Abstract
Ramoplanin is a lipoglycodepsipeptide antimicrobial active against clinically important Gram-positive bacteria including methicillin resistant Staphylococcus aureus. To proactively examine ramoplanin resistance, we subjected S. aureus NCTC 8325-4 to serial passage in the presence of increasing concentrations of ramoplanin, generating the markedly resistant strain RRSA16. Susceptibility testing of RRSA16 revealed the unanticipated acquisition of cross-resistance to vancomycin and nisin. RRSA16 displayed phenotypes, including a thickened cell wall and reduced susceptibility to Triton X-100 induced autolysis, which are associated with vancomycin intermediate resistant S. aureus strains. Passage of RRSA16 for 18 days in drug-free medium yielded strain R16-18d with restored antibiotic susceptibility. The RRSA16 isolate may be used to identify the genetic and biochemical basis for ramoplanin-resistance and further our understanding of the evolution of antibiotic cross-resistance mechanisms in S. aureus.
Keywords: ramoplanin, Staphylococcus aureus, vancomycin, VISA, nisin, cross-resistance
INTRODUCTION
Staphylococcus aureus is the frequent causative agent of hospital and community acquired infections. In 2005 there were an estimated 94,360 invasive methicillin resistant S. aureus (MRSA) cases and an estimated 18,650 deaths in the United States due to these infections (Klevens, et al., 2007). Most alarming is the observation that in 2005, the number of deaths in the United States (US) attributed to MRSA infections exceeded the total number of US deaths attributable to human immunodeficiency virus/AIDS (Bancroft, 2007, Klevens, et al., 2007).
Ramoplanin is a lipoglycodepsipeptide antibiotic active against clinically important Gram-positive bacteria including vancomycin resistant Enterococcus sp. (VRE), methicillin resistant S. aureus (MRSA) and vancomycin intermediate resistant Clostridium difficile (Neu & Neu, 1986, Jones & Barry, 1989, Biavasco, et al., 1991, Johnson, et al., 1992, Mobarakai, et al., 1994, Ristow, et al., 1995, Rolston, et al., 1996, Finegold, et al., 2004, Pelaez, et al., 2005). Preclinical studies have demonstrated that ramoplanin excerted a rapid bactericidal effect on S. aureus biofilms (Opperman, et al., 2003) and that a clinical vancomycin-resistant S. aureus strain containing the vanA gene was susceptible to ramoplanin (Bozdogan, et al., 2003). In the immediate past, ramoplanin was evaluated as a possible treatment for infection from these microorganisms, and in Asia, the use of the structurally-related antibiotic enduracidin has been in use as a growth promoting feed additive for livestock (Frankel, et al., 2003).
Treatment options for MRSA infections are limited as many MRSA strains are resistant to multiple antimicrobial agents (Ayliffe, 1997). Vancomycin remains the drug of last resort to combat infections from these microorganisms. However, the widespread use of vancomycin to treat MRSA infections has resulted in the increased frequency of isolation of vancomycin intermediate level resistant S. aureus (VISA) strains, from both clinical and community sources (Walsh & Howe, 2002). These data underscore the need for a better understanding of the molecular underpinnings of how resistance may arise to existing, and in particular, investigational antimicrobials (Mangili, et al., 2005). Generation of a S. aureus strain with reduced susceptibility to ramoplanin provides a model system to achieve greater insight into the mechanisms of ramoplanin action and the evolution of resistance mechanisms in Gram-positive bacteria.
Materials and Methods
Bacterial strain
S. aureus strain NCTC 8325-4 (also known as NRS135, (Novick, 1967)) was obtained from the repository maintained by the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA, www.narsa.net).
Isolation of ramoplanin-resistant S. aureus RRSA16
To generate ramoplanin-resistant S. aureus, a step pressure method was employed. Isolated colonies of S. aureus NCTC 8325-4 were inoculated into 5 mL aliquots of cation-adjusted Muller Hinton broth II supplemented with 0.02% Fraction V bovine serum albumin (CAMHB2+BSA) containing ramoplanin at concentrations of 0.1 to 10 μg/mL. The cultures were incubated at 37 °C with aeration for 48 h. At 48 h growth was observed in the culture containing 0.1 μg/mL ramoplanin. This culture was used to inoculate 5 mL CAMHB2+BSA containing ramoplanin at concentrations of 0.1 to 5 μg/mL at cell density of ~106 CFU/mL. These cultures were incubated for 24 to 72 h at 37 °C with aeration. The culture with growth in the highest concentration of ramoplanin was used to inoculate another series at a cell density of ~106 CFU/mL. Passage of the culture in this fashion was continued through the sixteenth series. In the sixteenth series growth was observed in a culture containing 5 μg/mL ramoplanin. A sample from this culture was plated on tryptic soy agar (TSA) with no antibiotic and incubated at 37 °C overnight. An isolated colony was selected and streaked onto TSA and grown overnight at 37 °C twice, then an isolated colony was then selected and named RRSA16. Oligonucleotide primers 16s_fw_sa (5′-CGTGCCTAATACATGCAAGTC-3′) and 16S_univ_rv (5′-ACGGGCGGTGTGTACAAG-3′) were used to amply a portion of the genes encoding 16s rRNA from genomic DNA prepared from each NCTC 8325-4 and RRSA16. Nucleotide sequences obtained from reactions performed with primers 16s_fw_sa and 16S_univ_rv on the amplified sequences from NCTC 8325-4 and RRSA16 were identical to each other and the published sequence of NCTC 8325-4.
Passage of RRSA16 on antibiotic-free media
An overnight culture of RRSA16 was sub-cultured into CAMHB2+BSA containing no antibiotics at a cell density of ~104 CFU/mL and incubated overnight at 37 °C with shaking. The overnight growth was used to inoculate a fresh CAMHB2+BSA culture containing no antibiotic at a cell density of ~104 CFU/mL and incubated overnight at 37 °C with shaking. Following the eighteenth passage in this manner, a sample from this culture was plated on TSA, and an isolated colony was selected (named R16-18d), to indicate its passage in non-selective media for 18 days). Genomic DNA was isolated from R16-18d and the sequence of the 16s rRNA gene was determined to be identical to the sequence from NCTC 8325-4 and RRSA16 as described above.
Broth microdilution determination of minimum inhibitory concentrations
Minimum inhibitory concentrations were determined on microdilution plates according to Weigand et al. (Wiegand, et al., 2008) using CAMHB2 as the growth media. Sodium chloride was added to a final concentration of 2% (w/v) when oxacillin was tested. BSA (0.02% w/v) was added to media when vancomycin, ramoplanin or nisin were tested to prevent peptide adhesion to polystyrene.
Calculation of doubling time
Doubling times were calculated as described (Cui, et al., 2003), with tryptic soy broth (TSB) cultures growing at 37 °C with aeration in the exponential phase (Eq. 1), where t1 and t2 are the times of measurement:
| (Equation 1) |
Time kill assays
S. aureus cultures were grown in TSB supplemented with 0.02% BSA (TSB+BSA) at 37 °C with shaking at 200 rpm to OD620 ≈ 0.4 and were then treated with antibiotic. The cultures were then incubated at 37 °C with shaking at 200 rpm. Samples were removed periodically for optical density measurements and viable counting.
Electron microscopy
S. aureus cultures were grown in TSB+BSA at 37 °C with aeration to an OD620 of ≈ 0.7. Samples were removed, pelleted and resuspended in 4% glutaraldehyde. The pellets were washed twice in 0.1 M sodium cacodylate buffer containing 7.5% sucrose and pre-embedded in 1% agar. The samples were washed twice with 0.1 M sodium cacodylate buffer containing 7.5% sucrose and post-fixed in 1.0% osmium tetroxide in 0.15 M sodium cacodylate buffer. Samples were washed for 10 min twice in 0.11 M veronal acetate buffer. Samples were then dehydrated in an ascending ethanol series and embedded in Epon resin. Sections were cut at 80 nm on a Reichert Ultracut S ultramicrotome and mounted on copper rhodium 200 mesh 3mm grids. Samples were stained with uranyl acetate for 30 min, rinsed three times in distilled water, stained with Reynold’s lead citrate stain prepared as described by Venable and Coggeshall (Venable & Coggeshall, 1965) for 5 min and rinsed three times in distilled water. Samples were viewed with a Philips/FEI CM12 transmission electron microscope at 80 kV. Cell-wall thickness was calculated as described elsewhere (Cui, et al., 2000). Twenty radial lines arranged regularly at angles of 18° were placed over the center of images of equatorially cut cells at final magnification of ×35,000 and the thickness of the cell wall measured from at least 10 different points. The thickness of the cell walls of 20 cells from each strain was measured. Results are reported as means ± standard deviation. The diameter of the 20 cells from each strain was measured as well using 20 radial lines arranged regularly at angles of 18° and placed over the center of equatorially cut cells; the results were reported as means ± standard deviation. The statistical significance of the data was evaluated by a Student’s t test.
Autolysis assay
Triton X-100 induced lysis assays were carried out as described elsewhere (Chatterjee, et al., 1976, Mani, et al., 1993). Briefly, 100 mL cultures of S. aureus growing exponentially (OD620 ≈ 0.6) in TSB medium at 37 °C with aeration were pelleted, washed twice in cold 0.05 M Tris-HCl (pH 7.2), then resuspended in 50 mL of 0.05 M Tris-HCl (pH 7.2) containing 0.05% (vol/vol) Triton X-100 (Sigma Chemical Co. St. Louis, Mo.). The cells were incubated at 37 °C with shaking and the OD620 was measured at 30 min intervals for 5 h. Values reported are averages of at least 3 independent experiments. The statistical significance of the data was evaluated by a Student’s t test.
RESULTS
Exposure of S. aureus NCTC 8325-4 to increasing concentrations of ramoplanin generates a strain with reduced susceptibility to ramoplanin, vancomycin and nisin
To proactively examine resistance to ramoplanin, we generated a resistant strain by serial passage of S. aureus NCTC 8325-4 in the presence of sub-minimum inhibitory concentrations of ramoplanin. The results from each passage of NCTC 8325-4 are shown in Table 1. In general, multiple passages were required for S. aureus to be able grow in the next higher concentration of ramoplanin. During the sixteenth passage, growth was observed in a culture containing 5 μg/mL ramoplanin. A sample from this culture was plated on TSA. An isolated colony was selected and passed twice on TSA, then a colony was selected and named RRSA16 for “ramoplanin resistant S. aureus sixteenth series.” The nucleotide sequence of the 16s rRNA genes of RRSA16 were identical to those of its S. aureus NCTC 8325-4 progenitor.
Table 1.
Serial passage of S. aureus in increasing concentrations of ramoplanin
| Passage # | Highest concentration of ramoplanin allowing growth (μg/mL) |
Growth time (hours)a |
|---|---|---|
| 1 | 0.1 | 48 |
| 2 | 0.1 | 24 |
| 3 | 0.25 | 24 |
| 4 | 0.5 | 48 |
| 5 | 0.5 | 24 |
| 6 | 0.5 | 24 |
| 7 | 1 | 24 |
| 8 | 1 | 24 |
| 9 | 2 | 24 |
| 10 | 2 | 24 |
| 11 | 3 | 48 |
| 12 | 3 | 72 |
| 13 | 3 | 24 |
| 14 | 3 | 24 |
| 15 | 4 | 24 |
| 16 | 5b | 48 |
Time required to visually observe growth in media containing highest concentration of ramoplanin that allowed growth when compared to negative control.
Growth was observed in a tube containing 5 μg/mL ramoplanin at 48 hours. No growth was observed at 48 hours in the tube containing the next highest amount of ramoplanin, 7.5 μg/mL.
The susceptibility of RRSA16 to a panel of antimicrobials focused on cell wall active compounds was determined by broth microdilution (Table 2). The ramoplanin minimum inhibitory concentration (MIC) increased from 0.75 μg/mL for NCTC 8325-4 to 8 μg/mL for RRSA16. Interestingly, RRSA16 had reduced susceptibility to two other antimicrobials that act by binding peptidoglycan lipid intermediate II, vancomycin and nisin. The vancomycin MIC increased from 1.25 μg/mL for NCTC 8325-4 to 9 μg/mL for RRSA16, a level classified as VISA. The nisin MIC increased from 10 μg/mL for NCTC 8325-4 to >32 μg/mL for RRSA16. The MIC for oxacillin, which inhibits peptidoglycan at the transpeptidation step, increased slightly from 0.25 μg/mL for NCTC 8325-4 to 0.5 μg/mL for RRSA16. No changes in the susceptibility were observed for bacitracin, phosphomycin, l-cycloserine, ciprofloxacin, erythromycin or rifampcin.
Table 2.
Susceptibility of NCTC 8325-4 and RRSA16 to various antibiotics as determined by microdilution susceptibility assays
| MIC (μg/mL) |
|||
|---|---|---|---|
| Compound | NCTC 8325-4 | RRSA16 | R16-18d |
| Ramoplanin | 0.75 | 8 | 3 |
| Vancomycin | 1.25 | 9 | 4 |
| Oxacillin | 0.25 | 0.5 | 0.25 |
| Nisin | 10 | >32 | >32 |
| Bacitracin | 32 | 32 | N/P |
| D-cycloserine | 64 | 64 | N/P |
| Phosphomycin | 8 | 8 | N/P |
| Ciprofloxacin | 0.25 | 0.25 | N/P |
| Erythromycin | 1 | 1 | N/P |
| Rifampicin | <0.06 | <0.06 | N/P |
The resistant RRSA16 was passed in antibiotic-free medium for 18 days, generating R16-18d, a strain that was more sensitive to ramoplanin and vancomycin than RRSA16 (Table 2). These values are still higher than the MICs observed for NCTC 8325-4. The nisin MIC of R16-18d remained higher than 32 μg/mL, the highest concentration tested.
RRSA16 has altered growth and ramoplanin time-kill characteristics
We next wished to examine RRSA16 for altered growth characteristics when grown in rich media. The doubling time of RRSA16 was 41 min, almost twice as long as the 23 min doubling time observed for NCTC 8325-4. The R16-18d doubling time of 26 min was similar to the doubling time of NCTC 8325-4.
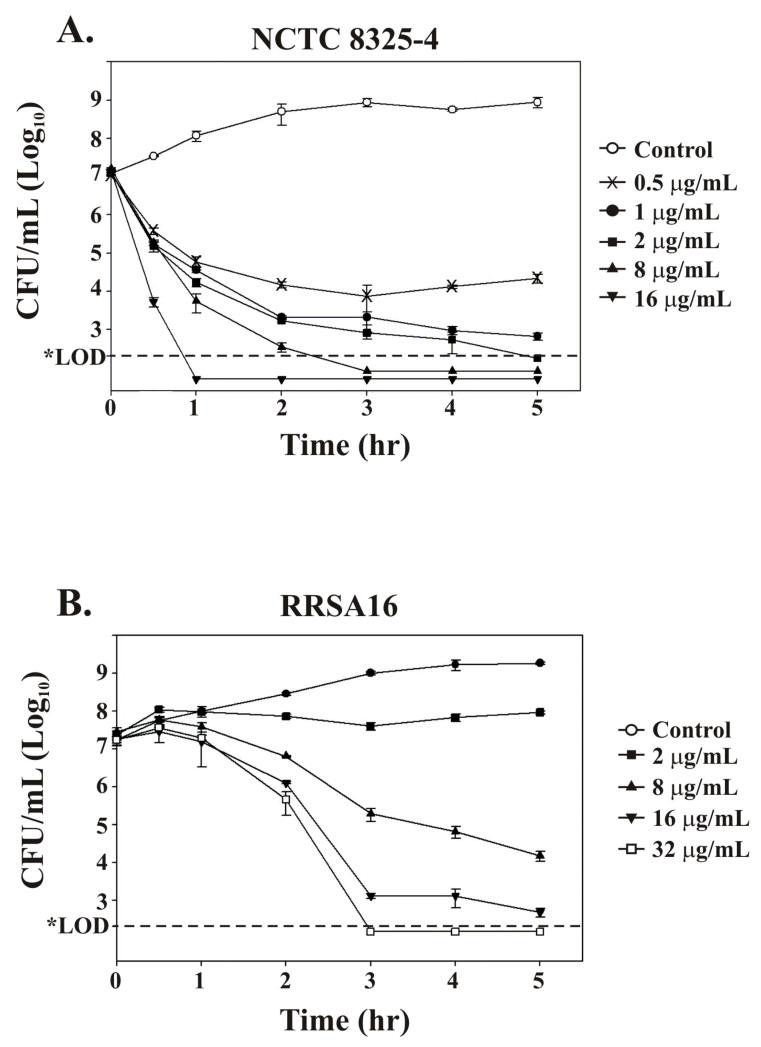
Exposure of NCTC 8325-4 to ramoplanin results in an immediate bactericidal effect even at the lowest concentration tested (0.5 μg/mL) (Figure 1A). When RRSA16 cultures were exposed to ramoplanin the bactericidal effect was delayed and reduced in severity (Figure 1B). Interestingly, at all the ramoplanin concentrations tested with RRSA16, including 32 μg/mL, viable counts were observed to increase at 30 min following ramoplanin addition.
Figure 1. Effect of ramoplanin on S. aureus viability is delayed and reduced in RRSA16.
S. aureus was grown to mid exponential phase (OD620 ≈ 0.4) and ramoplanin was added to the final concentrations indicated. No ramoplanin was added to control cultures. The horizontal dotted line indicates the viable count limit of detection of 300 CFU/mL. (A) Effect of ramoplanin on S. aureus NCTC 8325-4 viability. Note the drop in the viable counts of NCTC 8325-4 cultures following the addition of ramoplanin at all concentrations shown at 0.5 hr following antibiotic addition. (B) Effect of ramoplanin on S. aureus RRSA16 viability. Note that the viable counts of RRSA16 cultures were increased at 0.5 hr following ramoplanin addition at all antibiotic concentrations tested. Higher concentrations of ramoplanin and longer times were required to reduce RRSA16 viable counts three orders of magnitude.
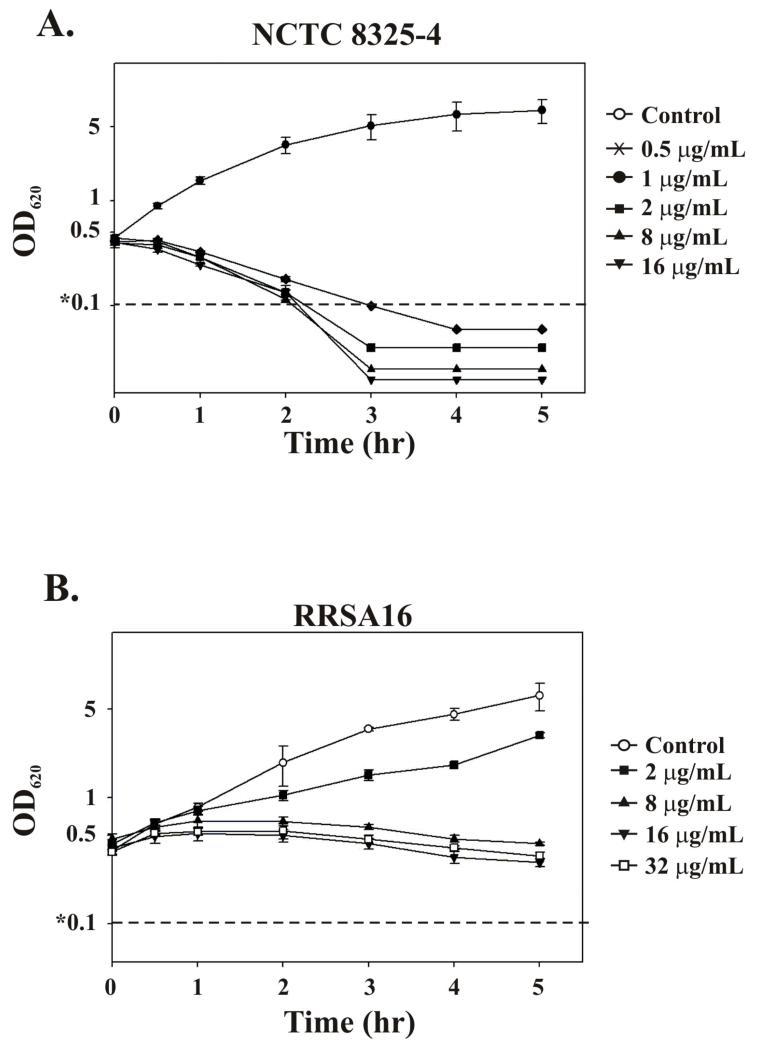
Rapid lysis was observed when NCTC 8325-4 was exposed to ramoplanin. Following the addition of 0.5 μg/mL ramoplanin at OD620 ≈ 0.4, the OD620 declined to less than 0.1 (the limit of detection) after 4 h (Figure 2A). This lytic effect appears to be independent of the concentration of ramoplanin since increasing the concentration of ramoplanin up to 16 μg/mL did not increase the rate of the OD620 decline. When RRSA16 was treated with ramoplanin no immediate lytic effect was observed (Figure 2B). Furthermore, the RRSA16 OD620 values increased at 30 min following ramoplanin addition. The OD620 values for RRSA16 then leveled out at 1 h following treatment and thereafter declined very slowly (except for RRSA16 exposed to 2 μg/mL ramoplanin for which the OD620 continued to increase). The increase in OD620 at 30 min following ramoplanin addition observed for RRSA16 agrees with the increase in viable count also observed. The very slow decline of OD620 at ramoplanin concentrations of 8, 16 and 32 μg/mL after 30 min combined with the loss of RRSA16 viability may indicate death without lysis (Figures 1B and 2B).
Figure 2. Bacteriolytic effect of ramoplanin not observed with RRSA16.
S. aureus was grown to mid exponential phase (OD620 ≈ 0.4) and ramoplanin was added to the indicated final concentration. No ramoplanin was added to control cultures. The horizontal dotted line indicates the spectrophotometer sensitivity limit of 0.1 AU. (A) Ramoplanin induces lysis of NCTC 8325-4. Optical density recorded at 620 nm of NCTC 8325-4 cultures when treated with all concentrations shown (B) Graph of the optical density at 620 nm of S. aureus RRSA16 as a function of time following addition of ramoplanin. Optical density does not drop rapidly following the addition of ramoplanin to RRSA16 cultures.
S. aureus RRSA16 has a thicker cell wall and smaller cell diameter than susceptible NCTC 8325-4
Since cell wall thickening is a common VISA phenotype (Cui, et al., 2003) and RRSA16 displayed a decreased susceptibility to vancomycin, we performed electron microscopy to determine if the cell wall had thickened. Electron micrographs of representative cells from each strain are shown in Figure 3 at ×35,000 magnification. Electron microscopy indicated that the RRSA16 cell wall thickness averaged 44.5 ± 4.0 nm, approximately twice as thick as the average NCTC 8325-4 cell wall thickness of 21.5 ± 4.2 nm (Figure 3). Additionally, RRSA16 cells were smaller than their NCTC 8325-4 progenitors, with an average diameter of 0.75 ± 0.06 μm as compared to the average diameter of NCTC 8325-4 cells, 1.09 ± 0.13 μm (Figure 3). Other than the thickened cell wall and reduced cell size RRSA16 cells had normal appearance including placement of septa (Figure 3).
Figure 3. Thickened cell wall and reduced cell size of RRSA16.
Representative transmission electron microscopy images at ×35,000 of NCTC 8325-4 and RRSA16 prepared as described in the Materials and Methods section. The average cell diameter and cell wall thickness (expressed as mean ± standard deviation of the mean) for each strain are indicated. A P-value of 5.7 × 10-20 was calculated using a two-tailed Student’s t test comparing the cell diameter measurements of NCTC 8325-4 and RRSA16 indicating that the cell diameters were significantly different. A P-value of 1.4 × 10-15 was calculated using a two-tailed Student’s t test comparing the cell wall thickness measurements of NCTC 8325-4 and RRSA16 indicating that the cell wall thicknesses were significantly different.
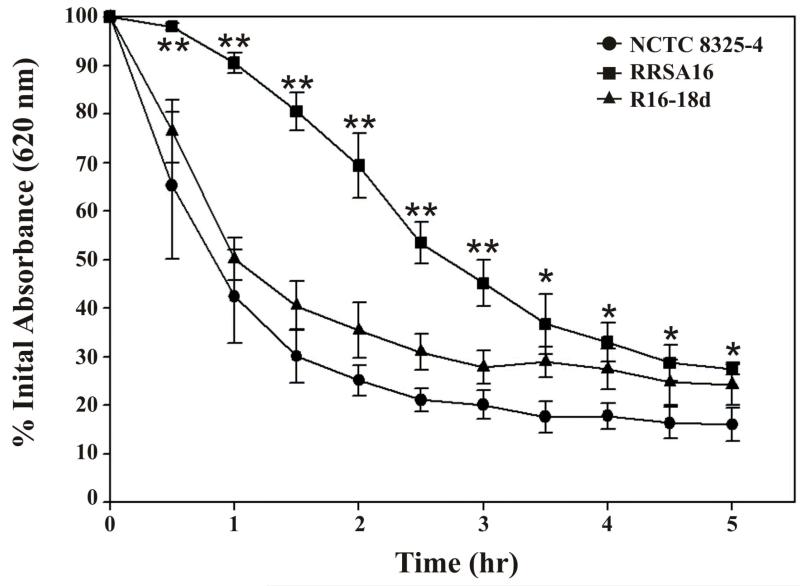
RRSA16 has decreased susceptibility to Triton X-100 induced lysis
Increased resistance to Triton X-100 induced lysis is indicative of alterations to the autolytic enzyme profile and is associated with decreased susceptibility to vancomycin (Lu, et al., 2005). Furthermore, the absence of lysis observed when RRSA16 was exposed to ramoplanin might indicate that autolytic enzyme activity was repressed. The amount of Triton X-100 induced autolysis of RRSA16 was significantly lower than NCTC 8325-4 at each time point measured, indicative of reduced activity of autolytic enzymes in the ramoplanin-resistant strain (Figure 4). The amount of Triton X-100 induced autolysis observed with R16-18d was similar to that of NCTC 8325-4.
Figure 4. Reduced susceptibility of RRSA16 to Triton X-100 induced lysis.
Cultures of S. aureus growing in exponential phase (OD620 ≈ 0.6) were resuspended in buffer containing 0.05% Triton X-100 as described in the Materials and Methods section. The decrease in OD620 as a function of time is shown for each strain. Note the slower and less complete lysis of RRSA16 in comparison to its progenitor strain NCTC 8325-4. Two-tailed Student’s t tests were performed to determine if the values observed for RRSA16 were significantly different from the values for NCTC 8325-4 and R16-18d at each time point except 0 hours. Error bars indicate the standard deviation of the mean for each value. Values observed for RRSA16 that are labeled with a double asterisk (**) were determined to be significantly different from both NCTC 8325-4 and R16-18d since the calculated P-values for both Student’s t tests at that point were < 0.05. Values observed for RRSA16 that are labeled with a single asterisk (*) were determined to be significantly different from only NCTC 8325-4 since the P-values returned from the Student’s t tests performed comparing RRSA16 and NCTC 8325-4 values were < 0.05 but the P-values returned from the Student’s t tests performed comparing RRSA16 and R16-18d values were > 0.05.
DISCUSSION
To proactively establish a model system to investigate ramoplanin resistance mechanisms in S. aureus, we subjected the NCTC 8325-4 strain to increasing concentrations of ramoplanin, generating strain RRSA16 that had a significantly decreased susceptibility to ramoplanin (Tables 1 & 2). To our knowledge this is the first report of ramoplanin resistance in clinical or laboratory settings. Ramoplanin treatment is thought to induce lysis by inhibiting the formation of new cell wall while autolytic enzymes responsible for cell wall turnover remain active, degrading the cell wall. Degradation of the cell wall leads to lysis caused by turgor pressure. When RRSA16 was exposed to ramoplanin, rapid lysis did not occur (Figure 2B), likely contributing to the delayed bactericidal effect (Figure 1B). The Triton X-100 induced autolysis assay demonstrated that autolytic enzymes had decreased activity in RRSA16 compared to its progenitor strain NCTC 8325-4 (Figure 4). Both the thickened cell wall layer (Figure 3) and decreased activity of autolytic enzymes in RRSA16 likely contribute to the observed loss of lysis following ramoplanin treatment and may contribute to the decreased susceptibility of RRSA16 to ramoplanin. However, it is unlikely that decreased autolytic activity was solely responsible for ramoplanin resistance as the R16-18d strain generated by passage of RRSA16 for 18 days in drug-free media had autolytic activity similar to that of NCTC 8325-4 (Figure 4) while its ramoplanin MIC was ~4 times higher than NCTC 8325-4 (Table 2).
An interesting finding of this study was that RRSA16 possessed a vancomycin MIC of 9 μg/mL, a level commensurate with VISA. VISA-type resistant strains display the phenotypes of a thickened cell wall (Hanaki, et al., 1998, Cui, et al., 2003, Howden, et al., 2006), reduced autolytic activity (Pfeltz, et al., 2000, Sieradzki & Tomasz, 2003, Howden, et al., 2006), reduced peptidoglycan cross-linking, and increased production of soluble N-acyl-d-Ala-d-Ala containing peptidoglycan fragments that are ligands for vancomycin (Sieradzki & Tomasz, 1997, Sieradzki & Tomasz, 1999, Cui, et al., 2003, Sieradzki & Tomasz, 2003, Cui, et al., 2006). VISA-type resistance cannot be attributed to the acquisition of a mobile genetic element nor can it be attributed to mutation of a single gene. Rather, VISA-type resistance arises from multiple mutations in many loci by a gradual adaptive process (Mwangi, et al., 2007, Howden, et al., 2008, Neoh, et al., 2008, Cui, et al., 2009). In this study we have demonstrated that RRSA16 had the VISA phenotypes of reduced autolytic activity (Figure 4) and a thickened cell wall (Figure 3). We suspect that increased cell wall material combined with reduced autolytic enzyme activity contributed to the increased ramoplanin resistance of RRSA16. Ramoplanin acts on vancomycin-resistant strains and targets a non-overlapping region of lipid intermediate II for sequestration (Cudic, et al., 2002), yet in RRSA16 marked vancomycin resistance emerged with ramoplanin resistance. Limited access to lipid II via restricted diffusion through the thickened cell wall to the outer membrane or by decoy titration through the overproduction of peptidoglycan precursors containing an intact pyrophosphate may explain the parallel resistance phenotypes observed. Since antibiotic susceptibility is significantly restored in the strain R16-18d, it is likely that a significant subset of events leading to ramoplanin resistant phenotype are transcriptionally controlled.
We also determined that RRSA16 had increased resistance to the lantibiotic nisin (Table 2). The site of nisin action is lipid II and similarly to ramoplanin, nisin binding requires the pyrophosphate moiety (Bonev, et al., 2004, Hsu, et al., 2004). However the primary mechanism of nisin action is not by substrate inhibition of transglycosylation; rather, stable pores composed of nisin and lipid II molecules are formed in the bacterial membrane resulting in lysis (Brotz, et al., 1998, Breukink, et al., 1999, van Heusden, et al., 2002, Breukink, et al., 2003, Hasper, et al., 2004). Decreased S. aureus susceptibility to nisin and other cationic peptide antimicrobials is confirmed by increased expression of the dlt operon resulting in increased d-alanylation of teichoic acids resulting in a more cationic cell envelope (Peschel, et al., 1999, Sass & Bierbaum, 2009). Increased d-alanylation of teichoic acids may influence susceptibility to ramoplanin as it is a cationic peptide, requiring ornithine at position 10 for molecular recognition of the lipid II pyrophosphate via an electrostatic interaction (Cudic, et al., 2002, Nam, et al., 2007). Furthermore, alteration of teichoic acid structure is known to modulate autolysin activity (Fedtke, et al., 2007). Since ramoplanin and nisin each bind the pyrophosphate moiety of lipid II and are both cationic one hypothesis that some component of the adaptations and mutations generated by serial passage in ramoplanin may have altered the ability of cationic peptides to associate with lipid II and/or the cell envelope. Further study of RRSA16 and R16-18d should provide insight into the molecular mechanism of ramoplanin resistance in S. aureus and may lead to strategies for the prevention of antimicrobial resistance during clinical use.
ACKNOWLEDGEMENTS
This work was generously supported by US Public Health Service grant AI46611 from the National Institutes of Health to D.G.M.
REFERENCES
- Ayliffe GA. The progressive intercontinental spread of methicillin-resistant. Staphylococcus aureus. Clin. Infect. Dis. 1997;24(Suppl 1):S74–79. doi: 10.1093/clinids/24.supplement_1.s74. [DOI] [PubMed] [Google Scholar]
- Bancroft EA. Antimicrobial resistance: it’s not just for hospitals. JAMA. 2007;298:1803–1804. doi: 10.1001/jama.298.15.1803. [DOI] [PubMed] [Google Scholar]
- Biavasco F, Manso E, Varaldo PE. In vitro activities of ramoplanin and four glycopeptide antibiotics against clinical isolates of Clostridium difficile. Antimicrob. Agents Chemother. 1991;35:195–197. doi: 10.1128/aac.35.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev BB, Breukink E, Swiezewska E, De Kruijff B, Watts A. Targeting extracellular pyrophosphates underpins the high selectivity of nisin. FASEB J. 2004;18:1862–1869. doi: 10.1096/fj.04-2358com. [DOI] [PubMed] [Google Scholar]
- Bozdogan B, Esel D, Whitener C, Browne FA, Appelbaum PC. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the Hershey Medical Center. J. Antimicrob. Chemother. 2003;52:864–868. doi: 10.1093/jac/dkg457. [DOI] [PubMed] [Google Scholar]
- Breukink E, Wiedemann I, van Kraaij C, Kuipers OP, Sahl H, de Kruijff B. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science. 1999;286:2361–2364. doi: 10.1126/science.286.5448.2361. [DOI] [PubMed] [Google Scholar]
- Breukink E, van Heusden HE, Vollmerhaus PJ, et al. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 2003;278:19898–19903. doi: 10.1074/jbc.M301463200. [DOI] [PubMed] [Google Scholar]
- Brotz H, Josten M, Wiedemann I, Schneider U, Gotz F, Bierbaum G, Sahl HG. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 1998;30:317–327. doi: 10.1046/j.1365-2958.1998.01065.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee AN, Wong W, Young FE, Gilpin RW. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J. Bacteriol. 1976;125:961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudic P, Kranz JK, Behenna DC, et al. Complexation of peptidoglycan intermediates by the lipoglycodepsipeptide antibiotic ramoplanin: minimal structural requirements for intermolecular complexation and fibril formation. Proc. Natl. Acad. Sci. USA. 2002;99:7384–7389. doi: 10.1073/pnas.102192099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Neoh HM, Shoji M, Hiramatsu K. Contribution of vraSR and graSR point mutations to vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2009;53:1231–1234. doi: 10.1128/AAC.01173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Murakami H, Kuwahara-Arai K, Hanaki H, Hiramatsu K. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 2000;44:2276–2285. doi: 10.1128/aac.44.9.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Iwamoto A, Lian JQ, Neoh HM, Maruyama T, Horikawa Y, Hiramatsu K. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2006;50:428–438. doi: 10.1128/AAC.50.2.428-438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Ma X, Sato K, et al. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 2003;41:5–14. doi: 10.1128/JCM.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedtke I, Mader D, Kohler T, et al. A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity. Mol. Microbiol. 2007;65:1078–1091. doi: 10.1111/j.1365-2958.2007.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, John SS, Vu AW, et al. In vitro activity of ramoplanin and comparator drugs against anaerobic intestinal bacteria from the perspective of potential utility in pathology involving bowel flora. Anaerobe. 2004;10:205–211. doi: 10.1016/j.anaerobe.2004.04.003. [DOI] [PubMed] [Google Scholar]
- McCafferty DG, Cudic P, Frankel BA, et al. Chemistry and biology of the ramoplanin family of peptide antibiotics. Biopolymers. 2002;66:261–84. doi: 10.1002/bip.10296. [DOI] [PubMed] [Google Scholar]
- Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 1998;42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- Hanaki H, Labischinski H, Inaba Y, Kondo N, Murakami H, Hiramatsu K. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 1998;42:315–320. doi: 10.1093/jac/42.3.315. [DOI] [PubMed] [Google Scholar]
- Hasper HE, de Kruijff B, Breukink E. Assembly and stability of nisin-lipid II pores. Biochemistry. 2004;43:11567–11575. doi: 10.1021/bi049476b. [DOI] [PubMed] [Google Scholar]
- Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2006;50:3039–3047. doi: 10.1128/AAC.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden BP, Stinear TP, Allen DL, Johnson PD, Ward PB, Davies JK. Genomic analysis reveals a point mutation in the two-component sensor gene graS that leads to intermediate vancomycin resistance in clinical Staphylococcus aureus. Antimicrob. Agents Chemother. 2008;52:3755–3762. doi: 10.1128/AAC.01613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu ST, Breukink E, Tischenko E, et al. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 2004;11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- Johnson CC, Taylor S, Pitsakis P, May P, Levison ME. Bactericidal activity of ramoplanin against antibiotic-resistant enterococci. Antimicrob. Agents Chemother. 1992;36:2342–2345. doi: 10.1128/aac.36.10.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Barry AL. In vitro evaluation of ramoplanin (A16686 or MDL62198). A new depsipeptide complex for potential topical use. Diagn. Microbiol. Infect. Dis. 1989;12:279–282. doi: 10.1016/0732-8893(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Lee SY, Hwa SY, Yang AH. Septic arthritis caused by vancomycin-intermediate Staphylococcus aureus. J. Clin. Microbiol. 2005;43:4156–4158. doi: 10.1128/JCM.43.8.4156-4158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangili A, Bica I, Snydman DR, Hamer DH. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2005;40:1058–1060. doi: 10.1086/428616. [DOI] [PubMed] [Google Scholar]
- Mani N, Tobin P, Jayaswal RK. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J. Bacteriol. 1993;175:1493–1499. doi: 10.1128/jb.175.5.1493-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarakai N, Quale JM, Landman D. Bactericidal activities of peptide antibiotics against multidrug-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 1994;38:385–387. doi: 10.1128/aac.38.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangi MM, Wu SW, Zhou Y, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc. Natl. Acad. Sci. USA. 2007;104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J, Shin D, Rew Y, Boger DL. Alanine scan of [L-Dap(2)]ramoplanin A2 aglycon: assessment of the importance of each residue. J. Am. Chem. Soc. 2007;129:8747–8755. doi: 10.1021/ja068573k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoh HM, Cui L, Yuzawa H, Takeuchi F, Matsuo M, Hiramatsu K. Mutated response regulator graR is responsible for phenotypic conversion of Staphylococcus aureus from heterogeneous vancomycin-intermediate resistance to vancomycin-intermediate resistance. Antimicrob. Agents Chemother. 2008;52:45–53. doi: 10.1128/AAC.00534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu HC, Neu NM. In vitro activity of A-16686, a new glycopeptide. Chemotherapy. 1986;32:453–457. doi: 10.1159/000238450. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Opperman TJ, Davey ME, Houseweart C, Rogers BL. Ramoplanin is effective against staphylococcal biofilms. Chicago, Ill: 2003. Abstract no. C1-1119. [Google Scholar]
- Pelaez T, Alcala L, Alonso R, Martin-Lopez A, Garcia-Arias V, Marin M, Bouza E. In vitro activity of ramoplanin against Clostridium difficile, including strains with reduced susceptibility to vancomycin or with resistance to metronidazole. Antimicrob. Agents Chemother. 2005;49:1157–1159. doi: 10.1128/AAC.49.3.1157-1159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- Pfeltz RF, Singh VK, Schmidt JL, et al. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob. Agents Chemother. 2000;44:294–303. doi: 10.1128/aac.44.2.294-303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow TA, Noskin GA, Warren JR, Peterson LR. In vitro activity of RP 59500 (quinupristin/dalfopristin) and ramoplanin against vancomycin-resistant Enterococcus faecium. Microb. Drug Resist. 1995;1:335–339. doi: 10.1089/mdr.1995.1.335. [DOI] [PubMed] [Google Scholar]
- Rolston KV, Dholakia N, Ho DH, LeBlanc B, Dvorak T, Streeter H. In-vitro activity of ramoplanin (a novel lipoglycopeptide), vancomycin, and teicoplanin against gram-positive clinical isolates from cancer patients. J. Antimicrob. Chemother. 1996;38:265–269. doi: 10.1093/jac/38.2.265. [DOI] [PubMed] [Google Scholar]
- Sass P, Bierbaum G. Native graS mutation supports the susceptibility of Staphylococcus aureus strain SG511 to antimicrobial peptides. Int. J. Med. Microbiol. 2009 doi: 10.1016/j.ijmm.2008.10.005. In press, doi: 10.1016/j.ijmm.2008.1010.1005. [DOI] [PubMed] [Google Scholar]
- Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzki K, Tomasz A. Gradual alterations in cell wall structure and metabolism in vancomycin-resistant mutants of Staphylococcus aureus. J. Bacteriol. 1999;181:7566–7570. doi: 10.1128/jb.181.24.7566-7570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzki K, Tomasz A. Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J. Bacteriol. 2003;185:7103–7110. doi: 10.1128/JB.185.24.7103-7110.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heusden HE, de Kruijff B, Breukink E. Lipid II induces a transmembrane orientation of the pore-forming peptide lantibiotic nisin. Biochemistry. 2002;41:12171–12178. doi: 10.1021/bi026090x. [DOI] [PubMed] [Google Scholar]
- Venable JH, Coggeshall R. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol. 1965;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TR, Howe RA. The prevalence and mechanisms of vancomycin resistance in Staphylococcus aureus. Annu. Rev. Microbiol. 2002;56:657–675. doi: 10.1146/annurev.micro.56.012302.160806. [DOI] [PubMed] [Google Scholar]
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]