwin, Yaochen Wang. Statisticians: Richard Gray, Hongchao Pan, Richard Peto. Former staff: A Beighton, M Forster, A Headon, C Hope, S Knight, P McGale, S Mozley, H Monaghan, A Muldal, A Naughten, S Turner. CTSU also prepared the software that randomised in four regional or national coordinating centres (Australia/New Zealand: National Health & Medical Research Council Clinical Trials Unit; Italy: Consorzio Mario Negri Sud; Japan: Tokyo University Department of Epidemiology and Biostatistics, then from 2001 Japan Clinical Research Support Unit; Spain: Institut d’Investigació Biomèdica Sant Pau, Barcelona [FIS PI020391]). CONAC (Corporación Nacional del Cáncer: Director C Agosin) distributed Nolvadex in Chile.
Data monitoring committee
Chair: R Souhami. Former chair: R Doll (deceased). Current members: D Altman, M Baum, R Collins, K I Pritchard, D Simpson. Former member: K Dickersin.
Steering committee
Co-chairs: R Arriagada, V Raina. Former chair: C Williams. International adviser: A Goldhirsch. Members: the national/regional coordinators (see below) and the Oxford Secretariat.
Collaborators
Collaborators are listed by country or region (names in brackets show the past and present national or regional coordinators, and names in parentheses show current national or regional administrator. Numbers show number of patients entered with any previous tamoxifen duration). Argentina [Mirta Abraham, Reinaldo Chacón], (Fany Pernas), 893—H A Abud (deceased), C M Alasino, C A Algamiz, J A Alvarado Velloso (deceased), A M Alvarez, A Alvarez Gardiol, E Alvarez Gardiol, R Arca, H Arocena, L A Barbera, C Bas, A J Benitez Gil, M Brown Arnold, A O Bustos, L B Cedaro, E L Cigno, F A Colo, A Colombo Berra, F S Coppola (deceased), R Delia, M E Dominguez, M Fages, L E Fein, A N Genovese, N A Giacomi, I Gomez, E Gonzalez Vera, A Hannois, A P Hunis, N La Palma (deceased), D E Levy, M R Machiavelli, A Marantz, M J Matwiejuk, M S Morante, C Paleta, G V Pascon (deceased), M E Pascual, E J Pejko (deceased), H R Requejo, M d l A Rodriguez (deceased), O Rodriguez Nievas, A Rossi (deceased), R L Santos, S Scalbi, F Sousa Martinez, J C Staringer, O J Verdier, S A Vigo, S Zunino. Australia and New Zealand [John F Forbes, ANZ Breast Cancer Trials Group; regional coordination Susan R Davis, Monash University Women's Health Program], (Dianne Lindsay, Jo Bradbury), 784—A S Abdelaal, E Abdi, S Ackland, V Ahern, C Atkinson, S Babington, C Baker, P Barker, S Baron-Hay, P Beale, M Beevors, S Begbie, J Beith, D Bell, R Bell, C Benjamin, D Birks, R Blum, A Bonaventura, R Bond, A Boyce, M Boyer, F Boyle, J Bradbury, B Brigham, N Bright, R Burcombe, I Byard, D Byram, L Castles, J Childs, M Chipman, J Chirgwin, A Coates, S Cox, P Craft, R de Boer, G Delaney, S Della-Fiorentina, J Dewar, P Donnelly, M Eastman, B Evans, A Falkov, B Fitzharris, M Francis, J Freund, M Friedlander, H Gan, S Gauden, V Gebski, D Gillette, A Goldrick, D Goldstein, R Gourlay, A Green, P Gregory, J Grygiel, I Gunn, I Haines, P Harnett, M Harris, R Harrup, S Hart, V Harvey, D Hastrich, A Hayden, T Hemanth, B Hickey, M Holocek, M Hughes, V Humeniuk, D Ingram, A Iseli, D Jackson, G Jacob, A Jamieson, M Jeffery, C Jose, G Kannourakis, R G Kay, J Kiffer, H Krawitz, D Leong, R Levy, C Lewis, R Linacre, L Lipton, D Logan, R Lowenthal, R Lynch, L Macnab, E Maguire, G Marx, R Masters, M McCrystal, J McKendrick, B McLeay, R McLennan, P Mercer, G Mitchell, P Mitchell, E Moylan, N Pavlakis, S Porges, D Porter, I Porter, T Porter, C Pyke, B Robinson, J Rutovitz, F Sardelic, M Schwarz, B Scott, E Segelov, R Sillar, J R Simes, B Smee, T Smith, N Spry, R Stanley, J F Stewart, J Stewart, D Stoney, A Sullivan, K Sunderland, P Thompson, D Thornton, M Tin, D Townend, S Turner, C Underhill, G van Hazel, D C A Walsh, H Wheeler, K White, S White, C Wynne, A Young, R Young. Belarus (Tatiana Kostetskaya), 146. Belgium [Martine Piccart], (Sylvie Bartholemus), 132—A Awada, F Cardoso, M Closon, D V F J Cocquyt, P Coucke, P DeFoort, I Deleu, D H Depypere, E Everaert, F Geurs, J Kains, P Neven, D R Serreyn, R van den Broecke. Brazil [Victor H Medeiros Alencar, previously H M Salvador Silva], (Elivania d O Vieira), 1465—W Almeida Jr, O Alves Barbosa, S S Araujo, I Archangelo Jr, I Arruda, C Nogueira Barreira J Batista Lucena, G Bezerra Pinheiro, M Caleffi, J T Campos Avelar, V d F Coutinho Maia Silva, E E Cronemberger, A B Diogenes, V R Emiliano, R d Freitas, A d Freitas Torres, R Farias, M D S C Freitas, M A A Funke, C Fuschino, D d Gama Dantes, C Gaspar, E Hoffman, S F Juacaba, S H Lopes Marques, F Lorenzato, A Maroja, R Maroja, E Marques, M d O Matias, G P Medeiros, P H Melo, F A Miranda Henriques, M Monteiro, J W Mourao de Farias, J Moura, A P Mousinho, A M Murad, L Murillo Britto, A A Nonata d Andrade, P Pascoto, J Peixoto, G Peixoto Lima, M A Pereira, G d S Pinheiro, T Prado Wanderley, J H Reis, R A Ribeiro, V Ritter, R M Sales, M S Alencar, R Santiago Almeida, N Stenger, C Studart Leal, C Tosello de Oliveira, L L Vianna, S Zerbini, D Coelho d Sá, I A guiar Porto, G A d Moraes. Chile [Octavio Peralta, Bettina Müller, previously Rodrigo Arriagada] 1603—E A Acevedo, J C Acevedo, R O Arevalo, R Baeza, A Belmar, M E Bravo, L Bronfman, J Camacho, M Campos, B Cardemil, P Carvajal, B Cerda, F Cisternas, E Contreras, J F Cornejo, A J Cubillos, C del Castillo, P Escobar, M Fernández, A Fica, M Fritis, G Gambi, O Giannini, L S Gomez, J Gutiérrez, C Hales, R Hasbún, P Huidobro, R Iglesis, O S Jury, H Krause, J M Lagos, A León, M León, J Madrid, C Mariani, R Martínez Lepe, R Martínez Rogers, L A Matamala, D Moreno, P Núñez, A Olcesse, P Olfos, L Orlandi, W Ortúzar, Y Pabst, H Paredes, E Pérez, J Pierart, V Pineda, A Pinto, Z Pizarro, G Rey, O Rivas, M Rodríguez, H Rojas, J Rosas, P Ruíz de Viñaspre, J M Russo, S San Martín, L Sepúlveda, J Solé, J Steinberg, M Toledo, C Torres, J M Torres, R Torres, S Torres, A Uribe, M Valenzuela, C Vásquez, I M Vigneaux, G Vigueras, A Vila, H Villagrán, M Villalón, V Zambrano, Z Zlatar, M Zúñiga. People's Republic of China [Beijing: Yongfu Shao, Xiang Wang, Oxford: Yaochen Wang], 147—F Fan, Y P Gong, J L Huang, Z H Jin, W L Li, Z W Lin, H J Liu, P Liu, Q L Liu, X T Ma, D Pang, X M Qiao, K D Shi, Y Q Sun, L Wang, Y Z Wang, T Wu, Z B Xie, B Zhang, W Q Zhang, H P Zhao, W P Zhou, J B Zhu. Colombia [Claudia Ramirez, previously Carlos Castro], 74. Croatia [Damir Vrbanec], 37: D Herceg, A Juretic, I Martinovic, V Paulinic-Diminic, D E Pezerovic, S Plestina. Cuba [Ramon d J Ropero, previously Rolando Camacho], 110—R I Alvarez, R M Amador, M A Arbesu, L Ballesta, M Catala, A de la Torre, M Domecq, X Escobar, S Franco (deceased), M O’Farril, I Perez, A Reyes de la Paz, I Rodriguez, R Rodriguez, I Sanchez, J L Soriano, Z Valdes. Czech Republic [Lubos Petruzelka, Olga Pribylova (deceased)], 942—D Adamkova-Krakorova, M Ambrus, J Barkmanova, L Barsova, J Bartos, O Bednarik, V Benesova, M Brychta, M Brychtova, S Cahova, M Chodacka, J Chovanec, V Cmejlova, P Coupek, K Cwiertka, J Finek, M Hacklova, L Hanus, E Helmichova, M Holikova, J Holub, H Honova, K Hovorkova, P Hrabetova, P Hubnerova, L Hudinkova, D Justrova, H Kankova, P Karasek, M Kaspar, E Kindlova, I Kiss, I Kocak, M Kohoutek, I Kolarova, B Konopaisek, J T Kozak, R Kozevnikovova, L Kozisek, K Krizan, K Kubackova, M Kubecova, M Kuta, P Lemez, I Lorenz, L Loukotkova, S Lukesova, M Lysy, H Macharova, D Mackova, P Mares, S Martin, V Muller, R Neumanova, F Novy, L Ostrizkova, L Pavel Pavlov, S Pluhacek, J Prausova, J Pribylova, J Ruzickova, M Safanda, J Salvet, E Sedlackova, S Semonska, Z Seneklova, M Smakal, M Soumarova, S Spelda, V Spurny, W Strzondala, P Tesarova, V Tomancova, M Tomanova, K Trskova, H Vesela, K Vondrackova, J Vydra, R Vyzula, M Zemanova, M Zimovjanova, M Zvolsky. Egypt [Hussein Khaled], (A Badran), 465—S E Abd-Elmoneim Khalil, F Abu Taleb, A Badran, H El Akkad, A El-Khodary, R M Gaafar, N Gad El Mawla (deceased), A Hablas, K Ismail (deceased), M Moneer, M S Zaghloul. Estonia [Vaino Ratsep], 12—A Kurvet, R Kutner, L Vahter. France [coordinated from Oxford Office], 34—P Bernard, C Hill. Greece [Christos Alexopoulos, Evangelia Razis], 12. Hong Kong Special Administrative Region [Wing Hong Kwan], 549—G K H Au, P Chan, R T Chan, A Cheng, C Chi Kin, W Foo, H-C Cheng, C Kwok, D Kwong, C Leung, W L Leung, R Liu King Yin, M Y Luk, R K Ngan, S K O, S W K Siu, J Suen, C C Tong, M Tong, S Y Tung, T K Yau, H Yiu. India [Vinod Raina, previously Indraneel Mittra], 3001—S H Advani, R A Badwe, B C Bakane, M N Bandyopadhyay, A Chandrasekharan, A Chaturvedi, A K D’Cruz, A C Deka, R Digumarti, M Dinesh, K A Dinshaw (deceased), J P Doshi, G Durgaprasad, R Gopal, V K Gupta, R Hawaldar, S Jain, K Jayakumar, S R Joharapurkar, S John, P K Julka, R Khanna, K C Kothari, M V Kumar, S Kumar, C Madhu, A Mathew, B S Mathew, B Mathor, A Mehta, F Mehta, G Mehta, N C Misra, S Misra, Y Nalini, H P Panchal, B Parekh, V Parmar, R Parshad, D D Patel, V Patel, S Premkumar, R Radhika, B Rajan, S K Sarkar, V Seenu, A Sengupta, P Shah, A Sharma, S C Sharma, K K Shenoy, H S Shukla, N K Shukla, S N Shukla, A Singh, K K Singh, A Srivastava, K Subrahmaniyam, P K Sur, H B Tongaonkar, R Vashisht, K V Veerendra Kumar, S Vijaya. Iran [Peiman Haddad], 247—K Dehshiri, F Amouzgar-Hashemi, H Madani, S H Mortazavi (deceased), M A Mousavizadeh, J Raafat, B Sadrolhefazi, M Tabatabaeefar. Ireland [T Finnegan], 1. Israel [Moshe Inbar, previously Noa Ben-Baruch, Adi Shani], 253—F Barak, N Ben-Zui, R Catane, M Dinerman, E Evron, A Figer, M Gips, H Hayat, E Idelevich, R Isacson, V Kopp, F Kovner, L Marchasin, D Matcejevsky, L Olga, E Perepechi, I G Ron, T Safra, D Sarid, S Shlanger, E Shumeli, E Tepper, N Yaal-Hahoshen (deceased). Italy [Antonio Nicolucci], (Miriam Valentini), 292—G Amiconi, F Ascione, S Banducci, G Baratelli, F Battistelli, R Bianchi, S Bravi, A Chiara, B Dall’Omo, A De Matteis, F Di Costanzo, L Di Lullo, B di Nardo, S Ferrari, R Fiore, G Fornari, G Gini, M Giordano, M Giovannini, A Gravina, L Isa, L Laudadio, G Luchena, G Mantovani, M Marcellini, E Mari, P Marpicati, A Martoni, E Massa, A M Molino, A Nuzzo, R Pedersini, E Piana, N L Pinna, M C Pirozzoli, F Recchia, A Riccardi, F S Robbiati, E Rossi, M Sannicoló, G Ucci, M C Valli, C Zamagni. Japan [Yasuo Ohashi, Tadashi Ikeda, previously Yasuo Nomura], (Emi Yoshida) 137—S Akashi, K Aogi, E Arita, H Aoyama, A Emi, J Fujisawa, K Fujiwara, T Fukutomi, R Haruta, Y Hata, T Hayashida, T Hojo, Y Hozumi, H Inoue, K Ishida, M Ishida, C Kanbayashi, D Kanke, A Kataoka, A Kato, N Kato, N Katsumata, Y Kawabuchi, H Kawaguchi, M Kitajima, S Kobayashi, M Kodaira, C Koga, S Mitsuyama, M Miyauchi, E Mori, S Murakami, K Nagao, Y Nakamura, S Nishimura, R Nishimura, M Ohba, T Onishi, S Ohno, T Osako, S Osumi, M Sakata, M Sano, T Sato, Y Sato, H Shigematsu, H Takahashi, Y Takahashi, S Takashima, H Takenaka, K Tamae, K Tanaka, T Toge, M Toi, Y Uchida, N Uchiyama, H Yamaguchi, N Yamamoto, H Yamashita, T Yoshiyama. Latvia [Juris Berzins], 92—M Bitina, I Gailite, G Keire, T Purkalne, M Ratiani, Z Zvirbule. Lithuania [Konstantine Valuckas], 112—M Aizenas, D Andzeviciene, V Caropaite, A Cesas, A Ciceniene, E Juodzbaliene, J Kurtinaitis, A Luksyte, N Satkauskiene, G Smailyte, L Tamoshaitite, A Zlabiene. Mexico [Juan Zinser], 82—A Erazo Valle, E Maafs, M T Ramirez. Netherlands [Emiel Rutgers, Otilia Dalesio], (Lidwina Wever) 76—G Algie, J W Baars, J Belderbos, R Boom, J A C Brakenhoff, O Dalesio, C A M de Swart, B de Valk, V Harskamp, O C Leeksma, K J Roozendaal, J Schrama, A K F Tanka, W E Terpstra, F Van Coevorden, D van Geldere, H Veen, S C Veltkamp, P Voogt. Oman [V Raina], 5—B A Bahrani. Paraguay [coordinated from Oxford], 2—R Abed. Poland [Tadesuz Pienkowski, Maryna T Rubach], (J Kielanowska), 890—H Bassara, D Boguszewska, A Brandys, E Brewczynska, M Chudzik, K Czyzewska, I Debicka, T Dobielinska-Eliszews, E Filipczyk-Cisarz, M Foszczynska-Kloda, L Frackowiak, A Garncarek, J Giermek, E Glinka-Malasnicka, M Gornas, W Hajdukiewicz, P Hudziec, A Jagiello-Gruszfeld, M Jonca, B Karczmarek-Borowska, J Kielanowska, P Koralewski, B Koscianska, A Kucharska, P Kukawski, M M Kurianowicz, M Litwiniuk, M Marczak-Zietkiewicz, R Muchacki, J Oberc, K Pajak, A Pawlaczyk, J Perual, A Pienkowski, W Piskorski, M Pysz, M Rubach, Rutkowski R, M Sikorska, A Smietana, A Songin, M Stolarek, G Stopyra, M Suszko-Kazarnowicz, E Szybicka-Fliskowska, M Talerczyk, A Walaszkowska, K Warzocha, B Wawrzynczak, J Wegrzyn, B Wlonska-Kusy, J Wojtacki, K Zabkowska, A Zielezinska, B A Ziemba, D Zuziak, I Zygulski. Portugal [Helena Gervasio], 68—J E Albano, T Carvalho, P Madeira, I Pazos. Russia [Vladimir Semiglazov], 320—N Barash, S Beljakov, A Bozhok, I Bulavina, O Burdaeva, S V Cheporev, M Cherenkova, V Chissov, L A Churilova, H Dmitrina, K Feodorov, T Fisanovich, v A Gorbounova, O Ivanova, L A Koroleva, S Kozhevnikov, A Lazarev, V Luppov, L Magcoeva, G Manikhas, D Melnikov, R Orlova, V Petrova, E Pogodina, V Predit, N Raevskaya, T Ricova, L Roman, I Seleznjeva, M V Shomova, S V Sidorov, J Spheglov, P Svetlouno, J Talovskiy, O M Vtoraya. Republic of South Africa [coordinated from Oxford, previously Elizabeth Murray, David Dent], 84—N Bental, R Claus, C Cox, S Giles, A Gudgeon, A Hemus, E McEvoy, D H Mokone, D Moodley, S Mundawarara, G Paris, B Pokharel, B L Rapoport, P Ruff, D Salton, E Sebastian, A Shelley, H Simonds, F Sitas, A Tarr, C Tarukandirwa, A L van Wijk, I D Werner. Spain [Xavier Bonfill], (Gerard Urrútia, Esther Canovas Martinez, Sera Tort), 1730—M V Abrio, C Adansa, J Aguiar Morales, A Albero, J Alfaro, V Alija, D Almenar Cubells, C Alonso, A Alonso Amigo, G Alonso Curbera, E Alonso Redondo, I Alvarez, J Andrade Santiago, R Andrés, S Antolín Novoa, M I Antón, M A Arcusa Lanza, A Arrivi, A Avellà, J P Avellanal Barral, M A Badia i Canto, P Ballesteros Garcia, M d M Barros, R Bastús-Piulats, J N Batista López, E Batiste-Alentorn, J Belón Carrión, M Benavides, R Bernabé Caro, R Blanco Guerrero, U Bohn Sarmiento, M Boleda, C Bosch, J J Bretón Garcia, M A Burillo Cordero, J Buxó i Costa, E Calvo, L Calvo, F Capdevila, F J Carabantes, T Cardona Hernández, E Carrasco, J Casado, A Casas, S Catot Tort, J I Chacón López-Muñiz, M Chaves Conde, L Cirera i Noguera, E Ciruelos, R Colomer, J Cruz, M A De la Cruz, L De La Cruz, H De La Cueva, R Del Moral, M Domènech, F Dominguez Cunchillos, O Donay, J Dorta Delgado, A Duque Amusco, A Escobedo, P España Saz, E Espinosa Arranz, C Esteban Esteban, A Etxeberria, X Fabregat Mayol (deceased), C Falo, E Fernández Bautista, A Fernández Ortega, E Fonseca Sánchez, R M Franquesa Granell, E Gallardo, O Gallego, P Gallurt Moreira, M García, I García Carbonero, M J García López, M García Pérez, A Garrido Saldana, L Garrigós, M Gay, J R Germà Lluch, M Gil, U Giménez, A Gómez Bernal, B González, A González Del Alba, R González Del Val, L M González de Sande, R González Mancha, M M Gordon, C Gravalos Castro, I Guasch i Jordan, M Guillot, J Hornedo, L Iglesias Pérez, M D Isla, M A Izquierdo, E Jiménez Orozco, L Jolis López, I Juez, J M Laín, R Lasso de la Vega, M L Limón, M Llanos, P López, L J López Gómez, J J López López, A Lorenzo Penuel, A M Lozano Barriuso, R Llorente, H Manzano, J Martín Broto, B Martínez, P Martínez del Prado, G Martín García, C Mendiola, F Molina, C Molins, A Montano, J Montesinos, J A Moreno Nogueira, J Múñoz, M Múñoz, V Muñoz Madero, M T Murillo, M Nogué, M Noguer, B Ojeda, S Olmos, J Oramas, L Palomar, P Pastor, R Pérez Carrión, M A Pérez Escutia, J Pérez Olaguer, M d M Pérez Pérez, J Pérez-Regadera Gómez, C Pericay, J Petrement Briones, J M Piera Pibernat, A Plazaola, J Rifà, J A Rivero, A Rodríguez, J M Rodríguez, R Rodríguez López, P Rodríguez Navarro, M Roget, M Ruiz Borrego, A Saenz, J Safiz, E Saigí, M A Sala González, C Salas Buzón, J Saldana, M Salgado, V Sanahuja, A Sánchez Ruiz, J Sanz Lacalle, M Sanz Martín, J J Satrústegi, J Schneider, M A Seguí, S Servitja, M Sureda González, J Terrasa, A Tres Sánchez, I Tusquets, G Urrútia, V Valentín, M Valladares Ayerbes, S Vázquez, A Velasco, P Vicente, M J Villanueva, J Virizuela Echaburu, P Zamora Aunon. Taiwan, China [Ming-Feng Hou], 253—K M Chen, C-Y Chung, S-S Du, C-S Huang, V C Kok, S-J Kuo, C Lin, S-H Tu, Y-G Soong, J Y Zhang. Tunisia, 2—K Rahal. Turkey [Cemalletin Topuzlu], 55—I Aslay, D E Baltali, U Berberoglu, H Bolukbusi, E Buyukunal, S Camlibel, M Dincer, E U Erkocak, D E Ozdedeli, N Ozturk. UK (1 South African patient followed up in UK)—C De Souza, L D Harris. USA [coordinated from Oxford], 138—A J Afrookteh, W Allgaier, R Andrade, A Arevalos, K Armstrong, A Barnes, H Barnes, S G Barnes, S P Bazeley, R Blum, R Bowles, S Branton, M L Breslin, D S Bryant, D W Bryant, R Bullock, T Campana, R L Carter, R Chlebowski, J Choper, K Coady, T Coe, S Cohen, M Cotner, L Crocket, A Cruz, A Cutugno, S Davidson, A Dedona, S E El-Eid, E Eskander, A Estabrook, S Feldman, P Forth, S Francella, E Frankel, R B Fritchley, M Gillis, R Goldberg, G I Grad, S Gran, M Grossbard, L Hamilton, S Hasan, A Hassan, C Ho, L B Holt, J O Hopkins, J Infantolino, C M Jones, S Jones, M R Kinney, R A Kloss, P Kozuch, J Lalli, N La Marche, C Lepis, W Lerner, E Longenbach, M L Machuca, M Makhmetov, C Makowski, B Malliah, O McBeth, P Mencel, M Mesnard, A Messeih, T Mirzoyev, M Moraes, D Morrison, J Mueller, K Nahum, D Neville, B M O’Connor, M Ohler, R Orwoll, T Pagana, W Peck, T Peterson, J Pezzimenti, J Potter, D T Poulis, P G Rausch, G River, D K Roeder, B Schaeider, M Schreiber, N Shah, R B Shaw, A Smith, M E Stark, S Steelman, P Tartter, D Taylor, E Thompson, K Thompson, W Todhunter, A Topilow, G Unruh, C Uzel, B A White, R Willcoxon, D Williams, B Wood.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Early Breast Cancer Trialists Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM. Tamoxifen: the long and short of it. J Natl Cancer Inst. 1996;88:1510–1512. doi: 10.1093/jnci/88.21.1516. [DOI] [PubMed] [Google Scholar]
- 4.Peto R. Five years of tamoxifen—or more? J Natl Cancer Inst. 1996;88:1791–1793. doi: 10.1093/jnci/88.24.1791. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Costantino JP, Wickerham DL. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Dignam J, Bryant J. The worth of 5 versus more than 5 years of tamoxifen therapy for breast cancer patients with negative nodes and estrogen-receptor positive tumors: an update of NSABP B-14. J Natl Cancer Inst. 1996;88:1529–1543. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 7.Tormey DC, Gray R, Falkson HC, for the Eastern Co-operative Oncology Group Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. J Natl Cancer Inst. 1996;88:1828–1833. doi: 10.1093/jnci/88.24.1828. [DOI] [PubMed] [Google Scholar]
- 8.Stewart HJ, Forrest AP, Everington D. Randomised comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. Br J Cancer. 1996;74:297–299. doi: 10.1038/bjc.1996.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US National Institutes of Health . National Cancer Institute Clinical Announcement: adjuvant therapy of breast cancer—tamoxifen update. National Institutes of Health; Bethesda, MD: 1995. [Google Scholar]
- 10.US National Institutes of Health NIH consensus statement. Adjuvant therapy for breast cancer. http://consensus.nih.gov/2000/2000AdjuvantTherapyBreastCancer114html.htm (accessed Oct 10, 2012). [PubMed]
- 11.Gray R, Davies C, Perry P. Tamoxifen for early breast cancer: better late than never. Ann Oncol. 2000;11:505–507. doi: 10.1023/a:1008323116265. [DOI] [PubMed] [Google Scholar]
- 12.Earl H, Gray R, Kerr D, Lee M. The optimal duration of tamoxifen treatment for breast cancer remains uncertain: randomize into aTTom. Clin Oncol (R Coll Radiol) 1997;9:141–143. doi: 10.1016/s0936-6555(97)80067-2. [DOI] [PubMed] [Google Scholar]
- 13.Gray RG, Rea DW, Handley K. ATTom: randomized trial of 10 versus 5 years of adjuvant tamoxifen among 6,934 women with estrogen receptor-positive (ER+) or ER untested breast cancer—preliminary results. Proc Am J Clin Oncol. 2008;26(suppl 10) abstr 513. [Google Scholar]
- 14.Davies C, McGale P, Peto R. Variation in use of adjuvant tamoxifen. Lancet. 1998;351:1487–1488. doi: 10.1016/S0140-6736(05)78869-3. [DOI] [PubMed] [Google Scholar]
- 15.WHO . International statistical classification of diseases and health-related problems, tenth revision. World Health Organization; Geneva: 1992. [Google Scholar]
- 16.Li CI, Kathleen E, Malone E, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–607. [PubMed] [Google Scholar]
- 17.Goss PE. Preventing relapse beyond 5 years: the MA.17 extended adjuvant trial. Semin Oncol. 2006;33(suppl 7):S8–S12. doi: 10.1053/j.seminoncol.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Early Breast Cancer Trialists' Collaborative Group . Treatment of early breast cancer: worldwide evidence, 1985–1990. Oxford University Press; Oxford: 1990. Introduction and methods.http://www.ctsu.ox.ac.uk/research/meta-trials/ebctcg/original-methods-for-ebctcg-meta-analyses (accessed Oct 10, 2012). [Google Scholar]
- 19.Love RR, Wiebe DA, Newcomb PA. Effects of tamoxifen on cardiovascular risk factors in post-menopausal women. Ann Intern Med. 1991;115:860–864. doi: 10.7326/0003-4819-115-11-860. [DOI] [PubMed] [Google Scholar]
- 20.McDonald CC, Stewart HJ, for the Scottish Breast Cancer Committee Fatal myocardial infarction in the Scottish Adjuvant Tamoxifen Trial. BMJ. 1991;303:435–437. doi: 10.1136/bmj.303.6800.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guetta V, Lush RM, Figg WD. Effects of the antioestrogen tamoxifen on low density lipoprotein concentrations and oxidation in post-menopausal women. Am J Cardiol. 1995;76:1072–1073. doi: 10.1016/s0002-9149(99)80302-6. [DOI] [PubMed] [Google Scholar]
- 22.Heart Protection Study Collaborative Group Effects on 11 year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals. Lancet. 2011;378:2013–2020. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration Nolvadex (tamoxifen citrate) http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154510.htm (accessed Oct 10, 2012).
- 24.Dowsett M, Cuzick J, Ingle J. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


