Infants with sickle cell disease have as much as a 100-fold increased rate of pneumococcal infection compared with the general population.1 Penicillin prophylaxis has been shown to reduce the risk of pneumococcal sepsis by 84%.2 Thus, sickle cell management guidelines include twice daily penicillin prophylaxis for infants and young children.3 While studies have described low rates of adherence to prophylactic antibiotic guidelines in older children, little is known about initiation of prophylactic antibiotics in early infancy, a period of great vulnerability.4 We describe adherence to guidelines for initiation of prophylactic antibiotics in a cohort of Tennessee Medicaid infants with sickle cell disease and examine risk factors for non-adherence to guidelines.
Methods
We conducted a 10-year retrospective cohort study, using data from Tennessee’s Medicaid (TennCare) Program. We used ICD-9 claims to identify children with Hemoglobin SS disease and pharmacy claims to determine if prophylactic antibiotics were filled.4 Maternal and infant demographic information was collected from birth certificates.
Infants were eligible for the cohort if they: had a diagnosis of sickle cell disease, were born between 1997-2006, and were enrolled in TennCare at birth. Infants born prior to 34 weeks gestational age were excluded. The final cohort included 407 infants.
The primary study endpoint was filling or not filling a recommended antibiotic prescription by 12 weeks of life, reflecting the language in the guidelines and clinical practice. Patients were classified according to potential confounders and effect modifiers, including infant factors, maternal factors, and geographic factors. We used Poisson regression with robust standard errors to investigate the association between risk factors and failure to fill an antibiotic prescription. Pearson’s chi-squared test was used to assess the unadjusted association of categorical risk factors with guideline non-adherence.
Results
Among 407 infants in the cohort, 60% failed to fill an antibiotic prescription in the first 12 weeks of life. Individual maternal, infant, and demographic factors were not predictive of non-adherence with prophylactic antibiotic guidelines.
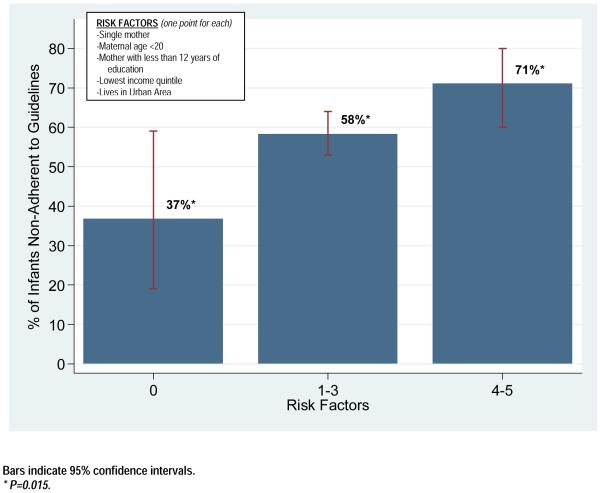
We generated a “risk score” for each infant, based on the presence of specific risk factors previously shown to predict non-adherence with pediatric preventive care (maternal age, maternal marital status, maternal education, income, and residence in an urban area).5,6 Among infants with 0 risk factors (n=19), 36.8% were non-adherent to guidelines, compared to 58.3% and 71.1% of those with 1-3 (n=312) or 4-5 risk factors (n=76), respectively. There was a significant difference in non-adherence to prophylactic antibiotic guidelines among infants in the three risk factor categories (p=0.02, Figure 1).
Figure 1.
Association of Multiple Risk Factors with Non-Adherence to Prophylactic Antibiotic Guidelines Among a Cohort of Tennessee Medicaid Infants with Sickle Cell Disease Born from 1997-2006.
Comment
Despite the availability of published guidelines for preventive care for infants with sickle cell disease, 60% of infants covered by Medicaid in Tennessee failed to fill a prophylactic antibiotic prescription within the recommended time period. In addition, the presence of multiple sociodemographic risk factors was associated with failure to fill prophylactic antibiotic prescriptions in our population.
Because we estimated non-adherence to antibiotic recommendations using pharmacy claims (a surrogate for adherence to guidelines), it is not possible to determine the reasons for non-adherence. It is possible that either parents did not fill prescriptions or that providers did not prescribe prophylactic medications as recommended. While uncertainty regarding the reasons for non-adherence does not influence the ultimate outcome, a better understanding of reasons for non-adherence will facilitate targeted interventions to improve compliance.
This study highlights an important problem—many infants with sickle cell disease in Tennessee do not receive guideline-recommended antibiotic prophylaxis. This is likely to increase the risk for serious, sometimes fatal, pneumococcal infections. Future efforts should focus on understanding the sources of non-adherence and implementing targeted interventions to improve adherence to prophylactic antibiotic guidelines in this population.
Acknowledgements
This study was supported by a National Institutes of Health National Research Service Award Grant, 5T32HD044328-05, and an AHRQ CERT Grant 5U18HS016974-02.
Footnotes
Michael Warren wrote the first draft of this research letter. None of the authors have a conflict of interest relating to this work. No reprints are requested.
Reference List
- (1).O’Brien KL, Swift A, Winkelstein JA, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 among infants with sickle cell disease. Pediatrics. 2000;106:965–972. doi: 10.1542/peds.106.5.965. [DOI] [PubMed] [Google Scholar]
- (2).Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. The New England Journal of Medicine. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- (3).National Institutes of Health National Heart Lung and Blood Institute The Management of Sickle Cell Disease. 2002 [Google Scholar]
- (4).Sox CM, Cooper WO, Koepsell TD, DiGiuseppe DL, Christakis DA. Provision of Pneumococcal Prophylaxis for Publicly Insured Children With Sickle Cell Disease. JAMA. 2003;290:1057–1061. doi: 10.1001/jama.290.8.1057. [DOI] [PubMed] [Google Scholar]
- (5).Bundt TS, Hu HM. National examination of compliance predictors and the immunization status of children: precursor to a developmental model for health systems. Mil Med. 2004;169:795–803. doi: 10.7205/milmed.169.10.795. [DOI] [PubMed] [Google Scholar]
- (6).Scholer SJ, Hickson GB, Ray WA. Sociodemographic factors identify US infants at high risk of injury mortality. Pediatrics. 1999;103:1183–1188. doi: 10.1542/peds.103.6.1183. [DOI] [PubMed] [Google Scholar]